Abstract
Absent in melanoma 2 (AIM2) plays an important role in innate immunity as a DNA sensor in the cytoplasm by triggering the assembly of an AIM2 inflammasome that results in caspase-1-mediated inflammatory responses and cell death. In recent years, studies have indicated that AIM2 can suppress cancer cell proliferation, and mutations in the gene encoding AIM2 are frequently identified in patients with colorectal cancer (CRC). However, the mechanism by which AIM2 restricts tumor growth remains unclear. We reconstructed AIM2 expression in HCT116 CRC cells by lentivirus transfection. Using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and flow cytometry, we demonstrated that expression of AIM2 inhibited the viability and increased the apoptosis rate of CRC cells, and cell cycle analysis suggested that AIM2 blocked cell cycle transition from G1 to S phase. Western blot analysis showed that AIM2 promoted apoptosis in CRC cells by suppressing the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) pathway. Our data suggest that AIM2 plays a critical role as a tumor suppressor and might serve as a potential therapeutic target in CRC.
Introduction
Colorectal cancer (CRC) is one of the leading causes of cancer-related death, and it is ranked as the second most common cancer in males and the third most common cancer in females worldwide according to Cancer Statistics for 2015.Citation1 Recent data have shown that the incidence of CRC in China has rapidly increased.Citation2 CRC is a heterogeneous disease with regard to molecular pathogenesis and clinical course, which makes it difficult to select patients who will benefit from therapies. Further study is required to identify new therapeutic targets in CRC.
Absent in melanoma 2 (AIM2) was originally reported as a tumor suppressor gene in melanoma in 1997 by DeYoung et al.Citation3 AIM2 is a member of the interferon-inducible PYRIN and HIN domain-containing (PYHIN) family proteins (also referred to as p200 family proteins).Citation4–Citation9 Recently, AIM2 was found to act as a DNA sensor in innate immunity. By direct binding to foreign double-stranded DNA in infected macrophages, AIM2 triggers the assembly of an AIM2 inflammasome, resulting in caspase-1-mediated inflammatory responses and cell death, and thus contributes to the host defense against bacterial and viral pathogens.Citation10–Citation15 Although the role of AIM2 in inflammasome activation is well accepted, its role in tumorigenesis is less clear. Exogenous AIM2 expression was shown to reduce human breast cancer cell proliferation by inhibition of nuclear factor kappa-B (NF-κB) transcriptional activity and to suppress mammary tumor growth in a mouse model.Citation16 A previous study showed that >50% of tumors from patients with small bowel cancer have frameshift mutations in the gene encoding AIM2.Citation17 A better understanding of the biological function of AIM2 in CRC is key to identifying therapies that could be used in the treatment of this disease.
Mutations in the gene encoding AIM2 are frequently identified in patients with CRC.Citation18,Citation19 Analysis of 414 colorectal tumors and matching control tissues further revealed that 67% of the tumors showed reduced expression of the gene encoding AIM2 relative to control tissues.Citation20 In this study, we aimed to identify the role of AIM2 expression in CRC cells and its underlying mechanism.
Materials and methods
Cell culture and lentivirus transfection
The human CRC HCT116 cell line was purchased from Genechem Co. (Shanghai, China) and cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C with 5% CO2. Lentiviral vector containing AIM2 was purchased from Genechem Co. HCT116 cells were infected with lentivirus to generate cell lines with stable expression of AIM2. HCT116 cells were incubated with infection medium containing recombinant lentivirus vectors at a multiplicity of infection of 20 for 16 hours, and then the medium was replaced with fresh complete medium. Green fluorescent protein (GFP) expression was observed by fluorescence microscopy to determine the proportion of GFP-positive cells at 72 hours. Empty vector was used as a negative control. The transfection efficiency was confirmed by quantitative real-time polymerase chain reaction (qRT-PCR) and Western blotting.
RNA isolation and qRT-PCR
Total RNA was extracted from HCT116 cells using TRI Reagent (Pufei International Trade Co., Ltd., Shanghai, China) according to the manufacturer’s instructions. Expression of target genes was detected by qRT-PCR using a QuantiFast SYBR Green PCR Kit (Qiagen, Germantown, MD, USA) following the manufacturer’s instructions. Primers for the AIM2 gene were as follows: forward 5′-CAGGAGGAGAAGGAGAAAGTTG-3′; reverse 5′-GTGCAGCACGTTGCTTTG-3′. Reactions of qRT-PCR were performed using MX3000p (Agilent, Santa Clara, CA, USA) according to the manufacturer’s instructions. A melting-curve analysis was performed to ensure specificity of the products. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was analyzed as an internal control. The relative mRNA levels of target genes were obtained by using the 2−ΔΔCt method with all assays performed in triplicate.
The program calculates the ΔΔCT value with the following formulas: ΔΔCt = ΔCt mean (AIM2 expression group) − ΔCt mean (negative control group); fold change of gene expression =2−ΔΔCt.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
The cell viability was measured using the MTT assay. Twenty-four hours after transfection, cells were seeded in 96-well plates at a density of 2,000 cells per well for 24, 48, 72, 96, and 120 hours. To determine cell proliferation, cells were incubated with 20 μL of 5 mg/mL MTT for 4 hours at 37°C and then 100 μL of dimethyl sulfoxide (DMSO) was added to solubilize the crystals with 20-minute incubation at room temperature. Optical density was measured at a wavelength of 490 nm. All experiments were performed three times, and average results were used to generate the growth curves.
Flow cytometry
For cell cycle analysis, 5×106 AIM2-expressing cells and negative control cells were harvested at log phase and fixed in 70% ethanol for 2 hours at 4°C. Cells were washed three times with cold phosphate-buffered saline (PBS) and then stained with 50 μg/mL propidium iodide (PI) and 100 μg/mL ribonuclease for 30 minutes each at 4°C in the dark. The proportion of cells in each phase of the cell cycle was analyzed using the Modft LT2.0 DNA assay (Becton Dickinson). Each experiment was performed in triplicate.
For the cell apoptosis assay, cells were harvested including nonadherent cells and washed in PBS, and 5×106 cells from each group were stained with Annexin V-Allophycocyanin using the Annexin V Apoptosis Detection Kit (eBioscience) according to the manufacturer’s protocol. The apoptosis rate was determined by flow cytometric analysis using CellQuest software. Each experiment was performed in triplicate.
Phosphatidylinositol 3-kinase (PI3K) activation
To investigate the role of the PI3K/Akt pathway in the suppressive effect of AIM2 on regulating the viability and apoptosis of CRC cells, we treated cells with insulin-like growth factor-1 (IGF-1), which activates the PI3K/Akt pathway and is upregulated in colon cancer.Citation21 AIM2-overexpressing HCT116 cells and negative control cells were plated overnight and then stimulated with 100 ng/mL IGF-1 (ab87177; Abcam). After incubation for another 24 hours, the apoptosis rate was analyzed by flow cytometric analysis, and the level of p-Akt was assessed by Western blotting.
Western blotting
For Western blotting analysis, cells in culture were lysed using radioimmunoprecipitation assay (RIPA) buffer and the protein concentration was measured by bicinchoninic acid assay. Proteins were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel electrophoresis and transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with 5% skim milk powder at room temperature, followed by the addition of primary antibodies against Akt1 (ab81283; Abcam) and GAPDH (sc-32233; Santa Cruz; as a control reference) and incubation overnight at 4°C on a shaking table. The membrane was washed three times, and then incubated with secondary antibodies for 1.5 hours at room temperature. Protein expression was detected using the enhanced chemiluminescence (ECL) substrate kit (Thermo Fisher Scientific, Rockford, IL, USA), and band intensity was quantified using ImageJ software.
Data analysis
Results were analyzed statistically using Student’s t-test for comparisons between two groups. Correlation parameters were subjected to Pearson and nonparametric Spearman correlations. P<0.05 was considered statistically significant.
Results
Establishment and characterization of AIM2-expressing HCT116 CRC cells
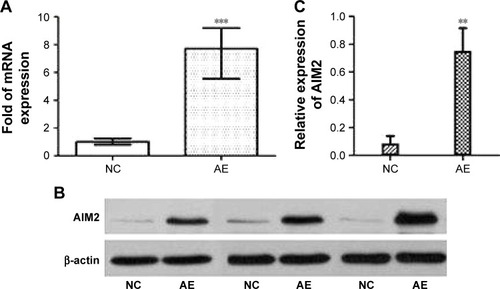
Previous reports have demonstrated that overexpression of AIM2 inhibits the progression of CRC. We reconstituted the expression of AIM2 in human HCT116 CRC cells, which lack functional AIM2,Citation19 by transfection with AIM2-bearing lentiviral vector. The transfection efficiency was evaluated by qRT-PCR. As shown in , the level of AIM2 mRNA was upregulated significantly (∼7.7-fold; ΔΔCt =−2.95) by transfection with lentivirus-AIM2 compared with the negative control cells. Western blot analysis confirmed the expression of AIM2 in transfected cells (), and the quantification showed that the relative protein level of AIM2 compared with β-actin was significantly higher in the AIM2-expressing cells compared with control cells (P<0.01, n=3; ). These results confirmed the successful construction of recombinant lentivirus containing the human AIM2 gene that was capable of efficiently infecting HCT116 cells and upregulating AIM2 protein expression.
Figure 1 Expression of AIM2 mRNA and protein measured by RT-PCR and Western blotting.
Abbreviations: AE, AIM2-expressing group; AIM2, absent in melanoma 2; NC, negative control; RT-PCR, real-time polymerase chain reaction.
Effects of AIM2 on CRC viability and apoptosis
To determine the effect of AIM2 on cell viability, the MTT assay was used to evaluate the viability of AIM2-HCT116 and negative control cells. The growth curves () showed that, relative to control cells, the viability of AIM2-HCT116 cells decreased significantly from day 1 to day 5 after transfection (n=3, P<0.01); the cell growth inhibition rates for days 1–5 were 22.84%, 33.43%, 26.15%, 27.30%, and 27.62%, respectively.
Figure 2 The effect of AIM2 on viability and apoptosis of HCT116 cells.
Abbreviations: AE, AIM2-expressing group; AIM2, absent in melanoma 2; APC, Allophycocyanin; IGF-1, insulin-like growth factor-1; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NC, negative control; OD, optical density.
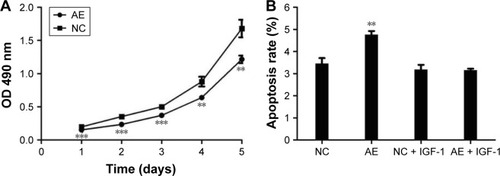
The cellular apoptosis rate in AIM2-HCT116 and negative control cells was 4.76%±0.17% vs 3.46%±0.24%, respectively (); thus, AIM2-HCT116 cells exhibited a significantly higher rate of apoptosis (n=3, P<0.01).
Effect of AIM2 on cell cycle progression of CRC cells
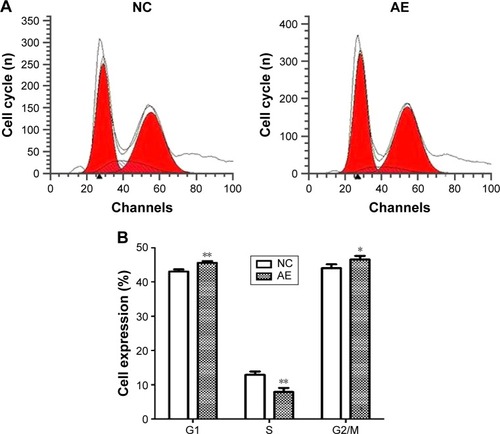
To further investigate the influence of AIM2 on the proliferative ability of HCT116 CRC cells, the distribution of cells in different phases of the cell cycle was analyzed by flow cytometry. As shown in , in AIM2-HCT116 CRC cells, the proportion of cells in G1 phase (45.56±0.51 vs 43.03±0.62, P<0.01) and G2/M phase (46.56±1.02 vs 44.04±1.11, P<0.05) was increased, whereas the proportion in S phase was decreased (7.88±1.1585 vs 12.93±0.94, P<0.01) relative to control-transfected cells.
Figure 3 Cell cycle distribution of HCT116 CRC cells.
Abbreviations: AE, AIM2-expressing group; AIM2, absent in melanoma 2; CRC, colorectal cancer; NC, negative control; PI, propidium iodide.
Relationship between AIM2 and the PI3K/Akt pathway
Although the abovementioned data suggest that AIM2 regulates the viability and apoptosis of CRC cells, the underlying mechanism remained unclear. Therefore, we examined the nature of the intracellular signaling induced by AIM2 in CRC cells. Specifically, we examined the PI3K–Akt pathway, which modulates cell cycle progression, apoptosis, and cell motility and is frequently mutated in human CRC.Citation22
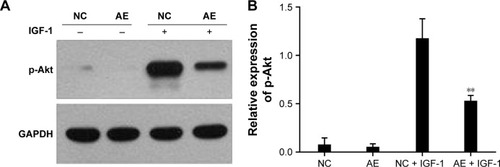
Without IGF-1, AIM2-HCT116 cells presented a higher apoptosis rate than the negative control cells, whereas after treatment with IGF-1 the apoptosis rate of AIM2-HCT116 cells was not significantly different from that of the negative control (). These data suggest that Akt activation inhibits the capacity of AIM2 to induce apoptosis in CRC cells.
Results of Western blotting analysis showed that Akt activation (p-Akt) was nearly the same in AIM2-expressing HCT116 cells and in empty vector-transfected control cells. Upon activation with IGF-1, the level of p-Akt was significantly lower in AIM2-HCT116 cells compared with negative controls (), indicating that the expression of AIM2 resulted in significant suppression of Akt activation.
Figure 4 Effect of AIM2 expression on activation of the PI3K/Akt pathway in HCT116 cells.
Abbreviations: AE, AIM2-expressing group; AIM2, absent in melanoma 2; Akt, protein kinase B; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IGF-1, insulin-like growth factor-1; NC, negative control; PI3K, phosphatidylinositol 3-kinase.
Overall, these data suggest that Akt activation (via phosphorylation) plays an important role in the inhibition of CRC progression by AIM2 and the proapoptotic effect of AIM2 is mediated via the inhibition of the PI3K/Akt pathway.
Discussion
Members of the pattern recognition receptor family are central players in the regulation of infections and inflammatory and metabolic diseases. AIM2 was recently discovered as a new member of this protein family. Studies have identified AIM2 as an innate immune DNA sensor that maintains intestinal homeostasis and prevents colitis and CRC, implicating a role as a tumor suppressor.Citation23,Citation24 Two recent studies comparing azoxymethane/dextran sodium sulfate-induced colitis-associated colon cancer development in AIM2-deficient B6 mice or wild-type mice concluded that AIM2 expression protects against colitis-associated colon cancer in mice.Citation25,Citation26 However, the mechanism of this effect remained unclear and controversial. The ability of AIM2 to tightly suppress overt cellular proliferation of intestinal epithelial cells may be the mechanism driving AIM2-dependent protection against tumorigenesis.
Our study showed that the AIM2 inhibited the viability and increased the apoptosis rate of CRC cells, in good agreement with reports that AIM2 inhibits the proliferation of a variety of human cancer cell lines, such as breast cancer cells, and murine fibroblast cells in vitro.Citation16,Citation27–Citation29 We demonstrate that AIM2-mediated inhibition of cell proliferation is associated with a decreased number of cells in S phase, indicating that expression of AIM2 may block cell cycle transition from G1 to S phase. Previous studies have demonstrated that the interferon-induced protein p202, which also belongs to the p200 family of proteins, has the ability to delay cell growth and retain cells in the G0/G1 phase.Citation30 PI3K/Akt signaling is important for many cellular activities including cell growth, survival, proliferation, and motility.Citation31–Citation34 Upon activation of the PI3K/Akt pathway, the apoptosis rate of AIM2-expressing cells was the same as that of non-expressing cells, indicating that activation of PI3K/Akt can suppress the proapoptotic effect of AIM2 in CRC cells. Moreover, Western blotting analysis demonstrated that expression of AIM2 suppressed the activation of PI3K/Akt. It can be concluded that AIM2 promotes apoptosis in CRC cells by suppressing the PI3K/Akt pathway. Hu et alCitation35 also studied the effect of AIM2 on the PI3K/Akt pathway and found that AIM2 protects intestinal integrity against Salmonella mucosal infection via Akt.
Conclusion
Our results show a potential antitumorigenic role for AIM2 in CRC that is likely mediated by inhibiting the PI3K/Akt pathway. This suggests that AIM2 plays a critical role as a tumor suppressor and might serve as a potential therapeutic target for future development of AIM2-based gene therapy for human CRC. Considering the complex nature of tumor growth and the biological diversity of CRCs, AIM2, like previously identified single markers, may be included in a set of multiple markers representing a characteristic expression profile of certain cancer subtypes. It will be important to discover whether different combinations of these marker sets result in more precise identification of patients who will benefit from specific therapies.
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- DaiZZhengRSZouXNAnalysis and prediction of colorectal cancer incidence trend in ChinaZhonghua Yu Fang Yi Xue Za Zhi20124659860322943913
- DeYoungKLRayMESuYACloning a novel member of the human interferon-inducible gene family associated with control of tumorigenicity in a model of human melanomaOncogene19971544534579242382
- RatsimandresyRADorfleutnerAStehlikCAn update on PYRIN domain-containing pattern recognition receptors: from immunity to pathologyFront Immunol2013444024367371
- AsefaBKlarmannKDCopelandNGGilbertDJJenkinsNAKellerJRThe interferon-inducible p200 family of proteins: a perspective on their roles in cell cycle regulation and differentiationBlood Cells Mol Dis200432115516714757431
- ChoubeyDDuanXDickersonEInterferon-inducible p200-family proteins as novel sensors of cytoplasmic DNA: role in inflammation and autoimmunityJ Interferon Cytokine Res201030637138020187776
- SchattgenSAFitzgeraldKAThe PYHIN protein family as mediators of host defensesImmunol Rev2011243110911821884171
- ChoubeyDDNA-responsive inflammasomes and their regulators in autoimmunityClin Immunol2012142322323122245264
- KhareSRatsimandresyRAde AlmeidaLThe PYRIN domain-only protein POP3 inhibits ALR inflammasomes and regulates responses to infection with DNA virusesNat Immunol201415434335324531343
- ShrivastavaGLeon-JuarezMGarcia-CorderoJMeza-SanchezDECedillo-BarronLInflammasomes and its importance in viral infectionsImmunol Res2016645–61101111727699580
- LamkanfiMDixitVMMechanisms and functions of inflammasomesCell20141571013102224855941
- SchroderKMuruveDATschoppJInnate immunity: cytoplasmic DNA sensing by the AIM2 inflammasomeCurr Biol2009196R262R26519321146
- Fernandes-AlnemriTYuJWJulianaCThe AIM2 inflammasome is critical for innate immunity to Francisella tularensisNat Immunol201011538539320351693
- RathinamVAJiangZWaggonerSNThe AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA virusesNat Immunol201011539540220351692
- ConnollyDJBowieAGThe emerging role of human PYHIN proteins in innate immunity: implications for health and diseaseBiochem Pharmacol201492340541425199457
- ChenIFOu-YangFHungJYAIM2 suppresses human breast cancer cell proliferation in vitro and mammary tumor growth in a mouse modelMol Cancer Ther2006511716432157
- SchulmannKBraschFEKunstmannEGerman HNPCC ConsortiumHNPCC-associated small bowel cancer: clinical and molecular characteristicsGastroenterology2005128359059915765394
- WoernerSMKloorMMuellerAGerman HNPCC ConsortiumMicrosatellite instability of selective target genes in HNPCC-associated colon adenomasOncogene200524152525253515735733
- WoernerSMKloorMSchwitalleYThe putative tumor suppressor AIM2 is frequently affected by different genetic alterations in microsatellite unstable colon cancersGenes Chromosomes Cancer200746121080108917726700
- DihlmannSTaoSEchterdiekFLack of absent in melanoma 2 (AIM2) expression in tumor cells is closely associated with poor survival in colorectal cancer patientsInt J Cancer2014135102387239624729378
- MaJPollakMNGiovannucciEProspective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3J Natl Cancer Inst19999162062510203281
- ParsonsDWWangTLSamuelsYColorectal cancer: mutations in a signalling pathwayNature2005436705279216094359
- HuSPengLKwakYTThe DNA sensor AIM2 maintains intestinal homeostasis via regulation of epithelial antimicrobial host defenseCell Rep20151391922193626655906
- RatsimandresyRAIndramohanMDorfleutnerAStehlikCThe AIM2 inflammasome is a central regulator of intestinal homeostasis through the IL-18/IL-22/STAT3 pathwayCell Mol Immunol201714112714227524110
- WilsonJEPetrucelliASChenLInflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and AktNat Med201521890691326107252
- ManSMZhuQZhuLCritical role for the DNA sensor AIM2 in stem cell proliferation and cancerCell20151621455826095253
- ChoubeyDWalterSGengYXinHCytoplasmic localization of the interferon-inducible protein that is encoded by the AIM2 (absent in melanoma) gene from the 200-gene familyFEBS Lett20004741384210828447
- LiuZYYiJLiuFEThe molecular mechanism of breast cancer cell apoptosis induction by absent in melanoma (AIM2)Int J Clin Exp Med201589147501475826628957
- PatsosGGermannAGebertJDihlmannSRestoration of absent in melanoma 2 (AIM2) induces G2/M cell cycle arrest and promotes invasion of colorectal cancer cellsInt J Cancer201012681838184919795419
- LiHLiuFGuoHZhuZJiaoYRole of interferon-inducible protein 202 (p202) in the regulation of adipogenesis in mouse adipose-derived stem cellsMol Cell Endocrinol2014382281482424246779
- BaderAGKangSZhaoLVogtPKOncogenic PI3K deregulates transcription and translationNat Rev Cancer200551292192916341083
- EngelmanJALuoJCantleyLCThe evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolismNat Rev Genet20067860661916847462
- KatsoROkkenhaugKAhmadiKWhiteSTimmsJWaterfieldMDCellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancerAnnu Rev Cell Dev Biol20011761567511687500
- VivancoISawyersCLThe phosphatidylinositol 3-Kinase AKT pathway in human cancerNat Rev Cancer2002248950112094235
- HuGQSongPXLiNAIM2 contributes to the maintenance of intestinal integrity via Akt and protects against Salmonella mucosal infectionMucosal Immunol2016951330133926838050
