Abstract
Triple-negative breast cancer (TNBC) is an aggressive subtype associated with frequent recurrence and metastasis. Unlike hormone receptor-positive subtypes, treatment of TNBC is currently limited by the lack of clinically available targeted therapies. Androgen signaling is necessary for normal breast development, and its dysregulation has been implicated in breast tumorigenesis. In recent years, gene expression studies have identified a subset of TNBC that is enriched for androgen receptor (AR) signaling. Interference with androgen signaling in TNBC is promising, and AR-inhibiting drugs have shown antitumorigenic activity in preclinical and proof of concept clinical studies. Recent advances in our understanding of androgenic signaling in TNBC, along with the identification of interacting pathways, are allowing development of the next generation of clinical trials with AR inhibitors. As novel AR-targeting agents are developed and evaluated in clinical trials, it is equally important to establish a robust set of biomarkers for identification of TNBC tumors that are most likely to respond to AR inhibition.
Introduction
Of the 250,000 new cases of breast cancer expected in the United States in 2017, 15% will be diagnosed as triple-negative breast cancer (TNBC).Citation1–Citation5 Accelerated growth, high recurrence rates, and frequent metastasis characterize the aggressiveness of TNBC and result in poor long-term patient survival.Citation2,Citation3 TNBC is defined by the lack of estrogen and progesterone receptors as well as absence of human epidermal growth factor 2 (HER2) overexpression/amplification. Despite successful use of targeted therapies in other subtypes of breast cancer, similar approaches in TNBC have not reached clinical practice. Because of the lack of targeted therapy, ~30%–40% of patients with early-stage TNBC develop metastatic disease and succumb to the cancer despite receiving standard multiagent adjuvant chemotherapy.Citation6,Citation7
Variable response to treatment has been a major challenge in developing targeted therapies in TNBC, where it points to an underlying heterogeneity within the subtype. Advances in gene expression profiling have revealed several complementary TNBC classification systems that may be associated with response to therapy.Citation5,Citation8–Citation11 New classifications have isolated a subset of TNBC that are enriched for AR expression.Citation5,Citation12 Given the tremendous clinical success of targeting estrogen receptor in hormone-positive breast cancer, AR positivity in TNBC may constitute a clinically targetable signaling pathway. In support of this approach, numerous preclinical studies have validated the use of AR modulation in limiting cell proliferation, and there are ongoing clinical trials evaluating the safety and efficacy of AR antagonists in breast cancer. The following review explores the role of AR in tumorigenesis and progression, and its role not only as a prognostic and predictive tool but also as a potential therapeutic target in TNBC.
Hormone signaling in tumorigenesis
Estrogen receptor (ER) and progesterone receptor (PR) can stimulate tumor growth and metastasis in breast cancer. Antihormone therapies, such as tamoxifen, aromatase inhibitors, and selective ER degraders, are efficacious in hormone-positive breast cancer. A third hormone receptor, AR, is present in all subtypes of breast cancer and is attracting attention as a potential therapeutic target in breast cancer.Citation13
The AR is an intracellular steroid receptor that dimerizes and translocates to the nucleus upon binding of androgen ligands, where it binds to androgen response elements (AREs) to promote target gene transcription in a tissue-specific manner (). Normal breast development is driven by AR interaction with the Wnt pathway, but AR is also known to regulate genes implicated in metastasis, and androgens have shown independent tumorigenic activity in vitro and in animal models.Citation14–Citation20
Figure 1 Ligand-dependent activation of androgen response elements.
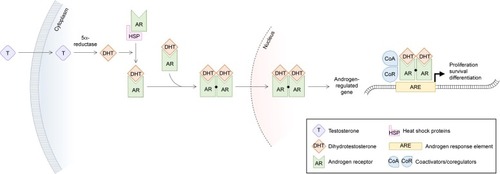
AR also interacts with other intracellular signaling pathways. Despite AR’s demonstrated tumorigenic activity, crosstalk with the ER pathway can have the paradoxical effect of limiting tumor proliferation. The ER regulates gene transcription in a similar manner by binding to estrogen response elements (EREs) in cis-regulatory elements of estrogen-regulated genes.Citation21,Citation22 AR can competitively bind to EREs and coactivators to suppress estrogen-mediated tumor proliferation.Citation18,Citation23 However, in the absence of ER, as is the case in TNBC, AR mainly interacts with AREs and stimulates tumor cell growth in an androgen-dependent manner.Citation24
Androgen signaling in breast cancer
Unlike ER and PR, AR is found in all major breast cancer subtypes and is estimated to be present in 53%–80% of all breast cancers.Citation4,Citation25–Citation31 AR+ breast tumors are diagnosed more commonly in older patients.Citation4,Citation24,Citation29,Citation31,Citation32 A positive AR tumor status appears to be associated with favorable clinical features such as lower tumor-node-metastasis stage, lower nuclear grade, less risk of lymph node involvement, and smaller tumor size at diagnosis.Citation4,Citation24,Citation25,Citation28–Citation33 AR expression significantly overlaps with ER+/PR+ status, lack of HER2 overexpression/amplification, and lower proliferative index.Citation18,Citation24,Citation27,Citation29–Citation32,Citation34,Citation35
In patients unselected for hormone receptor or HER2 expression, AR may be an indicator of favorable prognosis. Some studies have reported association of AR+ breast cancers with better response to endocrine therapy and longer disease-free survival (DFS) and overall survival (OS).Citation4,Citation18,Citation24–Citation27,Citation29,Citation31,Citation32,Citation36,Citation37
A dual role for AR, dependent on the relative strength of ER signaling, has been proposed by some groups, and would explain the varied prognoses among ER+AR+, ER+AR−, ER−AR+, and ER−AR− breast cancer.Citation14 The detrimental effect of discordant AR and ER expression suggests that androgen-mediated proliferation in breast cancer may be regulated by the relative availability of each receptor. When estrogen is low, testosterone is preferentially converted to estradiol, an ER ligand, instead of to 5α-dihydrotestosterone (DHT), an AR ligand, thus translating androgen supply into ER-driven tumorigenesis.Citation20,Citation38–Citation40 Accordingly, a higher tumor AR-to-ER ratio is independently associated with lymph node metastasis and poor survival.Citation20 When the AR-to-ER ratio is low, or when estrogens are available, androgen metabolism will activate AR to compete for EREs. In these circumstances, AR can be antitumorigenic.
Androgen signaling in TNBC
Association of AR status with clinicalpathological characteristics in TNBC
Using immunohistochemical (IHC) assessment, AR is present in 13%–37% of TNBC, serving as the sole hormone receptor in these cases.Citation4,Citation24,Citation25,Citation30,Citation33,Citation34,Citation41–Citation44 As ER and PR are absent in TNBC, the biological and therapeutic role of AR independent of other hormone receptors can be studied in this subtype. Association of AR with clinical and pathological features and ultimately prognosis in TNBC is not completely understood. AR positivity in TNBC is associated with older age at presentation, coinciding with the high circulating levels of androgens seen in postmenopausal breast cancer patients.Citation45 In TNBC, some studies have shown AR positivity to be associated with higher nuclear grade, higher tumor stage, and lymph node metastases, though others have found an association with lower nuclear grade or have failed to note any associations with clinical-pathological features.Citation20,Citation41–Citation44,Citation46 Conflicting reports of a higher vs lower proliferation index in AR+ TNBC have also been made.Citation42,Citation44 Several reports have noted overlap between AR positivity and apocrine histological features or apocrine gene expression signature in TNBC.Citation30,Citation34,Citation47,Citation48
Meta-analyses led by Qu and Wang, encompassing over 4,000 cases of TNBC, demonstrated AR+ status to be associated with better DFS and OS.Citation26,Citation49 Another meta-analysis by Gonzalez-Angulo et al, however, could only note a non-significant trend of better DFS and OS, and numerous other studies have observed no difference or a negative impact of AR status on outcomes.Citation20,Citation24,Citation25,Citation31,Citation32,Citation41,Citation42,Citation50
At present, there is no standardized method or cutoff for detection of AR expression, and AR assessment is not part of routine pathological testing for breast cancer. The majority of published literature has utilized AR IHC nuclear staining for determination of AR positivity, yet tissue processing methods and choice of AR antibody are not consistent among published literature on this topic. Thus, studies assessing the clinical correlations and prognostic impact of AR are limited by their retrospective nature, variability in techniques and cutoffs (>1% to ≥10% nuclear staining by IHC) used to determine AR positivity, and variations in the clinical and treatment characteristics of patient cohorts being evaluated. In summary, the prognostic value of AR expression in TNBC is not yet clear, and is likely complicated by lack of standardized testing methodology and heterogeneity within the patient population.
AR+ subtype presents a unique clinical course
Recent efforts in molecular characterization of TNBC have resulted in its classification into additional subtypes. Seminal gene expression profiling studies by Perou et al categorized breast cancer into four intrinsic molecular subtypes.Citation8 The basal-like subtype comprises a group of tumors characterized by low or absent ER expression, very low prevalence of HER2 overexpression/amplification, and expression of genes usually found in the basal or myoepithelial cells of the human breast. Although the majority of TNBCs fall into the basal-like intrinsic subtype, the overlap between immunohistochemically defined TNBC and basal-like intrinsic subtype is not complete.Citation50 Various studies demonstrate that 70%–80% of TNBC are basal-like and 20%–30% of non-TNBC are basal-like by molecular profiling.Citation51–Citation53 Proportion of non-basal-like subtypes within TNBC may be influenced by age at breast cancer diagnosis; Prat et al demonstrated a higher incidence of non-basal TNBC in women over 60 years (26%) of age as compared to those who are 40 years or younger (4.3%).Citation53 Approximately 7% of unselected TNBC tumors classify as luminal A or B subtype on intrinsic molecular profiling.Citation53 There may be a link between TNBC noted to be luminal on intrinsic profiling and AR overexpression; it is postulated that TNBCs classified as luminal A may be enriched with AR overexpression.Citation5
TNBC is a diverse entity for which additional subclassifications beyond basal and non-basal may be needed. Using gene expression from publically available data sets, Lehmann et al classified TNBC initially into seven molecular subtypes, and recently refined the classification into four molecular subtypes: basal-like 1, basal-like 2, mesenchymal, and luminal androgen receptor-like (LAR) based on gene expression profiles.Citation11 Based on identification of cell lines corresponding to each subtype, they also demonstrated that these subtypes may be responsive to different targeted therapies.Citation5 The methodology of Lehmann et al’s molecular classification has recently been adapted to a RNA-seq platform to better fit individual clinical samples.Citation54
Approximately 16% of TNBCs classify as LAR molecular subtype (). Retrospective studies have demonstrated LAR subtype to be associated with clinical-pathologic features, treatment response, and outcomes. LAR subtype tumors have lower pathological grade and are diagnosed in women of older ages compared to all other TNBC types. LAR tumors also demonstrate significant enrichment of axillary lymph node metastasis and preferential distant metastasis to bone.Citation11
Figure 2 Incidence of TNBC by TNBC type-4 classification.
Abbreviations: TNBC, triple-negative breast cancer; BL1, basal-like 1; BL2, basal-like 2; M, mesenchymal; LAR, luminal androgen receptor-like; UNC, unclasssified.
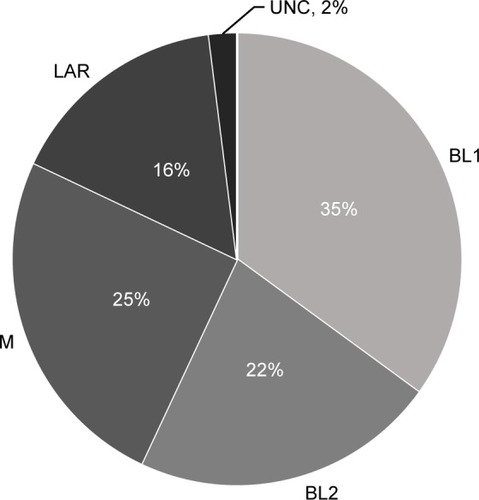
LAR tumors respond to neoadjuvant chemotherapy at much lower rates; recent studies have demonstrated that the LAR molecular subtype is associated with lower pathological complete response (pCR) rates compared to other TNBC subtypes.Citation11,Citation55 Similar associations of positive AR IHC expression with low pCR rates have also been reported in TNBC.Citation4,Citation56 Furthermore, some studies have also demonstrated better DFS in spite of low pCR in AR+ TNBC.Citation4 The discrepancy between neoadjuvant chemotherapy response and survival in the LAR subtype mimics the observations noted for ER-positive and/or luminal A breast cancerCitation57,Citation58 and further supports the notion of biological similarities between AR+/LAR subtype and luminal AR+/ER+ breast cancers.
Androgen signaling drives the LAR phenotype
Gene ontologies for LAR subtype are heavily enriched in hormonally regulated pathways including steroid synthesis, porphyrin metabolism, and androgen/estrogen metabolism. LAR subtype also shows enhanced activity of AR at the transcript level and correlations with nuclear AR IHC staining and protein levels.Citation5 Tumors within the LAR group are also found to express downstream AR targets and coactivators (DHCR24, ALCAM, FASN, FKBP5, APOD, PIP, SPDEF, and CLDN8).Citation5 AR expression in LAR subtype tumors is responsible for tumor cell viability and survival, as suggested by the significantly reduced ability of LAR cell lines to form colonies after knockdown of AR expression.Citation5
Androgen signaling is a targetable pathway in TNBC
Preclinical in vitro and xenograft studies have demonstrated that LAR cell lines are sensitive to AR inhibition.Citation5,Citation14,Citation19,Citation20 AR expression is also noted in cell lines representing other TNBC subtypes beyond the LAR subtype. AR inhibition can thus be a potential therapeutic strategy for other TNBC subtypes. Recent studies showed that AR inhibition with enzalutamide and bicalutamide significantly reduces baseline proliferation, anchorage-independent growth, migration, and invasion, and increases apoptosis in LAR and three non-LAR TNBC molecular subtypes (mesenchymal-like, mesenchymal stem-like, and basal-like 2).Citation19,Citation59 Enzalutamide inhibits DHT-driven tumor growth in ER-negative (MDA-MB-453) xenografts by increasing apoptosis.Citation20 Thus, the preclinical studies suggest that antagonism of the androgen signaling pathway could be a potential therapeutic approach for TNBC.
Androgen receptor targeting
Preclinical evidence of efficacy
Early androgen signaling inhibitors were first investigated as part of standard-of-care androgen deprivation therapy (ADT) for prostate cancer. Wong and Xie validated the suspected association between androgen exposure and mammary cancer in rats, demonstrating a role of androgens in inducing histological transformation, which was reversed with the androgen-blocking agent flutamide.Citation60 Subsequent studies in breast cancer employed the fourfold more potent agent bicalutamide, which in prostate cancer is capable of inducing accessory sex organ regression with minimal effect on serum hormone levels.Citation61 Bicalutamide has shown a paradoxical effect in breast cancer depending on ER expression, inducing apoptosis in ER− tumors and reversing androgen-driven cell death in ER+ tumors.Citation20,Citation62–Citation64 Lehmann et al have described bicalutamide sensitivity in the LAR molecular subtype of TNBC.Citation5 Bicalutamide’s apoptotic effects in other subtypes of TNBC have also been demonstrated in preclinical studies.Citation40,Citation59 In a noteworthy case report by Arce-Salinas et al, a patient with metastatic AR+ TNBC whose disease had progressed under heavy systemic chemotherapy achieved complete clinical response in chest wall disease after 4 months of oral bicalutamide therapy.Citation65 These preclinical data, along with the decades of safety and tolerability studies in prostate cancer, prompted a surge of clinical trials employing AR antagonists in breast cancer and TNBC in particular.
Bicalutamide is a nonsteroidal antiandrogen, which competitively inhibits the binding of androgens with AR.Citation66 It is commonly used in the treatment of locally advanced and metastatic prostate cancer, either as monotherapy or combined with a gonadotropin-releasing hormone agonist.Citation67,Citation68 Whereas bicalutamide inhibits transcription of AR-regulated genes by assembling corepressors rather than coactivators, the second-generation nonsteroidal antiandrogen enzalutamide has a fivefold greater affinity for AR and prevents nuclear translocation of ligand-bound AR.Citation69–Citation71 As described earlier, bicalutamide promotes proliferation in ER+ breast cancer, perhaps due to AR competitively binding to EREs in the nucleus, an effect which enzalutamide prevents by inhibiting nuclear entry.Citation20 Enzalutamide was shown to inhibit ER-mediated mammary tumor growth in ER+ as well as in ER− cancers, which supports the proposed mechanism of action. Work by Barton et al suggested that AR inhibition may be effective even in low AR-expressing TNBC, as evidenced by enzalutamide-induced apoptosis in one LAR and three non-LAR TNBC subtype cell lines.Citation19 AR inhibition alone may be insufficient in cases of advanced or chemoresistant TNBC. Abiraterone acetate is a selective inhibitor of cytochrome P450 17α-hydroxylase/17,20-lyase (CYP17), reducing adrenal and tumor androgen biosynthesis.Citation72 Castrate-resistant, taxane-resistant prostate cancer exhibits good clinical response to abiraterone acetate in conjunction with prednisone, an effect that is dependent on AR, at least in part.Citation73–Citation76 Use of CYP17 inhibitors to reduce androgen availability may increase the potency of agents targeting the AR in breast cancer.
Kwilas et al evaluated the ability of AR inhibition to reduce the growth and improve the immune-mediated killing of breast cancer cells with differing expression of the ER and AR.Citation77 They reported that while AR expression was required for the growth inhibitory effects of enzalutamide on breast cancer cells, both enzalutamide and abiraterone improved the sensitivity of breast cancer cells to immune-mediated lysis independent of detectable AR expression. Reduction in osteoprotegerin was noted to mediate the increase in sensitivity of AR− TNBC cells to immune-mediated killing. This data further supports investigation of AR inhibition in the AR+ TNBC and also in AR− TNBC, especially in combination with immunotherapy. In a mouse model of prostate cancer, the combination of enzalutamide and immunotherapy resulted in a significantly higher OS as opposed to each individual treatment.Citation78 These findings expand the treatment potential of enzalutamide and other androgen antagonists to both AR+ and AR− TNBC.
Clinical activity of antiandrogen monotherapy
Bicalutamide
AR status in TNBC patients has been utilized as a biomarker for preselection of patients for antiandrogen therapy trials. A Translational Breast Cancer Research Consortium Phase II study was the first to evaluate antiandrogen therapy in TNBC patients selected by AR status (NCT00468715).Citation62 Eligibility for the trial required either the primary or a metastatic tumor to be positive for AR (IHC >10% nuclear staining). Twelve percent (51 of 424) of screened patients demonstrated AR positivity. Patients received bicalutamide 150 mg orally daily. Among 26 evaluable patients, although there were no complete or partial responses, the 6-month clinical benefit rate (CBR) was 19%. Bicalutamide was well tolerated with no grade 4/5 treatment-related adverse events observed. This study demonstrated proof of principle for the efficacy of androgen blockade in a select group of patients with ER−/PR−/AR+ breast cancer.
Enzalutamide
A recent Phase II clinical trial evaluated single-agent enzalutamide in women with advanced AR+ TNBC (AR IHC >0%) (NCT001889238). Of the 75 patients who were evaluable, CBR was 35% and 29% at 16 and 24 weeks, respectively.Citation79 This trial also reported on the positive association of gene signature (PREDICT AR) for identification of TNBC patients most likely to benefit from this approach.Citation50 PREDICT AR positive status was noted in 50% of patients with metastatic TNBC in this study, and patients with positive signature experienced higher CBR at 16 and 24 weeks compared to those lacking this gene signature.Citation50,Citation79 AR IHC, on the other hand, did not correlate with response to enzalutamide in this study. This indicates that further development and refinement of biomarkers for identification of patients most likely to benefit from antiandrogen therapy are needed.
CYP17 inhibitors
Abiraterone acetate plus prednisone treatment was evaluated in a Phase II proof-of-concept trial for advanced AR+ TNBC (NCT01842321).Citation80 AR positivity for this trial was set at ≥10% IHC expression, and 37% of tested samples met this criterion. Among 30 evaluable patients, the 6-month CBR was 20% (one complete response and five patients with stable disease for ≥6 months). Although the study did not meet its predefined end point of a 25% CBR, abiraterone acetate may be effective for a selected subset of AR+ TNBC. Indeed, several ongoing Phase I–II trials are investigating the efficacy of CYP17 inhibitors, alone or combined with other pathway inhibitors ().
Table 1 Recently completed and recruiting clinical trials targeting androgen signaling in TNBC
Combination therapy
Preclinical data suggest that AR dependency may coexist with other oncogenic aberrations, suggesting potential value of combining AR targeting with other targeted agents.
AR inhibitors plus CDK4/CDK6 inhibitors
Cyclin-dependent kinases (CDK) 4 and 6 promote proliferation by removing retinoblastoma (RB) protein-driven suppression of cell cycle progression. CDK4/6 inhibitors such as palbociclib inhibit proliferation by arresting the cell cycle in the G1 phase.Citation81 Although RB protein is commonly lost in TNBC, RB has been associated with AR expression in TNBC, and sensitivity to palbociclib has been reported in three LAR cell lines.Citation82,Citation83 Combination therapy of CDK4/6 inhibitors and androgen deprivation treatment is being evaluated in two ongoing Phase I/II clinical trials ().
AR inhibitors plus PI3K inhibitors
Phosphatidylinositol-3-kinase (PI3K) and the downstream components Akt and mechanistic target of rapamycin (mTOR) are recognized as promising targets for treatment of breast cancer.Citation84,Citation85 Activating mutations of PIK3CA are seen in ~40% of AR+ TNBC tumors, as compared to 4% of AR− TNBC tumors.Citation86 Though PIK3CA inhibitors display antiproliferative effects on tumors with elevated PI3K activity, AR knockdown alone can allow cells to bypass the tumor suppressor activity of phosphate and tensin homolog (PTEN), which can promote proliferation.Citation17 Combination therapy with a PI3K inhibitor and AR inhibitor has an additive apoptotic effect in AR+ TNBC cell lines.Citation84,Citation86 Combination of the mTOR inhibitor rapamycin and the antiandrogen enzalutamide has also shown additive effect in LAR TNBC cell lines and in a LAR xenograft model.Citation87 Based on this preclinical evidence, clinical investigation of antiandrogen therapy with drugs targeting PI3K/mTOR pathway is underway. An ongoing Phase I study is assessing combinations of abiraterone with PI3K inhibitor or mTOR inhibitor in metastatic TNBC (NCT01884285).
AR inhibitors plus neoadjuvant chemotherapy
AR+ TNBC is associated with relative resistance to conventional neoadjuvant chemotherapy as demonstrated by lower rates of pCR.Citation5,Citation11,Citation55 This observation raises the question of whether combining AR inhibition with chemotherapy would improve response to chemotherapy in AR+ TNBC. A Phase II clinical trial is currently underway to assess rates of pCR or near-pCR in early-stage AR+ (≥10%) TNBC patients treated with enzalutamide and weekly paclitaxel (NCT02689427).
Future direction
The 6-month CBR of 19%–29% observed with antiandrogen monotherapy in clinical trials to date (NCT00468715, NCT001889238, and NCT01842321) is relatively modest. However, this degree of activity is not very different from early experience of targeting ER in metastatic breast cancer where diethylstilbestrol monotherapy yielded response rates of 4%–21%.Citation114,Citation115 Subsequently, decades of research that involved development of more efficacious agents to target ER and standardization of techniques to accurately identify ER-positive disease led to improved success in clinical trials. The low response rates seen with current AR targeting in clinical trials could also be related to resistance (primary or secondary) to antiandrogen therapy. In prostate cancer, failure of ADT has been linked to amplification of AR and/or increased expression of AR variants such as AR-V7 that lack the C-terminal ligand-binding domain and are thus constitutively active.Citation73,Citation88,Citation89 Constitutively active AR variants have recently been detected in breast cancers, and have been shown to induce in vitro proliferation in the presence of enzalutamide.Citation90 The antibody used in most clinical trials (AR441, Dako North America Inc, Carpinteria, CA, USA) is antigenic to the receptor’s N-terminus, implying that the total AR detected may be comprised of both full-length AR and ligand-independent truncated AR variants. The development of IHC antibodies that detect AR’s C-terminal ligand-binding domain may improve patient selection in future clinical trials by allowing quantitation of both full-length AR and truncated variants. Recent preclinical studies suggest that AR variant antagonists (HSP90 inhibitors, ROR-γ inhibitors) may reverse ADT resistance in tumors with constitutively active AR.Citation91,Citation92 Similarly, other agents that block dimerization, nuclear translocation, or DNA binding could prove effective against full-length and truncated AR isoforms alike. Future clinical trials targeting AR in TNBC will benefit from a better understanding of ADT resistance and the ability to further select patients who will benefit from antiandrogen therapy.
A second potential source of ADT resistance in AR+ TNBC is membrane-initiated androgen signaling. While the classical model of AR signaling requires intracellular AR ligand binding, nuclear translocation, and ARE recognition (), nongenomic AR activity may also play a role in androgen resistance. Actin skeleton reorganization, decreased cell motility, and increased apoptosis have been ascribed to membrane AR signaling in breast cancer cell lines.Citation93–Citation95 Further investigation is required, though, as activated membrane AR has also been shown to promote cell viability in other cell types.Citation96,Citation97 The discovery of nongenomic androgen activity suggests the possibility of modulated androgen signaling independently of intracellular AR activity. Various approaches are being explored in preclinical studies, including albumin-conjugated androgens that only activate membrane AR and cannot enter the cytosol and conversely, agents that inhibit membrane AR phosphorylation and downstream signaling.Citation98,Citation99 In summary, membrane-initiated androgen signaling may partially complement and partially compete with genomic AR activity, and though its role is incompletely understood, membrane AR represents a potentially targetable marker in TNBC and other cancers.
Studies using knockout rodent models have revealed a complex relationship between AR and the immune response. Intracellular AR is differentially expressed in immune cell subpopulations, and androgen signaling can suppress B and T lymphocyte development and conversely stimulate neutrophil production.Citation100,Citation101 In prostate cancer, AR-dependent cell lines are highly susceptible to TNF-α-induced apoptosis, and exposure to TNF-α induces hypersensitivity to androgen signaling.Citation102,Citation103 The cytokine interleukin-6 upregulates AR transcription and induces ligand-independent AR activation, which may promote inflammation and tumor growth.Citation104–Citation106 Mediators of these responses include NF-κB, MAPK, STAT3, and PI3K/Akt/mTOR.Citation102,Citation104–Citation111 Additionally, androgen signaling downregulates expression of certain toll-like receptors, which may inhibit the immune response and promote proliferation.Citation112
The AR is thus involved in an intricate web of both pro-and anti-tumor signaling with other pathways. These complex interactions are incompletely understood, yet they illustrate the need for additional biomarkers to evaluate androgen dependency in patients. While standardized methods for detecting AR in breast tumor samples are important for establishing its predictive and prognostic value, patients with similar tumor AR expression may not respond homogeneously to antiandrogen therapy due to coregulated signaling pathways. As illustrated in the above section ‘Combination therapy’, several clinical trials are underway to evaluate combinations of targeted therapy.
Summary
Androgen targeting has demonstrated early promise and is worthy of further evaluation in appropriately selected TNBC. Evaluation of AR targeting in clinical trials has thus far utilized AR IHC expression (with various cutoffs) as selection criteria. Preclinical studies investigating this therapeutic approach have, on the other hand, utilized AR IHC and gene expression-defined subtypes (luminal or LAR subtypes). It unlikely that IHC positivity for AR alone will accurately identify patients likely to respond to AR modulating therapies, due to the complexity of interacting signaling pathways.Citation79,Citation113 Several gene expression-defined subtypes to identify tumor AR dependency have come forth in recent years (PREDICT AR, LAR subtype, intrinsic luminal subtype).Citation5,Citation11,Citation50,Citation53,Citation54 However, these need to be evaluated in context of prospective trials before being translated to routine clinical use. Additional translational efforts should also focus on changes in tumor AR dependency under pressures of standard chemotherapy and whether primary or metastatic tumor androgen dependency is most likely to correspond to antiandrogen treatment response.
Use of single-agent AR inhibitors have exhibited modest efficacy in clinical trials. Recent advances in our understanding of androgenic signaling in TNBC, along with the identification of interacting pathways, are allowing development of next generation of clinical trials with AR inhibitors. As novel AR-targeting agents are developed and evaluated in clinical trials, it is equally important to establish a robust set of biomarkers for identification of TNBC tumors that are androgen dependent. Once achieved, this approach should guide successful study design and accrual efforts ().
Figure 3 Current and future landscape of AR pathway targeting in breast cancer.
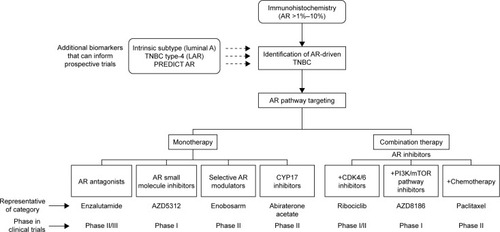
Though the role of androgen signaling in TNBC is complex, AR emerges as a potential therapeutic target for a subset of patients with this aggressive disease that otherwise lacks molecular targets. Antiandrogen agents are well tolerated, with acceptable and well-established safety profiles. Several antiandrogen agents are being evaluated in clinical trials either as single agents or in combination with other pathway inhibitors and cytotoxic compounds. The prospective development of standardized and reproducible methods for identifying AR-dependent tumors will allow investigators to tailor clinical trials to the correct subject population and allow clinicians to better predict patients’ response to these therapies in future.
Disclosure
The authors report no conflicts of interest in this work.
References
- American Cancer SocietyCancer Facts and Figures 2017Atlanta, GAAmerican Cancer Society2017
- DentRTrudeauMPritchardKITriple-negative breast cancer: clinical features and patterns of recurrenceClin Cancer Res20071315 Pt 14429443417671126
- BauerKRBrownMCressRDDescriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer registryCancer200710991721172817387718
- LoiblSMullerBMvon MinckwitzGAndrogen receptor expression in primary breast cancer and its predictive and prognostic value in patients treated with neoadjuvant chemotherapyBreast Cancer Res Treat2011130247748721837479
- LehmannBDBauerJAChenXIdentification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapiesJ Clin Invest201112172750276721633166
- HafftyBGYangQReissMLocoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancerJ Clin Oncol200624365652565717116942
- TanDSMarchioCJonesRLTriple negative breast cancer: molecular profiling and prognostic impact in adjuvant anthracycline-treated patientsBreast Cancer Res Treat20081111274417922188
- PerouCMSorlieTEisenMBMolecular portraits of human breast tumoursNature2000406679774775210963602
- SorlieTTibshiraniRParkerJRepeated observation of breast tumor subtypes in independent gene expression data setsProc Natl Acad Sci U S A2003100148418842312829800
- PratAParkerJSKarginovaOPhenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancerBreast Cancer Res2010125R6820813035
- LehmannBDJovanovicBChenXRefinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selectionPLoS One2016116e015736827310713
- LehmannBDPietenpolJAIdentification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypesJ Pathol2014232214215024114677
- RampurwalaMWisinskiKBO’ReganRRole of the androgen receptor in triple-negative breast cancerClin Adv Hematol Oncol201614318619327058032
- NiMChenYLimETargeting androgen receptor in estrogen receptor-negative breast cancerCancer Cell201120111913121741601
- LiuYNLiuYLeeHJActivated androgen receptor down-regulates E-cadherin gene expression and promotes tumor metastasisMol Cell Biol200828237096710818794357
- NaderiAHughes-DaviesLA functionally significant cross-talk between androgen receptor and ErbB2 pathways in estrogen receptor negative breast cancerNeoplasia200810654254818516291
- WangYRomighTHeXDifferential regulation of PTEN expression by androgen receptor in prostate and breast cancersOncogene201130424327433821532617
- PetersAABuchananGRicciardelliCAndrogen receptor inhibits estrogen receptor-alpha activity and is prognostic in breast cancerCancer Res200969156131614019638585
- BartonVND’AmatoNCGordonMAMultiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivoMol Cancer Ther201514376977825713333
- CochraneDRBernalesSJacobsenBMRole of the androgen receptor in breast cancer and preclinical analysis of enzalutamideBreast Cancer Res2014161R724451109
- BourdeauVDeschenesJMetivierRGenome-wide identification of high-affinity estrogen response elements in human and mouseMol Endocrinol20041861411142715001666
- CarrollJSMeyerCASongJGenome-wide analysis of estrogen receptor binding sitesNat Genet200638111289129717013392
- LanzinoMDe AmicisFMcPhaulMJEndogenous coactivator ARA70 interacts with estrogen receptor alpha (ERalpha) and modulates the functional ERalpha/androgen receptor interplay in MCF-7 cellsJ Biol Chem200528021204212043015772083
- AleskandaranyMAAbduljabbarRAshankytyIPrognostic significance of androgen receptor expression in invasive breast cancer: transcriptomic and protein expression analysisBreast Cancer Res Treat2016159221522727514395
- HuRDawoodSHolmesMDAndrogen receptor expression and breast cancer survival in postmenopausal womenClin Cancer Res20111771867187421325075
- QuQMaoYFeiXCThe impact of androgen receptor expression on breast cancer survival: a retrospective study and meta-analysisPLoS One2013812e8265024324816
- Vera-BadilloFETempletonAJde GouveiaPAndrogen receptor expression and outcomes in early breast cancer: a systematic review and meta-analysisJ Natl Cancer Inst20141061djt31924273215
- CollinsLCColeKSMarottiJDAndrogen receptor expression in breast cancer in relation to molecular phenotype: results from the Nurses’ Health StudyMod Pathol201124792493121552212
- ElebroKBorgquistSSimonssonMCombined androgen and estrogen receptor status in breast cancer: treatment prediction and prognosis in a population-based prospective cohortClin Cancer Res201521163640365025904752
- ParkSKooJParkHSExpression of androgen receptors in primary breast cancerAnn Oncol201021348849219887463
- ParkSKooJSKimMSAndrogen receptor expression is significantly associated with better outcomes in estrogen receptor-positive breast cancersAnn Oncol20112281755176221310761
- Gonzalez-AnguloAMStemke-HaleKPallaSLAndrogen receptor levels and association with PIK3CA mutations and prognosis in breast cancerClin Cancer Res20091572472247819276248
- HeJPengRYuanZPrognostic value of androgen receptor expression in operable triple-negative breast cancer: a retrospective analysis based on a tissue microarrayMed Oncol201229240641021264529
- SafarpourDPakneshanSTavassoliFAAndrogen receptor (AR) expression in 400 breast carcinomas: is routine AR assessment justified?Am J Cancer Res20144435336825057438
- AgoffSNSwansonPELindenHAndrogen receptor expression in estrogen receptor-negative breast cancerAm J Clin Pathol2003120572573114608899
- BryanRMMercerRJBennettRCAndrogen receptors in breast cancerCancer19845411243624406498736
- CastellanoIAlliaEAccortanzoVAndrogen receptor expression is a significant prognostic factor in estrogen receptor positive breast cancersBreast Cancer Res Treat2010124360761720127405
- HickeyTERobinsonJLCarrollJSMinireview: the androgen receptor in breast tissues: growth inhibitor, tumor suppressor, oncogene?Mol Endocrinol20122681252126722745190
- LabrieFEl-AlfyMBergerLThe combination of a novel selective estrogen receptor modulator with an estrogen protects the mammary gland and uterus in a rodent model: the future of postmenopausal women’s health?Endocrinology2003144114700470612960051
- MehtaJAsthanaSMandalCCA molecular analysis provides novel insights into androgen receptor signalling in breast cancerPLoS One2015103e012062225781993
- McGhanLJMcCulloughAEProtheroeCAAndrogen receptor-positive triple negative breast cancer: a unique breast cancer subtypeAnn Surg Oncol201421236136724046116
- MrklicIPogorelicZCapkunVExpression of androgen receptors in triple negative breast carcinomasActa Histochem2013115434434823031358
- SuttonLMCaoDSarodeVDecreased androgen receptor expression is associated with distant metastases in patients with androgen receptor-expressing triple-negative breast carcinomaAm J Clin Pathol2012138451151623010705
- TangDXuSZhangQThe expression and clinical significance of the androgen receptor and E-cadherin in triple-negative breast cancerMed Oncol201229252653321519872
- CauleyJALucasFLKullerLHElevated serum estradiol and testosterone concentrations are associated with a high risk for breast cancerAnn Intern Med19991304 Pt 127027710068384
- RakhaEAEl-SayedMEGreenARPrognostic markers in triple-negative breast cancerCancer20071091253217146782
- FarmerPBonnefoiHBecetteVIdentification of molecular apocrine breast tumours by microarray analysisOncogene200524294660467115897907
- MillsAMGottliebCWendrothSPure apocrine carcinomas represent a clinicopathologically distinct androgen receptor-positive subset of triple-negative breast cancersAm J Surg Pathol20164081109111627259012
- WangCPanBZhuHPrognostic value of androgen receptor in triple negative breast cancer: a meta-analysisOncotarget2016729464824689127374089
- ParkerJSPetersonACTudorICA novel biomarker may predict clinical activity from enzalutamide in triple-negative breast cancerPoster presented at: 2015 ASCO Annual MeetingMay 29–June 2; 2015Chicago, IL
- PratAPerouCMDeconstructing the molecular portraits of breast cancerMol Oncol20115152321147047
- BastienRRRodriguez-LescureAEbbertMTPAM50 breast cancer subtyping by RT-qPCR and concordance with standard clinical molecular markersBMC Med Genomics201254423035882
- PratAAdamoBCheangMCMolecular characterization of basal-like and non-basal-like triple-negative breast cancerOncologist201318212313323404817
- RingBZHoutDRMorrisSWGeneration of an algorithm based on minimal gene sets to clinically subtype triple negative breast cancer patientsBMC Cancer20161614326908167
- MasudaHBaggerlyKAWangYDifferential response to neo-adjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypesClin Cancer Res201319195533554023948975
- AsanoYKashiwagiSOnodaNClinical verification of sensitivity to preoperative chemotherapy in cases of androgen receptor-expressing positive breast cancerBr J Cancer20161141142026757422
- CortazarPZhangLUntchMPathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysisLancet2014384993816417224529560
- RouzierRPerouCMSymmansWFBreast cancer molecular subtypes respond differently to preoperative chemotherapyClin Cancer Res200511165678568516115903
- ZhuALiYSongWAntiproliferative effect of androgen receptor inhibition in mesenchymal stem-like triple-negative breast cancerCell Physiol Biochem20163831003101426938985
- WongYCXieBThe role of androgens in mammary carcinogenesisItal J Anat Embryol20011062 Suppl 111112511729946
- FurrBJValcacciaBCurryBICI 176,334: a novel non-steroidal, peripherally selective antiandrogenJ Endocrinol19871133R7R93625091
- GucalpATolaneySIsakoffSJPhase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancerClin Cancer Res201319195505551223965901
- SzeleiJJimenezJSotoAMAndrogen-induced inhibition of proliferation in human breast cancer MCF7 cells transfected with androgen receptorEndocrinology19971384140614129075695
- Toth-FejelSCheekJCalhounKEstrogen and androgen receptors as comediators of breast cancer cell proliferation: providing a new therapeutic toolArch Surg20041391505414718276
- Arce-SalinasCRiesco-MartinezMCHannaWComplete response of metastatic androgen receptor-positive breast cancer to bicalutamide: case report and review of the literatureJ Clin Oncol2016344e21e2424888812
- WirthMPHakenbergOWFroehnerMAntiandrogens in the treatment of prostate cancerEur Urol2007512306313 discussion 1417007995
- AndersonJThe role of antiandrogen monotherapy in the treatment of prostate cancerBJU Int200391545546112603397
- PilepichMVWinterKJohnMJPhase III radiation therapy oncology group (RTOG) trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostateInt J Radiat Oncol, Biol, Phys20015051243125211483335
- BaekSHOhgiKANelsonCALigand-specific allosteric regulation of coactivator functions of androgen receptor in prostate cancer cellsProc Natl Acad Sci U S A200610393100310516492776
- ChengSBrzostekSLeeSRInhibition of the dihydrotestosterone-activated androgen receptor by nuclear receptor corepressorMol Endocrinol20021671492150112089345
- TranCOukSCleggNJDevelopment of a second-generation antiandrogen for treatment of advanced prostate cancerScience2009324592878779019359544
- BedoyaDJMitsiadesNClinical appraisal of abiraterone in the treatment of metastatic prostatic cancer: patient considerations, novel opportunities, and future directionsOnco Targets Ther2013691823319868
- AntonarakisESLuCWangHAR-V7 and resistance to enzalutamide and abiraterone in prostate cancerN Engl J Med2014371111028103825184630
- AzadAAEiglBJMurrayRNEfficacy of enzalutamide following abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer patientsEur Urol2015671232925018038
- RomanelAGasi TandefeltDConteducaVPlasma AR and abiraterone-resistant prostate cancerSci Transl Med20157312312re10
- SalviSCasadioVConteducaVCirculating cell-free AR and CYP17A1 copy number variations may associate with outcome of metastatic castration-resistant prostate cancer patients treated with abirateroneBr J Cancer2015112101717172425897673
- KwilasARArdianiAGameiroSRAndrogen deprivation therapy sensitizes triple negative breast cancer cells to immune-mediated lysis through androgen receptor independent modulation of osteoprotegerinOncotarget20167172349823451127015557
- ArdianiAFarsaciBRogersCJCombination therapy with a second-generation androgen receptor antagonist and a metastasis vaccine improves survival in a spontaneous prostate cancer modelClin Cancer Res201319226205621824048332
- TrainaTMillerKYardleyDResults from a phase 2 study of enzalutamide (ENZA), an androgen receptor (AR) inhibitor, in advanced AR+ triple-negative breast cancer (TNBC)J Clin Oncol201533suppl abstr 1003
- BonnefoiHGrelletyTTredanOA phase II trial of abiraterone acetate plus prednisone in patients with triple-negative androgen receptor positive locally advanced or metastatic breast cancer (UCBG 12-1)Ann Oncol201627581281827052658
- FryDWHarveyPJKellerPRSpecific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenograftsMol Cancer Ther20043111427143815542782
- AsgharUHerrera-AbreuMTCuttsRIdentification of subtypes of triple negative breast cancer (TNBC) that are sensitive to CDK4/6 inhibitionJ Clin Oncol201533suppl abstr 11098
- TrereDBrighentiEDonatiGHigh prevalence of retinoblastoma protein loss in triple-negative breast cancers and its association with a good prognosis in patients treated with adjuvant chemotherapyAnn Oncol200920111818182319556322
- Cuenca-LopezMDMonteroJCMoralesJCPhospho-kinase profile of triple negative breast cancer and androgen receptor signalingBMC Cancer20141430224779793
- Cancer Genome Atlas NetworkComprehensive molecular portraits of human breast tumoursNature20124907418617023000897
- LehmannBDBauerJASchaferJMPIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitorsBreast Cancer Res201416440625103565
- RoblesAJCaiSCichewiczRHSelective activity of deguelin identifies therapeutic targets for androgen receptor-positive breast cancerBreast Cancer Res Treat2016157347548827255535
- DehmSMSchmidtLJHeemersHVSplicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistanceCancer Res200868135469547718593950
- VisakorpiTHyytinenEKoivistoPIn vivo amplification of the androgen receptor gene and progression of human prostate cancerNat Genet1995944014067795646
- HickeyTEIrvineCMDvingeHExpression of androgen receptor splice variants in clinical breast cancersOncotarget2015642447284474426554309
- FerraldeschiRWeltiJPowersMVSecond-generation HSP90 inhibitor onalespib blocks mRNA splicing of androgen receptor variant 7 in prostate cancer cellsCancer Res20167692731274227197266
- WangJZouJXXueXROR-gamma drives androgen receptor expression and represents a therapeutic target in castration-resistant prostate cancerNat Med201622548849627019329
- KallergiGAgelakiSMarkomanolakiHActivation of FAK/PI3K/Rac1 signaling controls actin reorganization and inhibits cell motility in human cancer cellsCell Physiol Biochem200720697798617982280
- KampaMNifliAPCharalampopoulosIOpposing effects of estradiol- and testosterone-membrane binding sites on T47D breast cancer cell apoptosisExp Cell Res20053071415115922725
- PelekanouVNotasGSanidasETestosterone membrane-initiated action in breast cancer cells: interaction with the androgen signaling pathway and EPORMol Oncol20104213514920189893
- KousteniSBellidoTPlotkinLINongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activityCell2001104571973011257226
- MigliaccioACastoriaGDi DomenicoMSteroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferationEMBO J200019205406541711032808
- AlevizopoulosKBacopoulosNPapadopoulouNPreclinical studies of MDX-12C, a selective membrane androgen receptor ligand with activity in prostate cancerJ Clin Oncol20082615 suppl abstr 14549
- MalaguarneraRSaccoAMorcavalloAMetformin inhibits androgen-induced IGF-IR up-regulation in prostate cancer cells by disrupting membrane-initiated androgen signalingEndocrinology201415541207122124437490
- ChuangKHAltuwaijriSLiGNeutropenia with impaired host defense against microbial infection in mice lacking androgen receptorJ Exp Med200920651181119919414555
- ViselliSMStanzialeSShultsKCastration alters peripheral immune function in normal male miceImmunology19958423373427751013
- ChopraDPMenardREJanuszewskiJTNF-alpha-mediated apoptosis in normal human prostate epithelial cells and tumor cell linesCancer Lett2004203214515414732222
- HaradaSKellerETFujimotoNLong-term exposure of tumor necrosis factor alpha causes hypersensitivity to androgen and anti-androgen withdrawal phenomenon in LNCaP prostate cancer cellsProstate200146431932611241555
- ChenTWangLHFarrarWLInterleukin 6 activates androgen receptor-mediated gene expression through a signal transducer and activator of transcription 3-dependent pathway in LNCaP prostate cancer cellsCancer Res20006082132213510786674
- HobischAEderIEPutzTInterleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptorCancer Res19985820464046459788616
- LinDLWhitneyMCYaoZInterleukin-6 induces androgen responsiveness in prostate cancer cells through up-regulation of androgen receptor expressionClin Cancer Res2001761773178111410519
- AarnisaloPPalvimoJJJanneOACREB-binding protein in androgen receptor-mediated signalingProc Natl Acad Sci U S A1998955212221279482849
- CinarBDe BenedettiAFreemanMRPost-transcriptional regulation of the androgen receptor by mammalian target of rapamycinCancer Res20056572547255315805247
- MatsudaTJunichoAYamamotoTCross-talk between signal transducer and activator of transcription 3 and androgen receptor signaling in prostate carcinoma cellsBiochem Biophys Res Commun2001283117918711322786
- NakajimaYDelliPizziAMMallouhCTNF-mediated cytotoxicity and resistance in human prostate cancer cell linesProstate19962952963028899002
- UedaTBruchovskyNSadarMDActivation of the androgen receptor N-terminal domain by interleukin-6 via MAPK and STAT3 signal transduction pathwaysJ Biol Chem200227797076708511751884
- Sanchez-HernandezMChaves-PozoECabasITestosterone implants modify the steroid hormone balance and the gonadal physiology of gilthead seabream (Sparus aurata L.) malesJ Steroid Biochem Mol Biol201313818319423743364
- ParkerJPetersonATudorIA novel biomarker to predict sensitivity to enzalutamide (ENZA) in TNBCJ Clin Oncol20153315 suppl abstr 1083
- CarterACSedranksNKelleyRMDiethylstilbestrol: recommended dosages for different categories of breast cancer patientsJAMA19772371920792085576887
- GockermanJPSpremulliENRaneyMLoganTRandomized comparison of tamoxifen versus diethylstilbestrol in estrogen receptor-positive or -unknown metastatic breast cancer: a Southeastern Cancer Study Group trialCancer Treat Rep19867010119912033530447
