Abstract
Increasing evidence has demonstrated that aberrant expressions of long non-coding RNAs (lncRNAs) are involved in various malignancies, including hepatocellular carcinoma (HCC). This study aimed to investigate the role of lncRNA colon cancer-associated transcript 2 (CCAT2) in the progression of HCC. Quantitative real-time polymerase chain reaction analysis confirmed that CCAT2 was upregulated in HCC cell lines and cancerous tissues compared with normal liver cell line and adjacent normal tissue samples. The level of CCAT2 was positively associated with tumor–node–metastasis stages and vessel invasion. Survival analyses revealed that high CCAT2 expression predicted poor prognostic outcomes, serving as an independent prognostic factor for HCC patients. Patients with high CCAT2 expression had a 1.849-fold increased risk of death compared with those with low CCAT2 expression. Moreover, we also found that knockdown of CCAT2 expression reduced cell migration and invasion in vitro. We further demonstrated that CCAT2 played a key role in enhancing the epithelial–mesenchymal transition (EMT) through the regulation of vimentin, E-cadherin and transcription factor snail2 expression. Taken together, our findings showed that high CCAT2 expression is associated with poor survival in HCC patients. CCAT2 promotes HCC progression by regulating Snail2-induced EMT. CCAT2 may be a prognostic biomarker and therapeutic target for HCC.
Introduction
Hepatocellular carcinoma (HCC) has become the most common leading cause of cancer-associated mortality globally.Citation1 Although in recent years there are mounting progresses in clinical treatment for HCC, the 5-year survival rate of HCC patients is still poor and exceeds 600,000 people dying of HCC each year.Citation2 Uncontrolled tumor metastasis, frequent intrahepatic propagation and extrahepatic progression are the main causes for the poor prognosis of HCC. Identification of the prognostic predictors and key molecular mechanisms that influence the metastasis and progression of HCC will be meaningful.
In the human genome, ~2% of transcripts can be translated into proteins, whereas 98% of transcripts are non-coding RNAs, including microRNAs (miRNAs) and long non-coding RNAs (lncRNAs).Citation3 The lncRNAs are >200 nucleotides in length and unable to be translated into proteins. Increasing evidence has demonstrated that lncRNAs can function as oncogenes or tumor suppressors,Citation4,Citation5 which could regulate downstream genes at different levels, including chromatin modification and transcriptional and posttranscriptional processing.Citation6–Citation8 Colon cancer-associated transcript 2 (CCAT2), a novel lncRNA mapping to 8q24 genomic region, was first discovered by Ling et al.Citation9 They showed that CCAT2 was highly overexpressed in microsatellite-stable colorectal cancer and promoted tumor growth, metastasis and chromosomal instability. Overexpression of CCAT2 can also promote the metastasis and invasion of breast, lung and stomach cancer cells and influence the prognosis of breast and stomach cancers.Citation10–Citation12 However, the biological function and clinical significance of CCAT2 in HCC remain unknown.
Therefore, we performed a study to investigate the role of CCAT2 in HCC and its potential prognostic value. We found that the expression of CCAT2 was upregulated in HCC cell lines and tissues, and high CCAT2 expression was associated with advanced tumor–node–metastasis (TNM) stage, frequent vessel invasion and poor patient prognosis. Decreased CCAT2 expression in HCC cells significantly suppressed cell migration and invasion. Furthermore, we also showed that alteration of CCAT2 expression can influence E-cadherin, vimentin and snail2 levels, indicating that CCAT2 affected HCC progression partly through snail2-induced epithelial–mesenchymal transition (EMT).
Materials and methods
Clinical specimens
A total of 96 pairs of HCC and matched paracancerous tissues were consecutively collected from patients who received surgical resection of HCC from September 2004 to October 2010 at Taizhou Hospital of Zhejiang Province, Affiliated Hospital of Wenzhou Medical University. The clinical and pathological characteristics were obtained from patient charts. None of the patients had received preoperative radiotherapy, chemotherapy or any other medical intervention. Tumors were staged according to the Seventh Edition of the Cancer Staging Manual by the American Joint Committee on Cancer. Among the patients, 53.1% (51/96) were diagnosed with TNM stage I, 37.5% (36/96) with TNM stage II, 9.4% (9/96) with TNM stage III and no case were in stage IV. The median follow-up for all patients was 39.5 months (range, 5.2–60 months), and 56 cancer-associated fatalities (58.3%) were recorded. The study was approved by the Ethics Committee on Human Research of Taizhou Hospital of Zhejiang Province, and written informed consent was obtained from all the patients.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNAs were extracted from paired HCC and non-cancerous tissues or cultured cells using Trizol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. RT and qPCR kits (Takara, Dalian, China) were used to determine the expression of CCAT2 in tissue samples and cultured cells. qRT-PCR was done using SYBR1 Premix Ex Taq II (Tli RNaseH Plus) (Takara) on ABI 7900HT (Applied Biosystems, Foster City, CA, USA). The primers used in this study are shown in . The relative expression of CCAT2 was normalized to reference glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and was calculated.
Table 1 Primers used for qRT-PCR and siRNA oligonucleotides
Cell lines, small interfering RNA(siRNA) and transfection of HCCcells
The HCC cell lines HepG2, SMMC772 and MHCC97H and the normal liver cell line MIHA were purchased from Fudan University Cancer Institute (Shanghai, China). The HCC cell lines were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (FBS) at 37°C in an atmosphere containing 5% CO2. HepG2 and MHCC97H cells were cultured in six-well plates and transfected with siRNA or negative control using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. The siRNA specifically targeting CCAT2 (siRNA-CCAT2) was synthesized (Invitrogen). The siRNA sequences are shown in .
Monolayer wound-healing assay
Cell migration was analyzed using a wound-healing assay. Treated HepG2 and MHCC97H cells were plated in six-well plates containing 10% FBS medium. Once the cells reached 90% density, the monolayer was scratched and then cultured in fresh medium for 48 h. Mitomycin C was applied to inhibit the cell division. The migration distances of the cells were measured from the images (five fields) that were obtained at the indicated time point.
Transwell invasion assay
The invasion ability of HCC cell was performed using 24-well Transwells (8 mm pore size; Corning Life Sciences) coated with 1 mg/mL Matrigel (BD Sciences). The cells were seeded in the upper chamber of the wells in 100 μL FBS-free medium, and 500 μL of 20% FBS medium was added to the lower chambers. Following incubation for 24 h, the cells remaining on the upper membrane were removed with cotton wool. Cells that had invaded through the membrane were stained with methanol and 0.1% crystal violet and photographed with a phase-contrast inverted microscope (Olympus, Tokyo, Japan). The cells from at least five random microscopic fields (×40) were counted.
Statistical analysis
SPSS 20.0 software (IBM, Armonk, NY, USA) was used for statistical analysis. The significance of differences between groups was estimated by Student’s t-test and χ2 test as appropriate. The survival curves were calculated by the Kaplan–Meier method with the log-rank test applied for comparison. Survival data were evaluated using univariate and multivariate Cox proportional hazards model. Variables with a value of P<0.05 in univariate analysis were used in subsequent multivariate analysis on the basis of Cox regression analyses. Pearson’s correlation analyses were used to investigate the correlation among CCAT2 with snail2, vimentin and E-cadherin. Two-sided P-values were calculated, and a P-value <0.05 was considered statistically significant.
Results
CCAT2 expression is upregulated in human HCC cell lines and tissues and correlated with prognosis of HCC patients
As shown in , CCAT2 level in three cancer cell lines (HepG2, SMMC772 and MHCC97H) was significantly higher than that of normal liver cell line (MIHA). Among all 96 pairs of HCC patients, CCAT2 expression level was significantly upregulated in 64% (61/96) of tumors compared with adjacent normal tissues (P<0.01) ().
Figure 1 Increased expression of CCAT2 in HCC tissues and cell lines and its prognostic significance.
Abbreviations: CCAT2, colon cancer-associated transcript 2; HCC, hepatocellular carcinoma; qRT-PCR, quantitative real-time polymerase chain reaction; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Cum survival, cumulative survival.
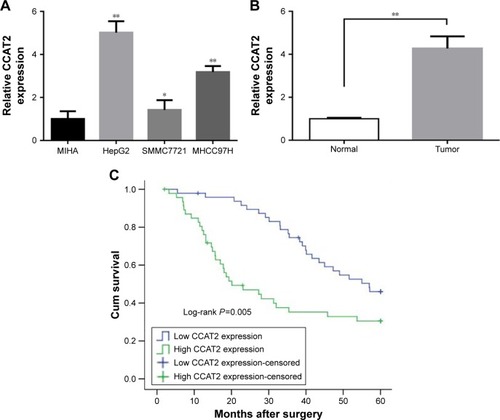
To assess the correlation of CCAT2 expression with clinicopathological characteristics, all cases were divided into low (n=48) or high (n=48) CCAT2 expression groups based on the median of CCAT2 expression level. As shown in , the high CCAT2 expression group showed higher TNM stage (P=0.041) and more frequent vessel invasion (P=0.026) than the low CCAT2 expression group. Besides, CCAT2 was significantly overexpressed in patients with alcoholism history (P=0.041). However, there was no significant correlation between CCAT2 expression and other clinicopathological parameters such as sex, age, tumor size and smoking (P>0.05).
Table 2 Association of CCAT2 expression with clinicopathological characteristics of HCC
is a Kaplan–Meier survival analysis depicting the relationship between CCAT2 expression and the prognosis of HCC. The mean survival time for low CCAT2 expression groups was 47.433±2.188 months, whereas that for high CCAT2 expression groups was only 31.267±3.196 months (P<0.01 by log-rank test).
Furthermore, a Cox proportional hazards model analysis was used to assess the prognostic parameters in patients with HCC. Multivariate analysis showed that, after TNM stage, vessel invasion, sex, age, tumor size and alcoholism were adjusted, CCAT2 expression was independently associated with OS, with a hazard ratio (HR) of 1.849 (95% confidence interval [95% CI], 1.064–3.213; P=0.029; ).
Table 3 Univariate and multivariate Cox regression analyses of CCAT2 for overall survival of patients with HCC (n=96)
CCAT2 promotes HCC cell migration and invasion in vitro
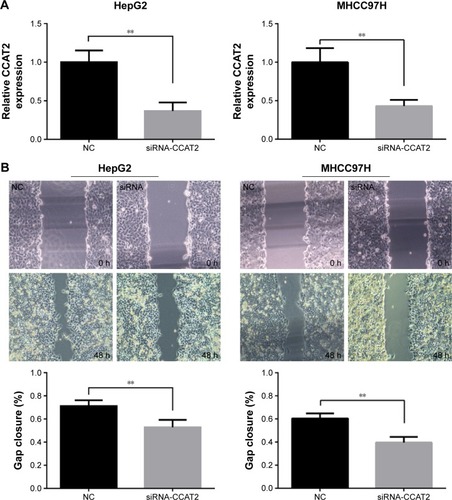
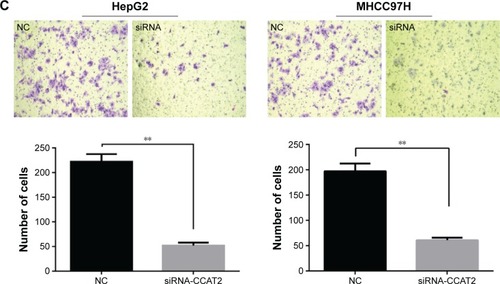
Migration and invasion ability strongly correlate with cancer progression and metastasis. To investigate whether CCAT2 had a functional role in facilitating HCC cell metastasis, we performed wound-healing assay and Transwell assay. CCAT2 expression was downregulated by siRNA-mediated gene silencing in both HepG2 and MHCC97H cells. The knockdown of CCAT2 expression in the cells was demonstrated by qRT-PCR (). As shown in , low CCAT2 expression in HepG2 and MHCC97H cells, which were transfected with siRNA-CCAT2, exhibited a significantly lower scratch closure rate than that in control cells (). Moreover, compared with the control cells, low CCAT2 expression in HCC cells also showed markedly repressed invasion ability (). These findings indicate that CCAT2 expression may play a pivotal role in the migration and invasion of HCC cells.
Figure 2 Effect of CCAT2 on HCC cells migration and invasion.
Abbreviations: CCAT2, colon cancer-associated transcript 2; HCC, hepatocellular carcinoma; siRNA, small interfering RNA; qRT-PCR, quantitative real-time polymerase chain reaction; NC, negative control.
CCAT2 promotes HCC cell EMT
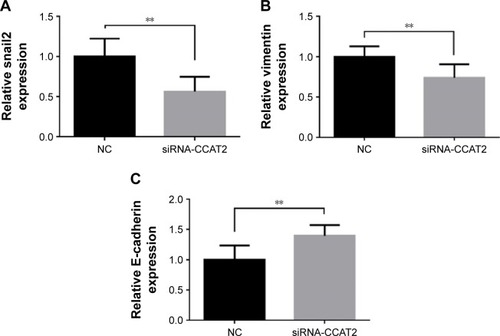
EMT plays crucial roles during cancer initiation and progression, especially in increasing invasion and migration ability of cancer cells.Citation13,Citation14 To further investigate the possible mechanism by which CCAT2 promotes HCC progression, the expression levels of EMT-related markers such as vimentin, E-cadherin and snail2 were detected by qRT-PCR in HCC tissues. As expected, the Pearson’s correlation analysis showed that the expression of CCAT2 in HCC lesions was positively correlated with vimentin (r=0.587, P<0.001) and snail2 (r=0.494, P<0.001) and negatively related to E-cadherin (r=−0.233, P=0.022). Next, we transfected CCAT2 siRNA into HepG2 cells and analyzed its effects on the expression of vimentin, snail2 and E-cadherin. We found that the expression levels of vimentin and snail2 were significantly reduced in cells treated with siRNA-CCAT2. However, the expression of E-cadherin was increased after inhibition of CCAT2 (). These data support our hypothesis that CCAT2 influences HCC progression by regulating snail2-induced EMT.
Figure 3 qRT-PCR analysis expression of snail2, vimentin and E-cadherin in HCC cells treated with siRNA-CCAT2.
Abbreviations: qRT-PCR, quantitative real-time polymerase chain reaction; HCC, hepatocellular carcinoma; siRNA, small interfering RNA; CCAT2, colon cancer-associated transcript 2; NC, negative control.
Discussion
Increasing evidence links dysregulation of lncRNAs to diverse human diseases, including HCC.Citation15–Citation18 In this study, we found that CCAT2 was upregulated in HCC tissues and cell lines, compared with adjacent normal tissues and normal liver cell line. High CCAT2 expression was significantly correlated with aggressive tumor characteristics (higher tumor stage and vessel invasion) and poor prognosis. Moreover, multivariate analyses showed that CCAT2 expression was an independent prognostic factor for HCC patients. These findings support the concept that the CCAT2 expression level could serve as a prognostic biomarker for HCC.
CCAT2 expression is exhibited in different manners in various types of tumors. Qiu et alCitation11 reported that CCAT2 was a lung adenocarcinoma-specific lncRNA and not correlated with sex, smoking, TNM stage, tumor size or lymph node metastasis. However, Wang et alCitation12 found that high CCAT2 expression was associated with higher incidence of lymph node metastasis and distance metastasis and predicted poor prognosis in gastric cancer. In addition, Redis et alCitation10 revealed that CCAT2 overexpression decreases the sensitivity of breast cancer cells to 5-fluorouracil (5-FU). Interestingly, CCAT2 expression in breast cancer was inversely correlated with nodal status, having the highest expression in lymph node-negative disease. Therefore, CCAT2 may exert cancer-type-dependent effects. Further studies are definitely required to examine the role of CCAT2 in different human tumors.
Recent studies indicate that lncRNAs play an important role in tumor metastasis. Knockdown of lncRNA SPRY4-IT1 inhibited the migration and invasion of colorectal cancer cells and induced cell cycle arrest.Citation19 The lncRNA FTX could inhibit HCC cell growth and metastasis both in vitro and in vivo.Citation20 CCAT2 has also been shown to promote proliferation and invasion in breast,Citation10 lungCitation11 and cervical cancer cells.Citation21 We then determined the effect of CCAT2 in HCC cells. We found that downregulated expression of CCAT2 inhibited cell migration and invasion, in line with the findings of Zhou et al.Citation22 However, compared with the study performed by Zhou et al, our study has two strengths: first, our study revealed that the expression of CCAT2 in HCC tissue is an independent prognostic factor for HCC; second, we found that, besides regulating migration, CCAT2 was involved in the pathogenesis of HCC by regulating EMT.
There are various mechanisms of CCAT2 being involved in the pathogenesis of cancer. Previous data showed that upregulation of CCAT2 might promote the proliferation and metastasis of cancer cells through enhancing the WNT pathway.Citation9,Citation23 CCAT2 could physically interact with TCF7L2, by which the expressions of miR-17-5p, miR-20a and MYC are regulated in colon cancer.Citation9 To date, the detailed molecular mechanism through which CCAT2 contributes to HCC progression is still unclear. Recent studies have revealed that some lncRNAs can regulate tumor cell metastasis by affecting the EMT process in HCC.Citation20,Citation24,Citation25 Therefore, we further analyzed the relationship between CCAT2 and EMT in HCC. Hallmarks of EMT are the aberrant expression of E-cadherin, vimentin and the transcription factor, snail. We found that CCAT2 expression was positively correlated with vimentin and snail2 and negatively related to E-cadherin in HCC tissues, basically supporting our hypothesis that CCAT2 was involved in the pathogenesis of HCC via regulating EMT. Then, we examined the expression levels of snail2, E-cadherin and vimentin following inhibition of CCAT2 in HCC cells. We found that depletion of CCAT2 inhibited snail2 and vimentin expression and promoted E-cadherin expression, indicating that CCAT2 mediated promoting effects on HCC cell metastasis possibly by affecting the Snail2-induced EMT. EMT is implicated in the promotion of tumor cell invasion and metastasis. Therefore, as an important regulator of EMT, CCAT2 could be a suitable candidate for intervention in the treatment of cancer, serving as a drug target. Drugs that could regulate the expression of CCAT2 have clinical application prospects.
Conclusion
We found that CCAT2 was upregulated in HCC tissues and cells and served as a negative prognostic factor in HCC patients. More importantly, we primarily confirmed the regulatory mechanism of lncRNA CCAT2 in HCC that CCAT2 promoted tumor progression through increasing cancer cell migration and invasion ability in part by regulating Snail2-mediated EMT. Further insights into the functional and clinical implications of CCAT2 and its targets may propel the development of novel therapeutic strategies for HCC.
Author contributions
Zheping Fang and Fabiao Zhang conceived and designed the study; Yongfu Xu and Binfeng Wang performed the majority of experiments and wrote the manuscript; Aidong Wang and Xuefeng Du contributed to the sample collection; Peng Hu and Yu Zhu performed the statistical analysis. All authors contributed toward date analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This project was supported by the Science Technology Program of Zhejiang Province on the Scientific Research Project (LY17H160069) and the Zhejiang Provincial Health Department Project (grant nos 2014KYA227 and 2017KY161).
Disclosure
The authors report no conflicts of interest in this work.
References
- FornerALlovetJMBruixJHepatocellular carcinomaLancet201237998221245125522353262
- MinguezBLachenmayerADiagnostic and prognostic molecular markers in hepatocellular carcinomaDis Markers201131318119022045404
- CechTRSteitzJAThe noncoding RNA revolution-trashing old rules to forge new onesCell20141571779424679528
- MitraSAMitraAPTricheTJA central role for long non-coding RNA in cancerFront Genet201231722363342
- WangJSunJWangJLong noncoding RNAs in gastric cancer: functions and clinical applicationsOnco Targets Ther2016968169726929639
- MercerTRDingerMEMattickJSLong non-coding RNAs: insights into functionsNat Rev Genet200910315515919188922
- WiluszJESunwooHSpectorDLLong noncoding RNAs: functional surprises from the RNA worldGenes Dev200923131494150419571179
- HanPChangCPLong non-coding RNA and chromatin remodelingRNA Biol201512101094109826177256
- LingHSpizzoRAtlasiYCCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic progression and chromosomal instability in colon cancerGenome Res20132391446146123796952
- RedisRSSieuwertsAMLookMPCCAT2, a novel long non-coding RNA in breast cancer: expression study and clinical correlationsOncotarget20134101748176224077681
- QiuMXuYYangXCCAT2 is a lung adenocarcinoma-specific long non-coding RNA and promotes invasion of non-small cell lung cancerTumour Biol20143565375538024504682
- WangCYHuaLYaoKHChenJTZhangJJHuJHLong non-coding RNA CCAT2 is up-regulated in gastric cancer and associated with poor prognosisInt J Clin Exp Pathol20158177978525755774
- ParkMYKimKRParkHSExpression of the serum response factor in hepatocellular carcinoma: implications for epithelial-mesenchymal transitionInt J Oncol20073161309131517982656
- ChenXLingalaSKhoobyariSNoltaJZernMAWuJEpithelial mesenchymal transition and hedgehog signaling activation are associated with chemoresistance and invasion of hepatoma subpopulationsJ Hepatol201155483884521334406
- WangTHLinYSChenYLong non-coding RNA AOC4P suppresses hepatocellular carcinoma metastasis by enhancing vimentin degradation and inhibiting epithelial-mesenchymal transitionOncotarget2015627233422335726160837
- ZhangJYWengMZSongFBLong noncoding RNA AFAP1-AS1 indicates a poor prognosis of hepatocellular carcinoma and promotes cell proliferation and invasion via upregulation of the RhoA/Rac2 signalingInt J Oncol20164841590159826892468
- ZhuXTYuanJHZhuTTLiYYChengXYLong noncoding RNA glypican 3 (GPC3) antisense transcript 1 promotes hepatocellular carcinoma progression via epigenetically activating GPC3FEBS J2016283203739375427573079
- ZhouTGaoYIncreased expression of LncRNA BANCR and its prognostic significance in human hepatocellular carcinomaWorld J Surg Oncol2016141826758762
- CaoDDingQYuWGaoMWangYLong noncoding RNA SPRY4-IT1 promotes malignant development of colorectal cancer by targeting epithelial-mesenchymal transitionOnco Targets Ther201695417542527621655
- LiuFYuanJHHuangJFLong noncoding RNA FTX inhibits hepatocellular carcinoma proliferation and metastasis by binding MCM2 and miR-374aOncogene201635415422543427065331
- WuLJinLZhangWZhangLRoles of long non-coding RNA CCAT2 in cervical cancer cell growth and apoptosisMed Sci Monit20162287587926983975
- ZhouNSiZLiTChenGZhangZQiHLong non-coding RNA CCAT2 functions as an oncogene in hepatocellular carcinoma, regulating cellular proliferation, migration and apoptosisOncol Lett201612113213827347113
- CaiYHeJZhangDLong noncoding RNA CCAT2 promotes breast tumor growth by regulating the Wnt signaling pathwayOnco Targets Ther201582657266426442763
- YuanJHYangFWangFA long noncoding RNA activated by TGF-beta promotes the invasion-metastasis cascade in hepatocellular carcinomaCancer Cell201425566668124768205
- WangTHYuCCLinYSLong noncoding RNA CPS1-IT1 suppresses the metastasis of hepatocellular carcinoma by regulating HIF-1alpha activity and inhibiting epithelial-mesenchymal transitionOncotarget2016728435884360327248828
