Abstract
Background
The prognostic significance of proliferating cell nuclear antigen (PCNA) expression in gastric cancer has long been assessed, yet results remain controversial. Therefore, we performed a meta-analysis to assess the prognostic value and clinicopathological significance of PCNA in gastric cancer.
Methods
A systematic literature search of PubMed, EMBASE, and the Cochrane Library databases was conducted. Summary odds ratios (ORs) and hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated to investigate the correlations between PCNA expression and clinicopathological features, overall survival (OS), and disease-free survival (DFS).
Results
A total of 19 studies involving 2,852 participants were included in our analysis. The pooled HR indicated that high PCNA expression was significantly associated with poor OS (HR 1.66, 95% CI 1.32–2.08) and DFS (HR 1.81, 95% CI 1.40–2.36). Subgroup analysis revealed that the association between PCNA and OS was also significant in Asian and European patients. In addition, the pooled ORs showed that high PCNA expression was significantly associated with deeper tumor invasion (OR 2.37, 95% CI 1.71–3.27), lymph node metastasis (OR 2.49, 95% CI 1.85–3.35), and advanced stage cancer (OR 1.89, 95% CI 1.36–2.63).
Conclusion
Our meta-analysis indicates that high PCNA expression might be a prognosticator of poor survival and a promising therapeutic target for gastric cancer patients.
Introduction
Gastric cancer is the fifth most common malignancy and the third leading cause of mortality worldwide. According to GLOBOCAN statistics, 951,000 new gastric cancer cases and 723,000 deaths from gastric cancer occurred globally in 2012.Citation1 Although comprehensive treatment is available, including adequate surgical resection supplemented by neoadjuvant treatments, the 5-year survival rate of gastric cancer remains <35%.Citation2,Citation3 Patients with the same clinical stage can have different prognoses, indicating that the clinical stage does not completely reflect the biological behavior of the tumor. Therefore, the identification of molecular biomarkers is warranted to improve clinical staging schemes and predict prognosis.Citation4 Prognostic biomarkers such as E-cadherin, STAT3, CD133, p53, MMP7, and lactate dehydrogenase have been explored in published articles.Citation5–Citation10 However, there is still a heated discussion on discovering a new biomarker to predict patient prognosis and to provide novel therapeutic targets for gastric cancer patients.
Proliferating cell nuclear antigen (PCNA) was originally discovered in 1978 by Miyachi et alCitation11 as the antigen to an autoimmune antibody in the sera of patients with systemic lupus erythematosus.Citation12 It was initially considered to be expressed during cell proliferation, with peak expression occurring during late G1 and S phases.Citation13,Citation14 However, in recent decades, PCNA has been shown to act as a molecular platform that coordinates a wide range of processes involved in genome maintenance, duplication, transmission, and cell-cycle regulation.Citation15,Citation16 Because cell proliferation is a requirement for tumor progression, and owing to the indispensable function of PCNA in cell proliferation, much attention has been paid to the role of PCNA in tumors.Citation17 Indeed, PCNA was found to be involved in the prognosis of cancer patients, including those with nasopharyngeal carcinoma, lung cancer, prostate carcinoma, and gastric carcinoma.Citation18–Citation21
A recent meta-analysis demonstrated that high PCNA expression was significantly associated with higher mortality, suggesting that it could be a useful prognostic biomarker in gliomas and cervical cancer.Citation22 However, controversy remains in gastric cancer about the impact of PCNA on patient survival and clinicopathological characteristics. Numerous publications have demonstrated that PCNA overexpression was associated with poor prognosis in gastric cancer patients,Citation23–Citation25 while some studies hold different views.Citation26,Citation27 To investigate this further, we conducted a meta-analysis to evaluate the association between PCNA expression and overall survival (OS), disease-free survival (DFS), and clini-copathological characteristics in gastric cancer.
Materials and methods
Search strategy and selection criteria
A comprehensive literature search of PubMed, EMBASE, and Cochrane Library databases was conducted with the MeSH terms and the following key words variably combined: “stomach”, “gastric”, “neoplasm”, “cancer”, “carcinoma”, “tumor”, “proliferating cell nuclear antigen”, and “PCNA”. The search was completed on May 20, 2016. Reference entries of eligible literature were scanned to minimize any deviation caused during the research process. This study is a meta-analysis, did not involve subjects, and was based on previous published articles; therefore, ethical approval was not required.
The inclusion criteria of studies in this meta-analysis were as follows: 1) patients diagnosed with gastric cancer by pathologists; 2) PCNA expression detected in primary tumor tissues; 3) an association between PCNA expression and parameters such as OS, DFS, or clinicopathological characteristics; 4) sufficient information to extract hazard ratios (HRs), odds ratios (ORs), and their 95% confidence intervals (CIs); and 5) full text, original research articles published in English. Reports of conferences and reviews were excluded. Only the most complete study was selected if duplicate data from other articles occurred. Two investigators (SY and ZL) independently screened all studies and identified those that were eligible for inclusion. Inconsistencies were resolved through negotiation and consultation.
Quality assessment
The methodological quality of the original studies was assessed by the Newcastle–Ottawa Scale (NOS),Citation28 which consisted of three factors: selection, comparability of subjects, and outcome. Each study received a score from 0 to 9 (allocated as stars), and scores higher than 6 were considered high quality. Two authors (SY and JH) independently performed this assessment, and discrepancies were resolved by discussion.
Data extraction
Two researchers (SY and ZL) used a predesigned form to extract the following data independently from qualified studies: authors, country, publication year, number of participants, patient’s age, patient’s gender, cutoff value, percentage of PCNA-positive patients, clinicopathological characteristics of patients (including histological differentiation, clinical stage, T stage, lymphatic invasion, lymph node metastasis, vessel invasion, and Lauren classification), follow-up information, and survival data. Inconsistencies were resolved by consultation with a third author (HX) when the two reviewers could not reach a consensus.
Statistical analysis
HR and its 95% CI were used to evaluate the correlation between PCNA expression and patient survival. If the HR with 95% CI were reported in the original study, we extracted the data directly. If not, we extrapolated HR from survival rates with P-values from log-rank tests or Kaplan–Meier survival curves using the method reported by Parmar et alCitation29 and Tierney et al.Citation30 ORs with 95% CIs were chosen to investigate the association between clinicopathological features and PCNA expression. Clinicopathological features included histological differentiation, clinical stage, T stage, lymphatic invasion, lymph node metastasis, vessel invasion, and Lauren classification. An observed HR or OR >1 implied a worse prognosis in the PCNA-positive group and was considered to be statistically significant if the 95% CI did not overlap 1.
Interstudy heterogeneity was tested by I2 statistics. I2>50% indicated that the studies showed significant heterogeneity, so a random-effects model was employed; otherwise, a fixed-effects model was implemented. Subgroup analysis and meta-regression were conducted to investigate the potential heterogeneity among studies. We also performed sensitivity analysis to evaluate the stability of the results. Potential publication bias was assessed by funnel plots and Egger’s linear regression test.Citation31 STATA statistical software (version 12.0, Stata Corporation, College Station, TX, USA) was used to perform data analyses. All P-values were two-sided and considered significant if <0.05.
Results
Search results
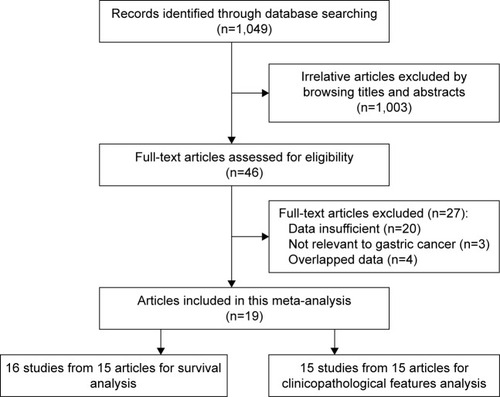
The processes of retrieval strategy for articles are described in . A total of 1,049 potential articles were identified for inclusion using the search strategies described in “Materials and methods” section. Through reviewing the title and abstracts, 1,003 papers were excluded. The remaining 46 were systematically evaluated by a full-text review. A further 27 were eliminated for the following reasons: the relationship between PCNA and tumors was not relevant to gastric cancer (n=3), insufficient data about survival or clinicopathological characteristics (n=13), nondichotomous variables of PCNA were excluded (n=7), and data overlapped those used in other studies (n=4). Finally, 19 studies involving a total of 2,852 gastric cancer patients met the requirements of our meta-analysis.Citation21,Citation23–Citation27,Citation32–Citation44
Study characteristics
The fundamental features of these 19 eligible articles are summarized in . Overall, 13 studies were conducted in patients from Asia and 6 from Europe. Sample sizes ranged from 32 to 841. All interstudies used the technique of immunohistochemistry (IHC) to detect the expression of PCNA. Ye et alCitation34 reported two independent data sets including familial gastric cancer and sporadic gastric cancer. Fifteen studies reported an association between PCNA expression and clinicopathological characteristics, and 16 articles contained studies investigating the effect of PCNA expression on survival (16 for OS and 3 for DFS).
Table 1 Characteristics of studies included in the meta-analysis
Impact of PCNA expression on OS and DFS
The correlation between PCNA expression and OS is shown in a forest plot (). Increased PCNA expression was shown to be significantly associated with an increased mortality risk by the random-effects model (pooled HR 1.66, 95% CI 1.32–2.08), with significant heterogeneity (I2=63.9%, P<0.001). Meta-regression and subgroup analyses were conducted based on study location, publication year, and cutoff value (). The source of heterogeneity could not be detected among these factors in meta-regression (all P>0.05). Subgroup analysis using pooled HRs showed that high PCNA expression was significantly associated with poor OS in both Asian (HR 1.69, 95% CI 1.28–2.23) and European (HR 1.52, 95% CI 1.11–2.08) patients. No significant heterogeneity was detected in European countries (I2=28.7%, P=0.240), in studies conducted after 2005 (I2=0%, P=0.655), or with cutoff <50% (I2=25.1%, P=0.254).
Figure 2 Forest plot of HR for the association between proliferating cell nuclear antigen expression and overall survival in gastric cancer patients.
Abbreviations: CI, confidence interval; HR, hazard ratio.
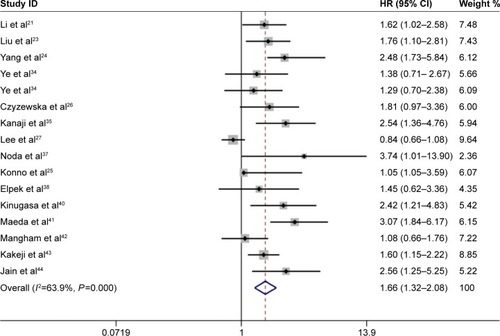
Table 2 Stratified analysis of PCNA expression with overall survival in gastric cancer patients
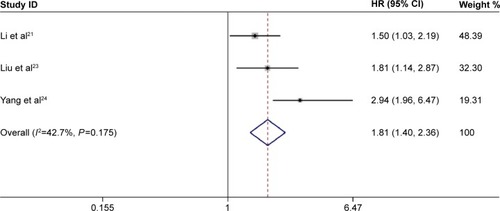
We also examined the impact of PCNA expression on DFS (). High expression of PCNA in primary gastric cancer was associated with a poor DFS in the fixed-effects model (pooled HR 1.81, 95% CI 1.40–2.36); moreover, no significant heterogeneity was detected (I2=42.7%, P=0.175).
Effect of PCNA expression on clinicopathological parameters
To further explore the biological role of PCNA, we investigated the correlation between PCNA expression and clinicopathological characteristics. First, we used the fixed-effects model to combine HR with 95% CI; if significant heterogeneity existed (I2>50%) among studies, the random-effects model was used. As illustrated in , increased PCNA expression was significantly correlated with the depth of invasion (T3/T4 vs T1/T2: OR 2.37, 95% CI 1.71–3.27), lymph node metastasis (positive vs negative: OR 2.49, 95% CI 1.85–3.35), and TNM stage (III–IV vs I–II: OR 1.89, 95% CI 1.36–2.63). No significant heterogeneity was observed (I2=0.0%–37.8%). However, PCNA expression was not associated with vascular invasion (positive vs negative: OR 1.32, 95% CI 0.70–2.48), histological grade (G3/G4 vs G1/G2: OR 1.04, 95% CI 0.72–1.50), or the Lauren classification type (intestinal vs diffuse: OR 1.14, 95% CI 0.70–1.86).
Table 3 Meta-analysis of PCNA high expression and clinicopathological features in gastric cancer
Sensitivity analysis
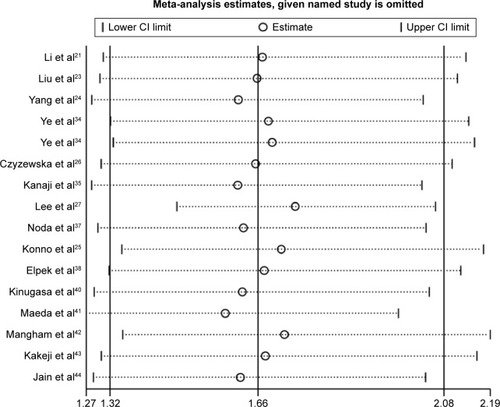
We performed a sensitivity analysis to assess the stability of our results regarding OS, DFS, and clinicopathological characteristics in gastric cancer patients. We compared the fixed-effects and random-effects models, but found no significant difference in OS (fixed-effects model: HR 1.43, 95% CI 1.26–1.62). Furthermore, the plots illustrated that our results were robust because pooled HRs or ORs were not significantly influenced by excluding any single study ().
Publication bias
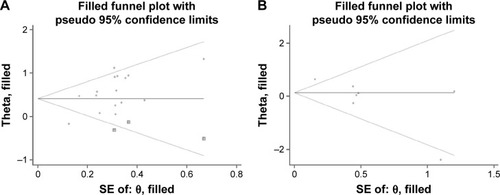
Begg’s funnel plot showed an asymmetric distribution, and the P-values from Egger’s tests indicated that there was significant publication bias in OS (P=0.003) and Lauren classification (P=0.024). To evaluate the potential impact of publication bias, a trim-and-fill analysis was performed. The adjusted pooled HR still showed a significant association between PCNA expression and OS (HR 1.51, 95% CI 1.22–1.88), whereas the adjusted pooled OR revealed a similar correlation between PCNA expression and Lauren classification to the above meta-analysis results (OR 1.14, 95% CI 0.70–1.85). After incorporating additional studies, the funnel plots were shown to be symmetrical (). This symmetry and the P-values from Egger’s tests indicated that there was no significant publication bias for pooled depth of invasion (P=0.9), lymph node metastasis (P=0.054), TNM stage (P=0.125), histological grade (P=0.268), or vascular invasion (P=0.865).
Discussion
PCNA is indispensable for DNA replication and the maintenance of genomic integrity in actively growing cells.Citation45 In replication machinery, the PCNA sliding clamp acts as a central scaffold to control the dynamic engagement of multiple factors at the heart of the replication fork.Citation16 It also forms a docking platform to recruit factors during the DNA damage response and replication surveillance.Citation16 Because of its role in cancer cell proliferation, PCNA has been widely used as a tumor marker. However, data are conflicting regarding the association between PCNA expression in tumor tissues and patient prognosis.Citation17 Previous studies suggested that high PCNA expression is an indicator of poor prognosis in cervical cancer or gliomas.Citation22 However, the equivalent data for patients with gastric cancer have not been reported.
Our meta-analysis of 19 individual studies involving 2,852 patients explored the relationship between PCNA and prognosis, as well as clinicopathological parameters in gastric cancer. The results indicate that high expression of PCNA predicts a poor OS and DFS in gastric cancer patients. Meanwhile, we observed significant heterogeneity among the studies regarding OS. Although the random-effects and fixed-effects models were used to pool data, neither model identified the source of heterogeneity. Meta-regression analysis showed that none of the factors thought to be the source, such as study location, publication year, and cutoff value, had a significant association with heterogeneity (all P>0.05). However, subgroup analysis indicated that heterogeneity was successfully removed in the subgroups of European countries, publication year >2005, and cutoff <50%. In addition, subgroup analysis revealed that high PCNA expression is also significantly associated with poor OS in Asian and European countries. We also evaluated the impact of PCNA expression on clinicopathological features. No significant heterogeneity was observed, so the fixed-effects model was used to show that increased PCNA expression was correlated with deeper tumor invasion, lymph node metastasis, and advanced TNM stage. These findings further verified the association between high PCNA expression and poor OS, which is consistent with our earlier results.
As we know, cancer is caused by multiple mechanisms that often appear error in DNA replication. And tumor progression cannot be separated from the proliferation and metastasis of tumor cells. PCNA is an indispensable factor for DNA replication, repair of DNA damage, chromatin structure maintenance, and cell-cycle progression, which also regulates tumor cell proliferation at both primary and metastatic sites.Citation17,Citation45 Interestingly, it is reported that a cancer-specific isoform of PCNA (csPCNA),Citation46 with methyl esterification on aspartic and glutamic acid residues, is expressed in tumor tissues but not in normal tissues.Citation45 However, its biochemical and molecular mechanisms are still unclear and further investigations will help clarify its roles in cellular malignant transformation and progression. In clinical research, a meta-analysis revealed that PCNA overexpression was correlated with advanced FIGO stage and poor survival in patients with cervical cancer, and PCNA overexpression was an important prognostic factor in glioma.Citation22 Our results also suggested that high PCNA expression was associated with poor survival and advanced clinicopathological features in gastric cancer patients. All these results demonstrate that PCNA might be an indicator of survival for cancer patients.
It is encouraging that some targeting PCNA inhibitors have been reported recently, which open the door to potential therapeutic targeting of PCNA. There are two types of PCNA-targeting inhibitors including peptides and small molecules.Citation45 One of the posttranslational modifications of PCNA for cell proliferation inhibition is phosphorylation of tyrosine residue 211 (pY211) of PCNA, which can be inhibited directly by peptide Y211F.Citation47 Y211F peptide could inhibit the synthesis of DNA, which was shown as the cell-cycle arrest at the S phase and apoptosis in vitro. Similarly, intratumoral injection of the Y211F peptide had been showed to significantly inhibit tumor growth and reduce tumoral pY211-PCNA in xenograft tumor models.Citation48,Citation49 In addition, PCNA-I, one of the small molecules targeting PCNA inhibitors, interferes with PCNA functions by influencing trimerization of PCNA formation. Treatment with PCNA-I resulted in downregulation of chromatin-associated PCNA, inhibition of DNA replication, and suppression of the proliferation of a variety of cancer cell lines.Citation50 Therefore, these promising approaches could be further exploited to targeting of cancer, and gastric cancer patients with a high PCNA expression might obtain a survival benefit from it.
Our analysis has a number of limitations. First, PCNA expression in gastric cancer tissues was detected by IHC in all included studies, but the accuracy of this method is dependent on the types of antibodies and their dilutions. As not all studies used the same primary antibody or antibody dilutions, this led to a potential bias. Subgroup analyses could not explore the effect of this difference on results because too few studies used the same antibodies and dilution ratios. Second, there was no uniform standard optimal threshold for evaluating PCNA IHC staining results. Cutoff values defining gastric cancer with high or low expression of PCNA were artificially set and varied from 23.8% to 75%, which might result in heterogeneity. As revealed in the subgroup analysis, heterogeneity was eliminated in the group with a cutoff value <50%. Third, each of the eligible studies had various parameters including sample size, age of participants, proportions of patients with high PCNA expression, and follow-up durations. Finally, we observed that studies reporting significant findings were more likely to be published in English language journals, whereas negative results were mostly published in native language journals, which were difficult to obtain and, thus, were excluded from our analysis.Citation51 Egger’s test revealed significant publication bias in studies on OS and Lauren classification. The results of trim-and-fill analysis on pooled HRs or ORs indicated that our results are relatively stable and reliable.
Conclusion
In conclusion, this meta-analysis revealed that the increased PCNA expression is significantly associated with poor OS and DFS, as well as with clinicopathological characteristics, including deeper tumor invasion, lymph node metastases, and more advanced stage in gastric cancer patients. This suggests that PCNA might be a useful biomarker to predict patient prognosis and could be a valuable therapeutic target for gastric cancer.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No 81372550) and Key Laboratory Programme of Liaoning Province (LZ2015080).
Disclosure
The authors report no conflicts of interest in this work.
References
- FerlayJSoerjomataramIDikshitRCancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012Int J Cancer20151365E359E38625220842
- Van CutsemESagaertXTopalBHaustermansKPrenenHGastric cancerLancet Epub201655
- AmedeiABenagianoMdella BellaCNiccolaiED’EliosMMNovel immunotherapeutic strategies of gastric cancer treatmentJ Biomed Biotechnol2011201143734822253528
- SawadaTYashiroMSentaniKNew molecular staging with G-factor supplements TNM classification in gastric cancer: a multicenter collaborative research by the Japan Society for Gastroenterological Carcinogenesis G-Project committeeGastric Cancer201518111912824488015
- XingXTangYBYuanGThe prognostic value of E-cadherin in gastric cancer: a meta-analysisInt J Cancer2013132112589259623169395
- WuPWuDZhaoLPrognostic role of STAT3 in solid tumors: a systematic review and meta-analysisOncotarget2016715198631988326959884
- YimingLYunshanGBoMCD133 overexpression correlates with clinicopathological features of gastric cancer patients and its impact on survival: a systematic review and meta-analysisOncotarget2015639420194202726503471
- YildirimMKayaVDemirpenceOGunduzSBozcukHPrognostic significance of p53 in gastric cancer: a meta-analysisAsian Pac J Cancer Prev201516132733225640374
- Soleyman-JahiSNedjatSAbdiradAHoorshadNHeidariRZendehdelKPrognostic significance of matrix metalloproteinase-7 in gastric cancer survival: a meta-analysisPLoS One2014104e012231625919283
- PetrelliFCabidduMCoinuAPrognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studiesActa Oncol201554796197025984930
- MiyachiKFritzlerMJTanEMAutoantibody to a nuclear antigen in proliferating cellsJ Immunol1978121622282234102692
- MathewsMBBernsteinRMFranzaBRJrGarrelsJIIdentity of the proliferating cell nuclear antigen and cyclinNature198430959663743766145097
- BravoRCelisJEA search for differential polypeptide synthesis throughout the cell cycle of HeLa cellsJ Cell Biol19808437958026892640
- BravoRFeySJBellatinJLarsenPMArevaloJCelisJEIdentification of a nuclear and of a cytoplasmic polypeptide whose relative proportions are sensitive to changes in the rate of cell proliferationExp Cell Res198113623113197308310
- SrinivasanMJewellSDQuantitative estimation of PCNA, c-myc, EGFR and TGF-alpha in oral submucous fibrosis – an immunohistochemical studyOral Oncol200137546146711377235
- MoldovanGLPfanderBJentschSPCNA, the maestro of the replication forkCell2007129466567917512402
- StoimenovIHelledayTPCNA on the crossroad of cancerBiochem Soc Trans200937Pt 360561319442257
- WangLFChaiCYKuoWRTaiCFLeeKWHoKYCorrelation between proliferating cell nuclear antigen and p53 protein expression and 5-year survival rate in nasopharyngeal carcinomaAm J Otolaryngol200627210110516500472
- LiuCLiuJWangXPrognostic impact of nm23-H1 and PCNA expression in pathologic stage I non-small cell lung cancerJ Surg Oncol2011104218118621495034
- BantisAGiannopoulosAGonidiMExpression of p120, Ki-67 and PCNA as proliferation biomarkers in imprint smears of prostate carcinoma and their prognostic valueCytopathology2004151253114748788
- LiNDengWMaJPrognostic evaluation of Nanog, Oct4, Sox2, PCNA, Ki67 and E-cadherin expression in gastric cancerMed Oncol201532143325491144
- LvQZhangJYiYProliferating cell nuclear antigen has an association with prognosis and risks factors of cancer patients: a systematic reviewMol Neurobiol20165396209621726558632
- LiuMLiJSTianDPHuangBRosqvistSSuMMCM2 expression levels predict diagnosis and prognosis in gastric cardiac cancerHistol Histopathol201328448149223329420
- YangCWenYLiHOverexpression of minichromosome maintenance 2 predicts poor prognosis in patients with gastric cancerOncol Rep201227113514221947329
- KonnoSTakebayashiYAibaMAkiyamaSOgawaKClinicopathological and prognostic significance of thymidine phosphorylase and proliferating cell nuclear antigen in gastric carcinomaCancer Lett2001166110311111295293
- CzyzewskaJGuzinska-UstymowiczKPryczyniczAKemonaABandurskiRImmunohistochemical evaluation of Ki-67, PCNA and MCM2 proteins proliferation index (PI) in advanced gastric cancerFolia Histochem Cytobiol200947228929619995716
- LeeKELeeHJKimYHPrognostic significance of p53, nm23, PCNA and c-erbB-2 in gastric cancerJpn J Clin Oncol200333417317912810831
- StangACritical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analysesEur J Epidemiol201025960360520652370
- ParmarMKTorriVStewartLExtracting summary statistics to perform meta-analyses of the published literature for survival endpointsStat Med19981724281528349921604
- TierneyJFStewartLAGhersiDBurdettSSydesMRPractical methods for incorporating summary time-to-event data into meta-analysisTrials200781617555582
- PetersJLSuttonAJJonesDRAbramsKRRushtonLComparison of two methods to detect publication bias in meta-analysisJAMA2006295667668016467236
- PotecaTPotecaASajinMComanescuMBiological prognostic parameters in gastric carcinomasChirurgia (Bucur)2014109334735424956340
- KuangRGWuHXHaoGXWangJWZhouCJExpression and significance of IGF-2, PCNA, MMP-7, and alpha-actin in gastric carcinoma with Lauren classificationTurk J Gastroenterol20132429910823934455
- YeYWDongRZZhouYPrognostic analysis of familial gastric cancer in Chinese populationJ Surg Oncol20111041768221400534
- KanajiSSaitoHTsujitaniSExpression of polo-like kinase 1 (PLK1) protein predicts the survival of patients with gastric carcinomaOncology200670212613316645325
- WuKZhaoLLiYShanYJWuLJEffects of vitamin E succinate on the expression of Fas and PCNA proteins in human gastric carcinoma cells and its clinical significanceWorld J Gastroenterol200410794594915052671
- NodaHMaeharaYIrieKKakejiYYonemuraTSugimachiKIncreased proliferative activity caused by loss of p21(WAF1/CIP1) expression and its clinical significance in patients with early-stage gastric carcinomaCancer20029472107211211932915
- ElpekGOGelenTAksoyNHKarpuzogluTKelesNMicrovessel count, proliferating cell nuclear antigen and Ki-67 indices in gastric adenocarcinomaPathol Oncol Res200061596410749590
- DanesiDTSpanoMFabianoAFlow cytometric DNA ploidy, p53, PCNA, and c-erbB-2 protein expressions as predictors of survival in surgically resected gastric cancer patientsCytometry2000421273410679740
- KinugasaSAbeSTachibanaMOverexpression of transforming growth factor-beta1 in scirrhous carcinoma of the stomach correlates with decreased survivalOncology19985565825879778627
- MaedaKChungYSTakatsukaSTumour angiogenesis and tumour cell proliferation as prognostic indicators in gastric carcinomaBr J Cancer19957223193237543771
- ManghamDCRowlandsDCNewboldKMReynoldsGMFieldingJWHallisseyMTExpression of proliferating cell nuclear antigen (PCNA) in gastric carcinoma: no evidence for prognostic valueJ Clin Pathol19944754734747913101
- KakejiYMaeharaYAdachiYProliferative activity as a prognostic factor in Borrmann type 4 gastric carcinomaBr J Cancer19946947497537908217
- JainSFilipeMIHallPAWaseemNLaneDPLevisonDAPrognostic value of proliferating cell nuclear antigen in gastric carcinomaJ Clin Pathol19914486556591679766
- WangSCPCNA: a silent housekeeper or a potential therapeutic target?Trends Pharmacol Sci201435417818624655521
- MalkasLHHerbertBSAbdel-AzizWA cancer-associated PCNA expressed in breast cancer has implications as a potential bio-markerProc Natl Acad Sci U S A200610351194721947717159154
- ZhaoHChenMSLoYHThe Ron receptor tyrosine kinase activates c-Abl to promote cell proliferation through tyrosine phosphorylation of PCNA in breast cancerOncogene201433111429143723542172
- ZhaoHLoYHMaLTargeting tyrosine phosphorylation of PCNA inhibits prostate cancer growthMol Cancer Ther2011101293621220489
- YuYLChouRHLiangJHTargeting the EGFR/PCNA signaling suppresses tumor growth of triple-negative breast cancer cells with cell-penetrating PCNA peptidesPLoS One201384e6136223593472
- TanZWortmanMDillehayKLSmall-molecule targeting of proliferating cell nuclear antigen chromatin association inhibits tumor cell growthMol Pharmacol201281681181922399488
- EggerMZellweger-ZahnerTSchneiderMJunkerCLengelerCAntesGLanguage bias in randomised controlled trials published in English and GermanLancet199735090743263299251637