Abstract
Background
Vascular endothelial growth factor (VEGF) is a key mediator that plays an important role in angiogenesis, tumor growth, and tumor metastasis. The associations between five polymorphisms of VEGF (rs3025039, rs699947, rs10434, rs1570360, and rs2010963) and renal cell carcinoma (RCC) risk have been extensively investigated, but the currently available results are inconsistent and inconclusive. To obtain a more accurate assessment of the associations, we conducted a meta-analysis in this study.
Materials and methods
Relevant studies were collected systemically from the following three electronic databases: MEDLINE, Web of Science, and CNKI (Chinese National Knowledge Infrastructure). Statistical analyses were performed using Review Manager 5.2 in a fixed- or random-effects model. Pooled odds ratios (ORs) with 95% confidence intervals (95% CIs) were calculated to establish the strength of associations.
Results
A total of eight case–control studies with 1,936 RCC cases and 2,770 controls fulfilling the inclusion criteria were selected for this meta-analysis. The pooled OR indicated that rs699947 polymorphism was significantly associated with RCC risk in all genetic models. A significant association was also found between the rs3025039 polymorphism and RCC risk in a homozygous model (TT vs CC: OR =1.38, 95% CI =1.11–1.72, P=0.004), a dominant model (CT+TT vs CC: OR =1.21, 95% CI =1.05–1.39, P=0.01), and a recessive model (TT vs CC+CT: OR =1.28, 95% CI =1.04–1.57, P=0.02). After a subgroup analysis of ethnicity in the allele contrast model of rs3025039 polymorphism, we found a significant relationship in the allele contrast model (T vs C: OR =1.21, 95% CI =1.05–1.40, P=0.007) in the Asian population. With regard to rs10434 polymorphism, significant association was observed only in a homozygous model (GG vs AA: OR =0.75, 95% CI =0.57–0.98, P=0.03). As to rs1570360 or rs2010963, we did not observe any relationship between the two polymorphisms and RCC risk in our study.
Conclusion
Our meta-analysis confirmed the fact that rs699947, rs3025039, and rs10434 polymorphisms were significantly relevant to elevated RCC risk. In the meanwhile, this study also demonstrated that the allele contrast model of rs3025039 polymorphism was likely to be associated with risk of RCC in the Asian population.
Introduction
Renal cell carcinoma (RCC) is the most common type of kidney cancer and accounts for >90% of all diagnosed cases.Citation1 It has been reported that RCC has the highest mortality rate among the genitourinary cancers, and the incidence of RCC has increased sequentially, especially in young patients and in patients with high-grade disease.Citation2,Citation3 The current statistical report shows that there are ~209,000 newly diagnosed RCC cases and 102,000 deaths caused by RCC per year worldwide.Citation4 A study indicates that ~40% of RCC patients die of metastatic disease, because metastases are often present at diagnosis and recurrence stage, which are also common after complete resection of the primary tumor.Citation5 It has been revealed that smoking,Citation6,Citation7 overweight and obesity,Citation8,Citation9 hypertension,Citation10 and potential environmental causesCitation11 are risk factors for the development and progression of RCC. However, the pathogenic mechanisms of RCC underlying the established risk factors still remain vague.
Recent molecular studies have suggested that gene polymorphism contributes to tumorigenesis.Citation12–Citation15 Vascular endothelial growth factor (VEGF), an endothelial cell-specific mitogen, has been deemed as a major contributor to the development of RCC.Citation16,Citation17 VEGF is a vital stimulator of pathological and physiological angiogenesis that acts on vascular endothelial cells and promotes human blood vessel growth during tumor growth, enabling invasion and metastasis,Citation18,Citation19 and the role of angiogenesis has been a recurrent denominator of RCC connected to VEGF.Citation20 Therefore, it will be useful to understand better the molecular pathogenesis of RCC, which can help to predict RCC and develop targeted agents to treat this disease.
VEGF is located on chromosome 6p21.3 with 8 exons and 7 introns, spanning ~14 kb.Citation21 It is highly polymorphic, especially in the promoter, the 5′-untranslated region (UTR), and the 3′-UTR.Citation22 There are several common single nucleotide polymorphisms (SNPs) in the VEGF gene, including rs3025039, rs699947, rs10434, rs1570360, and rs2010963 positions, which could increase the risk of developing diseases characterized by deranged angiogen-esis and alter gene expression and protein production.Citation15,Citation23,Citation24 Up to now, many studies have investigated the association between SNPs of the VEGF gene and RCC in different populations.Citation25–Citation32 However, the results have been inconsistent and inconclusive. In 2013, Zhang et alCitation33 conducted a meta-analysis to present the genetic knowledge on the VEGF gene polymorphisms and RCC risk in humans based on the published evidence. However, due to the limitations of the included study, the results of this meta-analysis indicated that the VEGF rs3025039(+936C/T), rs699947(−2578C/A), rs10434(1612G/A), rs1570360(+405C/G), and rs2010963 (−1154G/A) gene polymorphisms are not associated with the risk of RCC. Therefore, in this study, we performed an updated meta-analysis with eight studies for rs3025039, rs699947, rs10434, rs1570360, and rs2010963 polymorphisms to further clarify the associations.
Materials and methods
Literature and search strategy
A comprehensive systematic search was conducted for published articles using MEDLINE, Web of Science, and CNKI (Chinese National Knowledge Infrastructure); the years were limited from 1993 to 2016. The following keywords and MeSH terms were used: (“renal cell carcinoma” OR “renal cell cancer” OR “RCC”) AND (“vascular endothelial growth factor” OR “VEGF”) AND (“polymorphisms” OR “mutations” OR “variants” OR “single nucleotide polymorphisms” OR “SNP”). All included articles were published in English language. At the same time, the reference lists of retrieved papers and recent reviews were manually searched.
Study inclusion and exclusion criteria
Studies eligible for inclusion in our meta-analysis should meet the following criteria: 1) must be independent case–control or cohort design studies; 2) the article pertained to the abovementioned VEGF polymorphisms (rs3025039, rs699947, rs10434, rs1570360, or rs2010963) and RCC risk; 3) patients have clinically confirmed RCC; 4) the studies provided the number of cases and controls for various genotypes and sufficient data for calculating odds ratios (ORs) with 95% confidence intervals (CIs); and 5) genotype distributions of polymorphism of the control population were consistent with Hardy–Weinberg equilibrium (HWE). Accordingly, the exclusion criteria of the meta-analysis were 1) meta-analyses, reviews, case reports, or no healthy control population; 2) animal studies; 3) non-conformity with the criteria for RCC; 4) there are no sufficient data to estimate the ORs and 95% CIs; and 5) duplication of previous publications.
Data extraction
According to the inclusion and exclusion criteria, the following information was independently extracted from eligible studies by two investigators (Yu Tian and JingJing Song): name of the first author, year of publication, ethnicity (such as Asian or Caucasian), genotype method, sample sizes of cases and controls, genotype frequency of cases and controls, and P-value for HWE of controls. Any disagreements were figured out by discussion until a consensus was achieved.
Statistical analysis
HWE was assessed with the chi-square test using the genotypes of the controls in each study. The pooled ORs with the corresponding 95% CIs were used to investigate whether there existed associations between VEGF polymorphisms and the risk of RCC based on allele contrast, homozygote, heterozygote, dominant, and recessive models as follows: 1) rs3025039(+936C/T) polymorphism (T vs C, TT vs CC, CT vs CC, TT+CT vs CC, and TT vs CT+CC, respectively); 2) rs699947(−2578C/A) polymorphism (A vs C, AA vs CC, CA vs CC, AA+CA vs CC, and AA vs CA+CC, respectively); 3) rs10434(1612G/A) polymorphism (G vs A, GG vs AA, GA vs AA, GG+GA vs AA, and GG vs GA+AA, respectively); 4) rs1570360(−1154G/A and −1156G/A) polymorphism (A vs G, AA vs GG, GA vs GG, AA+GA vs GG, and AA vs GA+GG, respectively); and 5) rs2010963(+405C/G and −634G/C) polymorphism (C vs G, CC vs GG, CG vs GG, CC+GC vs GG, and CC vs GC+GG, respectively).
Heterogeneity was quantifiably measured using Cochran’s Q test and I Citation2 statistic combined with the corresponding P-value.Citation34 If I2 value exceeded 50% or P<0.10, the heterogeneity was significant, and a random-effects model was employed. Otherwise, the ORs were calculated by fixed-effects model (I2<50% or P>0.10).Citation35 To consider potential ethnicity variation, subgroup analysis was conducted on the basis of ethnicity. To test the stability of the result, we performed the sensitivity analysis by excluding one study in turn. Visual inspection of asymmetry in Begg’s funnel plots and Egger’s test was carried out to assess the potential publication bias. Those statistical analyses or data syntheses were calculated using Review Manager 5.2 and STATA version 11. All P-values were two sided, and P<0.05 was defined to be statistically significant.
Results
Study characteristics
The initial search yielded 1,304 references. Of these articles, we excluded 1,250 studies based on titles and/or abstracts. Fifty-four texts were then reviewed for a further evaluation. In accordance with the study inclusion criteria, 46 articles were excluded for different reasons: 30 studies were about VEGF as a predictive and/or prognostic biomarker; two studies were review; 12 studies were obviously irrelevant; one study was a meta-analysis, and one study was about animal experiment. Finally, eight studies were included in this meta-analysis.Citation25–Citation32 A flow diagram about the literature search and study selection process is presented in .
Figure 1 Flow diagram of the study selection process.
A total of 4,706 subjects were involved in this meta-analysis, including 1,936 RCC patients and 2,770 healthy controls. For the rs3025039(+936C/T) polymorphism, six literatures with a total number of 1,450 cases and 2,343 controls were included.Citation25–Citation27,Citation29,Citation30,Citation32 For the rs699947(−2578C/A) polymorphism, five literatures with a total number of 1,397 cases and 2,102 controls were included.Citation25–Citation27,Citation29,Citation31 For the rs10434(1612G/A) polymorphism, four literatures with a total number of 985 cases and 1,861 controls were included.Citation25,Citation26,Citation29,Citation32 For the rs2010963(+405C/G and −634G/C) polymorphism, five literatures with a total number of 1,305 cases and 2,198 controls were included.Citation25,Citation27,Citation29,Citation30 While for the rs1570360(−1154G/A and −1156G/A) polymorphism, only three literatures with a total number of 660 cases and 1,055 controls were included.Citation25,Citation28,Citation30 Overall, three of these included studies were conducted in Caucasian populationCitation27,Citation28,Citation30 and the other five studies were conducted in Asian population.Citation25,Citation26,Citation29,Citation31,Citation32 DNA samples that are used to detect the VEGF genetic polymorphisms were extracted from blood in all included studies. Methods used for genotyping of the eight case–control studies include Taqman, polymerase chain reaction (PCR), and PCR-restriction fragment length polymorphism (PCR-RFLP). Genotype distributions among the controls of all studies were consistent with HWE. The detailed study characteristics of the included studies are displayed in .
Table 1 Baseline characteristics of the included studies in the meta-analysis
Meta-analysis results
On the whole, the pooled ORs and 95% CIs of RCC were considered under allele contrast, homozygous, heterozygous, dominant, and recessive genetic models. A summary of our meta-analysis results for the five studied polymorphisms and RCC risk is provided in .
Table 2 Meta-analysis results for the five studied polymorphisms and RCC risk
Pooled effects for the rs3025039(+936C/T) polymorphism and RCC risk
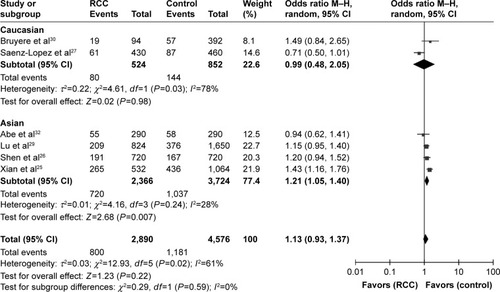
The pooled results of all analyses showed that a significant association between the rs3025039(+936C/T) polymorphism and RCC risk was demonstrated under homozygous, dominant, and recessive models (TT vs CC: OR =1.38, 95% CI =1.11–1.72, I2=25%, P =0.004; CT+TT vs CC: OR =1.21, 95% CI =1.05–1.39, I2=39%, P=0.001; TT vs CC+CT: OR =1.28, 95% CI =1.04–1.57, I2=0%, P=0.02). And there was no relationship in the other two models (T vs C: OR =1.13, 95% CI =0.93–1.37, I2=61%, P=0.61; CT vs CC: OR =1.17, 95% CI =1.00–1.37, I2=25%, P=0.06). According to the heterogeneity (I2.50%) in the allele contrast model, we performed a subgroup analysis of ethnicity. The result of this subgroup analysis was shown as follows: Caucasian (T vs C: OR =0.99, 95% CI =0.48–2.05, I2=78%, P=0.98) and Asian (T vs C: OR =1.21, 95% CI =1.05–1.40, I2=28%, P=0.007). We surprisingly found that rs3025039(+936C/T) polymorphism was significantly associated with RCC for Asians in the allele contrast model (; ). Furthermore, no publication bias was found, showing that the results are statistically robust (T vs C, P=0.468; TT vs CC, P=0.877; CT vs CC, P=0.765; TT+CT vs CC, P=0.707; TT vs CT+CC, P=0.881).
Pooled effects for the rs699947(−2578C/A) polymorphism and RCC risk
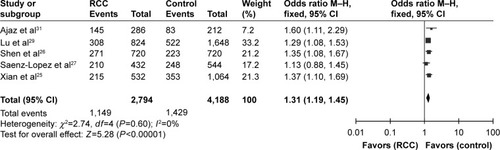
When it came to the rs699947(−2578C/A) polymorphism, we detected significant associations under all genetic models (A vs C: OR =1.31, 95% CI =1.19–1.45, I2=0%, P<0.00001; AA vs CC: OR =1.69, 95% CI =1.37–2.07, I2=0%, P<0.00001; CA vs CC: OR =1.31, 95% CI =1.12–1.52, I2=47%, P=0.0006; CA+AA vs CC: OR = 1.39, 95% CI = 1.21–1.61, I2=35%, P<0.00001; AA vs CC+CA: OR =1.43, 95% CI =1.19–1.73, I2=0%, P=0.0002), as indicated in and . Funnel plot symmetry was performed to estimate publication bias, and the results were validated by Egger’s test (A vs C, P=0.520; AA vs CC, P=0.642; CA vs CC, P=0.142; AA+CA vs CC, P=0.209; AA vs CA+CC, P=0.305).
Pooled effects for the rs10434(1612G/A) polymorphism and RCC risk
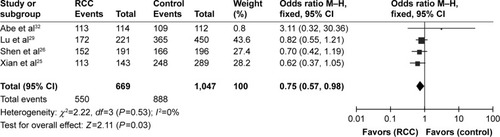
The meta-analysis results showed that the rs10434(1612G/A) polymorphism increased the risk of RCC in the homozygous model (GG vs AA: OR =0.75, 95% CI =0.57–0.98, I2=0%, P=0.03), but no significant associations were found in the heterozygous and dominant models where the results were as follows: G vs A: OR =0.89, 95% CI =0.8–1.00, I2=0%, P=0.06; GA vs AA: OR =0.82, 95% CI =0.63–1.07, I2=0%, P=0.15; GA+GG vs AA: OR =0.79, 95% CI =0.61–1.01, I2=0%, P=0.06; GG vs GA+AA: OR =0.89, 95% CI =0.77–1.04, I2=0%, P=0.14 (; ). No publication bias was detected in the funnel plot and Egger’s test (G vs A, P=0.274; GG vs AA, P=0.348; GA vs AA, P=0.355; GG+GA vs AA, P=0.331; GG vs GA+AA, P=0.235).
Pooled effects for the rs1570360(−1154G/A and −1156G/A) polymorphism and RCC risk
No associations were observed between rs1570360(−1154G/A and −1156G/A) polymorphism and RCC risk in any genetic models. The results were as follows: A vs G: OR =1.05, 95% CI =0.9–1.22, I2=25%, P=0.55; AA vs GG: OR =1.10, 95% CI =0.81–1.50, I2=0%, P=0.55; GA vs GG: OR =1.07, 95% CI =0.83–1.37, I2=0%, P=0.60; GA+AA vs GG: OR =1.08, 95% CI =0.86–1.36, I2= 19%, P=0.52; AA vs GG+GA: OR =1.04, 95% CI =0.81–1.32, I2=0%, P =0.78. Moreover, the results of Egger’s test suggested no publication (A vs G, P=0.487; AA vs GG, P=0.557; GA vs GG, P=0.272; AA+GA vs GG, P=0.499; AA vs GA+GG, P=0.920).
Pooled effects for the rs2010963(+405C/G and −634G/C) polymorphism and RCC risk
Our meta-analysis did not show any significant correlations between the rs2010963(+405C/G and −634G/C) polymorphism and RCC risk in all the genetic models in the total population (C vs G: OR =1.04, 95% CI =0.94–1.15, I2=31%, P=0.49; CC vs GG: OR =1.07, 95% CI =0.86–1.32, I2=28%, P=0.56; GC vs GG: OR =1.09, 95% CI =0.92–1.28, I2=0%, P=0.32; GC+CC vs GG: OR =1.09, 95% CI =0.93–1.27, I2=0%, P=0.29; CC vs GC+GG: OR =1.00, 95% CI =0.84–1.19, I2 =0%, P=1.00). Besides, the results of Egger’s test were as follows: C vs G, P=0.587; CC vs GG, P=0.819; CG vs GG, P=0.180; CC+GC vs GG, P=0.909; CC vs GC+GG, P=0.740.
Sensitive analysis
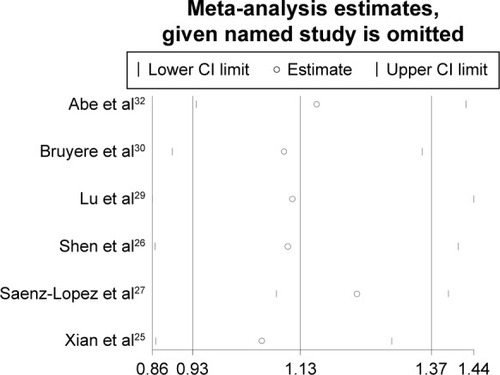
To assess whether the single study influenced the pooled results, we conducted the sensitivity analysis by excluding each single study one by one. The results of pooled ORs indicated that the overall significance of the ORs was not altered by any single study for the rs3025039(+936C/T) polymorphisms (). It is demonstrated that the pooled result of our study was of relatively high reliability and stability.
Publication bias
No significant publication bias was detected for the studies on rs3025039, rs699947, rs10434, rs1570360, and rs2010963 polymorphisms. On one hand, the shapes of the funnel plots were symmetrical by visual inspection. On the other hand, the P-value of all genetic models exceeded 0.05 in the Egger’s test.
Discussion
VEGF is one of the most important cytokines in angiogenesis, which has attracted extensive attention. High expression of VEGF in the primary tumor has been found in certain types of malignant tumors such as breast cancer, gastric cancer, and glioma.Citation36–Citation38 The high expression of VEGF was demonstrated by the vast majority of fresh frozen tumor samples in RCC as well,Citation39 and it was confirmed that von Hippel–Lindau (VHL) tumor suppressor gene inactivation could lead to VEGF overexpression in the majority of clear cell RCC tumors.Citation40
A large number of studies are focusing on the associations among protein expression/activity, gene variants (particularly SNPs), and tumor formation. In 2002, Abe et alCitation32 conducted the first case–control study of the association between SNPs in the 3′-UTR of the VEGF gene and RCC in the Japanese population and indicated that the C702T, C936T, and G1612A polymorphisms in the 3′-UTR of the VEGF gene are not associated with the risk of RCC. Since then, a series of case–control studies have been conducted, but the available results are inconsistent and inconclusive. In 2013, Zhang et alCitation33 conducted a meta-analysis with only five case–control studies and observed that polymorphisms or haplotypes in the VEGF gene did not modify the risk of RCC. To date, three additional studies have revealed the connections between VEGF polymorphisms and RCC risk.Citation25,Citation26,Citation29 Our present meta-analysis aims to update the previous meta-analysis and to provide a more reliable and credible conclusion on the associations between five common functional polymorphisms (rs3025039, rs699947, rs10434, rs1570360, and rs2010963) in VEGF gene and RCC susceptibility.
In our meta-analysis, eight eligible case–control studies were included with a total number of 1,936 RCC patients and 2,770 healthy controls. When all the eligible studies were pooled into the meta-analysis, the results showed that rs699947(−2578C/A) and rs3025039(+936C/T) polymorphisms significantly increased the risk of RCC. One possible reason for these inconsistent results could be explained that rs699947(−2578C/A) and rs3025039(+936C/T) polymorphisms were more influential than other SNPs on VEGF gene expression and protein production and indicate that various polymorphisms yield various effects on gene function, even when they are located at the same unit. Furthermore, in the subgroup analysis by ethnicity for the rs3025039(+936C/T) polymorphism, a statistically significantly increased RCC risk in Asians was found in the allele contrast model of rs3025039(+936C/T) polymorphism. For ethnicity variation, the possible reasons may be great disparities in common VEGF gene polymorphisms and the natural selection and genetic drift that are most likely to influence the risk of RCC. For one thing, most of our included studies were conducted in Asian population, and only three studies were performed in Caucasian population. For the other thing, the small sample size of some included studies could also lead to the discrepancy. In addition, we explain that the reasons why there was no relationship between rs3025039 polymorphism and RCC risk in the heterozygous model might be the insufficient studies and limited sample sizes. Therefore, we need further studies conducted in more different ethnicities, and experiments of enough samples are necessary to evaluate the associations between VEGF polymorphisms and RCC risk. As to rs10434(1612G/A) polymorphism, a significant association was observed in a homozygous model although all ORs in the separate studies were not statistically significant, which may due to the new research data and a large sample size.
Some possible limitations of our meta-analysis should be taken into consideration. First, even though a comprehensive search strategy was applied to determine eligible studies, it was probable that some eligible studies were not brought into, which could make our results have bias. Second, only eight eligible studies were collected, so the limited number of studies may have an influence on the analysis of the correlation between the five VEGF polymorphisms and the risk of RCC. Third, as a retrospective study, the meta-analysis may encounter recall or selection bias; thereby it is a possible reason to affect the reliability of our results. Fourth, for the rs10434(1612G/A) polymorphism, all studies included were conducted in Asian population. With a single Asian race was examined, ethnicity bias may exist and the conclusion may not be the same with other races. Fifth, various risk factors are crucial determining factors of tumor recurrence, such as smoking, hypotension, and other genetic factors. Thus, the unadjusted databases in this meta-analysis could not explain the inherent pathogenic mechanisms clearly.
Accumulating evidence indicates that the pathogenic mechanisms of many diseases including RCC were determined by a unique combination of endogenous and exogenous factors, resulting in various molecular and pathological subtypes of this disease.Citation41 Molecular pathological epidemiology (MPE) is an integrative field that is based on the disease continuum theory and the unique disease principle, which can give clues to analyze the risk factors of diseases.Citation42,Citation43 For example, an MPE study assessed the joint effects of genetic polymorphisms in the mammalian target of rapamycin pathway and epidemiologic risk factors on RCC risk and showed that obesity played a vital role in enhancing RCC risk during the life course.Citation44 Together, the MPE research can provide etiologic and pathogenic insights to precision medicine and public health, which will be applied to reveal the carcinogenic mechanism as a future direction of the field.
In spite of these potential limitations, this meta-analysis has its advantages and innovations. First, extensive search strategy and manual search allowed the eligible studies included as possible as we can. Second, compared to previous meta-analysis, our meta-analysis contained new research data and a large sample size. Third, according to the existence of heterogeneity, we conducted a subgroup analysis in our meta-analysis, which is not performed in previous meta-analysis. Therefore, we can consider that the conclusions are credible and reliable to explore the relationship between the VEGF polymorphisms and RCC risk.
Conclusion
Our meta-analysis reevaluates the relationship between VEGF polymorphisms and RCC risk and suggests that there is a significant association between rs699947(−2578C/A)/rs3025039(+936C/T) polymorphism and RCC. Moreover, our study also demonstrates that the allele contrast model of rs3025039 polymorphism is likely to be associated with risk of RCC in Asian population and the homozygous model of rs10434 polymorphism is statistically significantly relevant to elevated RCC risk, while no significant association is detected between rs1570360(−1154G/A and −1156G/A) or rs2010963(+405C/G and −634G/C) polymorphism and risk of RCC. Considering the limitations mentioned earlier, further well-designed studies including more different ethnicities and larger sample sizes are needed to confirm our results and explore these associations.
Disclosure
The authors report no conflicts of interest in this work.
References
- MickleyAKovalevaOKzhyshkowskaJGratchevAMolecular and immunologic markers of kidney cancer-potential applications in predictive, preventive and personalized medicineEPMA J201562026500709
- KingSCPollackLALiJKingJBMasterVAContinued increase in incidence of renal cell carcinoma, especially in young patients and high grade disease: United States 2001 to 2010J Urol201419161665167024423441
- CairnsPRenal cell carcinomaCancer Biomark201091–646147322112490
- PanDXuLLiuHInterleukin-11 receptor predicts post-operative clinical outcome in patients with early-stage clear-cell renal cell carcinomaJpn J Clin Oncol201545220220925420690
- RiniBICampbellSCEscudierBRenal cell carcinomaLancet200937396691119113219269025
- HuntJDvan der HelOLMcMillanGPBoffettaPBrennanPRenal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studiesInt J Cancer2005114110110815523697
- YuanJMCastelaoJEGago-DominguezMYuMCRossRKTobacco use in relation to renal cell carcinomaCancer Epidemiol Biomarkers Prev1998754294339610793
- van DijkBASchoutenLJKiemeneyLAGoldbohmRAvan den BrandtPARelation of height, body mass, energy intake, and physical activity to risk of renal cell carcinoma: results from the Netherlands Cohort StudyAm J Epidemiol2004160121159116715583368
- BjorgeTTretliSEngelandARelation of height and body mass index to renal cell carcinoma in two million Norwegian men and womenAm J Epidemiol2004160121168117615583369
- McLaughlinJKChowWHMandelJSInternational renal-cell cancer study. VIII. Role of diuretics, other anti-hypertensive medications and hypertensionInt J Cancer19956322162217591207
- McCredieMPommerWMcLaughlinJKInternational renal-cell cancer study. II. AnalgesicsInt J Cancer19956033453497829242
- MengFDMaPSuiCGTianXJiangYHAssociation between cytochrome P450 1A1 (CYP1A1) gene polymorphisms and the risk of renal cell carcinoma: a meta-analysisSci Rep20155810825630554
- WangZWeiMRenYmiR149 rs71428439 polymorphism and risk of clear cell renal cell carcinoma: a case-control studyTumour Biol20143512121271213025213695
- MengFMaPSuiCThe association between VDR polymorphisms and renal cell carcinoma susceptibility: a meta-analysisTumour Biol20143566065607224609903
- MaNLiLWChengJLPredictive value of vascular endothelial growth factor polymorphisms on the clinical outcome of renal cell carcinoma patientsOncol Lett20159265165625621033
- YangSMHuangCYShiueHSJoint effect of urinary total arsenic level and VEGF-A genetic polymorphisms on the recurrence of renal cell carcinomaPLoS One20151012e014541026701102
- JosephRWParasramkaMEckel-PassowJEInverse association between programmed death ligand 1 and genes in the VEGF pathway in primary clear cell renal cell carcinomaCancer Immunol Res20131637838524778130
- KumarBRayKBReddyGVVEGF-C differentially regulates VEGF-A expression in ocular and cancer cells; promotes angiogenesis via RhoA mediated pathwayAngiogenesis201114337138021698469
- CarmelietPVEGF as a key mediator of angiogenesis in cancerOncology200569suppl 341016301830
- BadalSAAikenWDChinSNMolecular targets and angiogenesis in renal cell carcinoma, a multi-target approach: mini reviewCurr Drug Targets Epub201652
- VincentiVCassanoCRocchiMPersicoGAssignment of the vascular endothelial growth factor gene to human chromosome 6p21.3Circulation1996938149314958608615
- WatsonCJWebbNJBottomleyMJBrenchleyPEIdentification of polymorphisms within the vascular endothelial growth factor (VEGF) gene: correlation with variation in VEGF protein productionCytokine20001281232123510930302
- PasqualettiGDanesiRDel TaccaMBocciGVascular endothelial growth factor pharmacogenetics: a new perspective for anti-angiogenic therapyPharmacogenomics200781496617187509
- BianconiMFaloppiLLoretelliCAngiogenesis genotyping in the selection of first-line treatment with either sunitinib or pazopanib for advanced renal cell carcinomaOncotarget2016725375993760727175586
- XianWZhengHWuWJPredictive value of vascular endothelial growth factor polymorphisms on the risk of renal cell carcinomasGenet Mol Res20151437634764226214443
- ShenBLQuQSMiaoSZZhangYXAssociation between SNPs in vascular endothelial growth factor polymorphisms and risk of renal cell carcinoma: a case-control studyGenet Mol Res2015143111191112526400342
- Saenz-LopezPVazquezFCozarJMCarreteroRGarridoFRuiz-CabelloFVEGF polymorphisms are not associated with an increased risk of developing renal cell carcinoma in Spanish populationHum Immunol20137419810323073296
- RickettsCZeegersMPLubinskiJMaherERAnalysis of germline variants in CDH1, IGFBP3, MMP1, MMP3, STK15 and VEGF in familial and sporadic renal cell carcinomaPLoS One200946e603719551141
- LuGDongYZhangQJiaoLYangSShenBPredictive value of vascular endothelial growth factor polymorphisms on the risk of renal cell carcinomas: a case-control studyTumour Biol201536118645865226044558
- BruyereFHovensCMMarsonMNVEGF polymorphisms are associated with an increasing risk of developing renal cell carcinomaJ Urol201018441273127820723915
- AjazSKhaliqSA bidAAssociation of a single-nucleotide polymorphism in the promoter region of the VEGF gene with the risk of renal cell carcinomaGenet Test Mol Biomarkers201115965365721491998
- AbeASatoKHabuchiTSingle nucleotide polymorphisms in the 3′ untranslated region of vascular endothelial growth factor gene in Japanese population with or without renal cell carcinomaTohoku J Exp Med2002198318119012597245
- ZhangYLiSXiaoHQHuZXXuYCHuangQVascular endothelial growth factor gene polymorphisms and renal cell carcinoma: a systematic review and meta-analysisOncol Lett2013641068107824137466
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ2003327741455756012958120
- HigginsJPThompsonSGQuantifying heterogeneity in a meta-analysisStat Med200221111539155812111919
- ChenZXuSXuWExpression of cluster of differentiation 34 and vascular endothelial growth factor in breast cancer, and their prognostic significanceOncol Lett201510272372926622560
- WangXLAiZSFangJPTangRYChenXMExpression of vascular endothelial growth factors (VEGF)-A, -C and -D and their prognostic significance and relationship with angio- and lymphangiogenesis in gastric cancerZhonghua Zhong Liu Za Zhi2008301183784319173829
- ChenWHeDLiZZhangXPanDChenGOverexpression of vascular endothelial growth factor indicates poor outcomes of glioma: a systematic review and meta-analysisInt J Clin Exp Med2015868709871926309522
- LeeJSKimHSJungJJParkCSLeeMCExpression of vascular endothelial growth factor in renal cell carcinoma and the relation to angiogenesis and p53 protein expressionJ Surg Oncol2001771556011344484
- NeufeldGCohenTGengrinovitchSPoltorakZVascular endothelial growth factor (VEGF) and its receptorsFASEB J19991319229872925
- OginoSLochheadPChanATMolecular pathological epidemiology of epigenetics: emerging integrative science to analyze environment, host, and diseaseMod Pathol201326446548423307060
- HamadaTKeumNNishiharaROginoSMolecular pathological epidemiology: new developing frontiers of big data science to study etiologies and pathogenesisJ Gastroenterol201752326527527738762
- OginoSNishiharaRVanderWeeleTJReview article: the role of molecular pathological epidemiology in the study of neoplastic and non-neoplastic diseases in the era of precision medicineEpidemiology201627460261126928707
- ShuXLinJWoodCGTannirNMWuXEnergy balance, polymorphisms in the mTOR pathway, and renal cell carcinoma riskJ Natl Cancer Inst2013105642443223378641