Abstract
Decoy receptor 3 (DcR3) has been recently described as an antiapoptosis and prometastasis factor since it can competitively bind to FasL, TL1A, and LIGHT, and it is highly expressed in many malignant tumors. Downregulation of DcR3 can promote tumor cell apoptosis and inhibit metastasis. A previous study demonstrated that reduction of DcR3 could induce tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis in pancreatic cancer cells. However, whether such an effect is seen in hepatocellular carcinoma (HCC) remains to be explored. This study was designed to investigate the sensitivity of HCC cells to TRAIL after silencing DcR3, and this was done by evaluating the expression of DcR3 in HCC cells and the effect on TRAIL-mediated apoptosis after downregulation of DcR3. Our data showed that DcR3 was highly expressed in HepG2, BEL-7402, Hep3B, Huh-7, MHCC97H, and SMCC7721 cell lines compared with normal liver cell line LO-2. Both HepG2 and BEL-7402 were tolerant to TRAIL-mediated apoptosis, and the tolerance was negatively correlated to the expression of DcR3. Silencing of DcR3 with shRNA and treatment with TRAIL induced obvious apoptosis in HepG2 and BEL-7402, with more cancer cells found in the G1 phase. SiDcR3 combined with TRAIL could induce activation of caspases-3, -8, and -9, raise the expression of the apoptotic protein Bax, and reduce the expression of antiapoptotic proteins (Bcl-2, Mcl-1, Bcl-XL, IAP-2, and survivin). Caspase-8 inhibitor Ac-IETD-CHO significantly decreased the activation of caspase cascade, indicating that the extrinsic pathway may have a vital role in the apoptotic events induced by SiDcR3/TRAIL. Furthermore, our results showed that the TRAIL death receptor 5 (DR5) was upregulated and that DR5 neutralizing antibody abrogated the effect of SiDcR3. Our results demonstrated that downregulation of DcR3 could enhance TRAIL-mediated apoptosis in HCC through the death receptor pathway. In the future, this might be useful as a clinical treatment method of liver cancer.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors in the world. The incidence of HCC ranks sixth among all cancers with approximately 600,000 people affected.Citation1 HCC is among a group of tumors that have the highest mortality rate.Citation1 Since the early symptoms of liver cancer are not obvious, early diagnosis is difficult. When diagnosed, HCC is almost at the intermediate or the advanced stage. Clinical treatments for liver cancer include liver resection, liver transplantation, chemotherapy, and radiotherapy. For liver cancer, the rate of 5-year survival is very low because of poor response to various kinds of treatments. Development of new cancer treatments is gradually becoming a research hotspot in recent years.Citation2–Citation5
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), a member of the TNF superfamily, was first cloned from human peripheral blood and heart cDNA library.Citation6 Similar to other TNFs, when bound with its receptor, TRAIL can induce tumor cell apoptosis.Citation7,Citation8 So far, five receptors, DR4, DR5, Decoy receptor 1 (DcR1), DcR2, and OPG, have been found.Citation9–Citation12 Binding of DR4 and DR5 to TRAIL activates the death receptor pathway, produces DISC, and activates caspase-8, resulting in the activation of caspase-3, caspase-6, and caspase-7, inducing death pathways into mitochondria, and further activating the Bcl-2 family and other molecules. This process induces apoptosis.Citation13 The TRAIL pathway kills tumor cells but is harmless to normal cells, which makes it a more promising treatment target.Citation14 However, many tumor cells show tolerance to TRAIL,Citation15 and liver cancer is no exceptionCitation16 as there is sufficient evidence showing that various liver cancer cell lines have different degrees of tolerance to TRAIL. Therefore, reducing the tolerance of TRAIL will be of great help to the development of new treatments for HCC.
DcR3, a member of the soluble TNF receptor (TNFR) superfamily, sharing a similar sequence with OPG, TNF2, and Fas, can competitively bind to FasL, TL1A, and LIGHT, inhibit apoptosis, modulate immune function, and induce angiogenesis.Citation17–Citation19 DcR3 has been found to be highly expressed in many malignant tumors.Citation20–Citation23 Silencing of DcR3 can affect the proliferation, invasion, and metastasis ability of tumor cells and sensitize tumor cells to Fas-mediated apoptosis.Citation24,Citation25 A previous study has reported that reduction of DcR3 can increase the sensitivity of pancreatic cancer cells to TRAIL-mediated apoptosis.Citation25 However, DcR3 has not been studied in HCC cell lines. The purpose of the present study was to investigate whether reduction of DcR3 can potentiate TRAIL-mediated apoptosis in HCC cells and to explore the molecular mechanisms of this process.
Materials and methods
Cell culture
Human HCC cell lines HepG2, Huh-7, and BEL-7402 were purchased from the Cell Bank of Shanghai Institute of Biochemistry and Cell Biology (Shanghai, People’s Republic of China). MHCC97H and SMCC7721 were purchased from the cell bank of Zhongshan Hospital, Fudan University (Shanghai, People’s Republic of China). Hep3B and LO-2 were purchased from the Cancer Hospital of the Chinese Academy of Medical Sciences (Beijing, People’s Republic of China). Cells were maintained in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum (Gibco, Waltham, MA, USA), 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA, USA) in 5% CO2 at 37°C.
Antibodies and reagents
DcR3 polyclonal antibody, recombinant human TRAIL (rhTRAIL), and neutralizing antibodies against TRAIL-R1 (DR4) and TRAIL-R2 (DR5) were purchased from R&D Systems (Minneapolis, MN, USA). Rat monoclonal antibodies against Mcl-1, Bcl-XL, Bax, survivin, IAP-2, DR4, DR5, and GAPDH were purchased from Abcam (Shanghai, People’s Republic of China). Ac-DEVD-AMC, Ac-IETD-AFC, Ac-LEHD-AMC, caspase-8 inhibitor IETD-CHO, and caspase-9 inhibitor LEHD-CMK were purchased from BD Biosciences (San Jose, CA, USA).
Lentiviral shRNA-mediated DcR3 silencing
Target shDcR3 was cloned into pLKO.1.-CMV vector. Target and nontarget vectors were packaged into lentiviral particles by BIOREE (Beijing, People’s Republic of China). Each particle was transduced into appropriate human liver cancer lines and selected by antibiotic resistance. These clones were screened using quantitative real-time polymerase chain reaction (PCR) for DcR3 expression, and the results were confirmed by enzyme-linked immunosorbent assay (ELISA).
ELISA and reverse transcriptase PCR (RT-PCR)
Supernatants of cell culture medium were collected and analyzed by sandwich ELISA using DcR3 ELISA Kit (R&D Systems) according to the manufacturer’s instructions. For DcR3 messenger RNA level, total RNA was extracted with Trizol reagent (Invitrogen, Beijing, People’s Republic of China) according to the manufacturer’s instructions. cDNA was synthesized from total RNA with Goscript™ Reverse Transcription System (Promega, Beijing, People’s Republic of China) and random hexamers. PCR was performed using Bio-Rad PCR System (BIORAD, Beijing, People’s Republic of China). Relative quantification was determined by normalization to the amount of actin. Primers used for real-time PCR, 5′-CTCTTCCTCCCATGACAC-3′ and 5′-CTGGAAAGCCACAAAGTC-3′ for DcR3 (112 bp), and 5′-ccaaccgcgagaagatga-3′ and 5′-ccagaggcgtacagggatag-3′ for actin (97 bp), were designed by Primer Express 3.0 and synthesized by Invitrogen, Waltham, MA, USA. PCR-amplified products were examined by electrophoresis on 2% agarose gel with ethidium bromide staining.
Cell viability assay
The effect of TRAIL on HCC cell viability was analyzed using Cell Counting Kit-8 (CCK-8) (Dojindo, Kumamoto, Japan). Cells were incubated in different concentrations of TRAIL (0, 25, 50, and 100 ng/mL) for 24 h, then CCK-8 was added and incubated at 37°C for 2 h. Ultraviolet spectrophotometer was used to measure the absorbance of each treatment group at 450 nm. Cell viability index was calculated: (experiment optical density (OD)450−blank OD450)/(control OD450− blank OD450) ×100%.
Cell cycle and apoptosis analysis
Apoptotic changes were detected by fluorescein isothiocyanate (FITC)-Annexin V staining. Propidium iodide (PI) was used to discriminate between apoptotic and necrotic cells among the Annexin V-positive cells.
Cells were treated with TRAIL (100 ng/mL) or vehicle dimethyl sulfoxide (DMSO) for 24 h and washed and resuspended in 100 μL binding buffer solution (Annexin-V-FITC Kit, Cwbiotech, Beijing, People’s Republic of China). Annexin V-FITC (5 μL) and PI (5 μL) were then added to the cell suspension for a 10-min incubation followed by fluorescence-activated cell sorting (FACS) analysis. For cell cycle analysis, cells were harvested and fixed using 75% ethanol overnight at 4°C. Cells were then incubated with RNase A for 30 min at 37°C and stained with PI. Cell cycle was determined by flow cytometry.
Caspase assays
Substrates Ac-DEVD-AMC, Ac-IETD-AFC, and Ac-LEHD-AMC were used to assess the activities of caspase-3, caspase-8, and caspase-9 in protein extractions using a fluorimeter according to the manufacturer’s instructions (BD Biosciences).
Western blotting
Cells were lysed using radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology, Haimen, People’s Republic of China), and the protein concentrations were quantified using the BCA Kit (Cwbiotech, Beijing, People’s Republic of China). Equal amounts of proteins (40 μg) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). The membranes were blocked with 5% nonfat milk at room temperature for 1 h and then incubated with individual primary antibody overnight at 4°C. After extensive washes, the secondary antibody (Abcam, Beijing, People’s Republic of China) was added to the system. Finally, immunoreactive protein bands were determined using the enhanced chemiluminescence system (Thermo Fisher Scientific, Waltham, MA, USA).
Treatment of cells with neutralizing antibodies
HepG2-LV-SiDcR3 cells were treated with neutralizing antibodies of DR4 and DR5 for 1 h and incubated with TRAIL (100 ng/mL) for 24 h.
Statistical analysis
All data are expressed as mean ± standard deviation. One-way analysis of variance test or unpaired Student’s t-test was used to perform comparison in all groups. Differences were considered significant at P<0.05. Three independent experiments were performed for each treatment. All statistical analyses were conducted using Graphpad Prism 6.0 (Graphpad Software, La Jolla, CA, USA).
Results
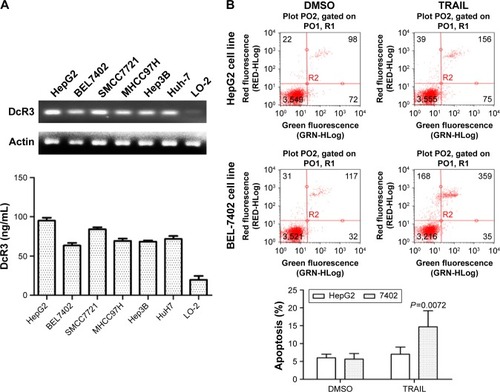
Expression of DcR3- and TRAIL-mediated apoptosis
The expression of DcR3 was measured using six liver cancer cell lines, HepG2, Huh-7, BEL-7402, Hep3B, MHCC97H, and SMCC7721 and human embryonic liver cell line LO-2. As shown in , ELISA and RT-PCR confirmed that expression of DcR3 increased to a different extent in six liver cancer cell lines compared with the normal liver cell line LO-2; HepG2 had the highest increase and BEL-7402 had the lowest (P<0.05). We chose HepG2 and BEL-7402 for further investigation. Cells were incubated in TRAIL 100 ng/mL for 24 h, as shown in . Apoptosis analysis with Annexin V/PI staining revealed that the degree of apoptosis of HepG2 was lower than that of BEL-7402. According to the findings of a previous study,Citation26 both of the HCC cell lines are tolerant to TRAIL. In this analysis, we found that the sensitivity of TRAIL-mediated apoptosis was negatively correlated to the expression of DcR3. Therefore, we hypothesized that DcR3 is a key factor of the resistance of liver cancer cell lines to TRAIL.
Figure 1 Expression of DcR3 in hepatocellular carcinoma cell lines and sensitivity to TRAIL for HepG2 and BEL-7402.
Abbreviations: DcR, decoy receptor; ELISA, enzyme-linked immunosorbent assay; qPCR, quantitative polymerase chain reaction; SD, standard deviation; TRAIL, tumor-necrosis factor-related apoptosis-inducing ligand; DMSO, dimethyl sulfoxide.
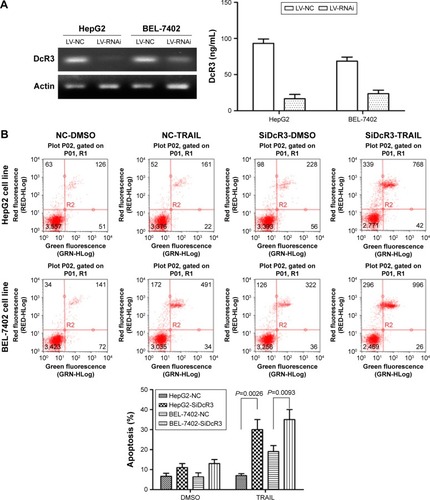
Reduced DcR3 expression could enhance the effects of TRAIL on apoptosis
Stable DcR3-knockdown HepG2 and BEL-7402 cell lines, ie, HepG2-LV-SiDcR3 and BEL-7402-LV-SiDcR3, were established to investigate whether downregulation of DcR3 could enhance the sensitivity of HCC cells toward TRAIL- mediated apoptosis. As show in , RT-PCR and ELISA confirmed that DcR3 expression markedly declined in HepG2-LV-SiDcR3 and BEL-7402-LV-SiDcR3.
Figure 2 Expression of DcR3 and apoptosis in hepatocellular carcinoma cell lines with different treatments.
Abbreviations: DcR, decoy receptor; ELISA, enzyme-linked immunosorbent assay; qPCR, quantitative polymerase chain reaction; SD, standard deviation; TRAIL, tumor-necrosis factor-related apoptosis-inducing ligand; DMSO, dimethyl sulfoxide.
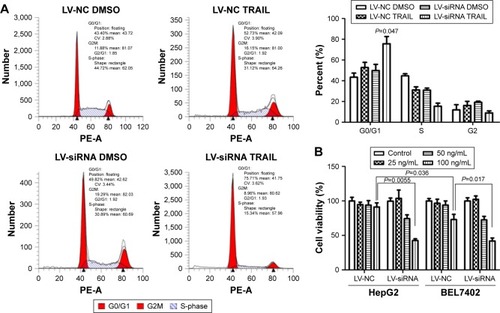
HCC cell lines treated with different levels of TRAIL (0, 25, 50, 100 ng/mL) for 24 h were used to measure cell viability by CCK-8 assay. As showed in , cell viability of HepG2-LV-SiDcR3 and BEL-7402-LV-SiDcR3 was restrained significantly (P<0.05) in a dose-dependent manner compared with control cells. Annexin-V/PI assay was used to measure apoptosis in HepG2 cell lines treated with TRAIL (100 ng/mL, 24 h); DMSO was used as control. Data demonstrated that apoptosis increased significantly () in HepG2-LV-SiDcR3/TRAIL (P<0.05). Cell cycle was measured by flow cytometry; compared with HepG2-LV-NC/TRAIL, a clear increase in G0/G1 phase cells was observed in HepG2-LV-SiDcR3/TRAIL. These results suggested that knockdown of DcR3 expression could enhance the apoptotic effects of TRAIL on HCC cells.
Figure 3 Effect of TRAIL on cell viability and cell cycle.
Abbreviations: CCK8, cell counting kit 8; CV, variable coefficient; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; DMSO, dimethyl sulfoxide.
SiDcR3 combined with TRAIL activated extrinsic and intrinsic signaling pathways
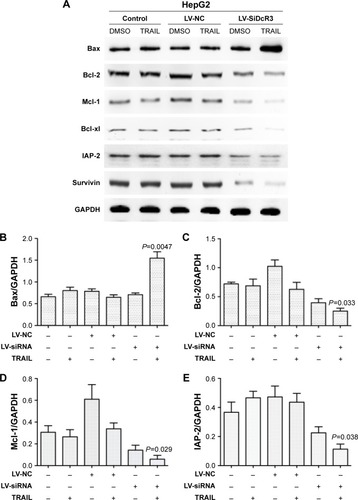
To explore the mechanism of cell death involved in SiDcR3/TRAIL, we first investigated whether the synergistic effect of DcR3/TRAIL was due to the increase of caspase activity. The activities of caspases-3, -8, and -9 increased obviously in HepG2-LV-SiDcR3/TRAIL () compared with the treatment of SiDcR3 or TRAIL (100 ng/mL) alone. This suggested that downregulation of DcR3 combined with TRAIL could activate both extrinsic and intrinsic signaling pathways. Then, we explored the role of caspase-8 and caspase-9 in SiDcR3/TRAIL-caspase activation. HepG2-LV-NC and HepG2-LV-SiDcR3 were incubated in the presence or absence of caspase-8 inhibitor (IETD-CHO) and caspase-9 inhibitor (LEHD-CMK). The activation of caspases-3, -8, and -9 was inhibited obviously in the IETD-CHO group; however, only the activation of caspase-9 was inhibited in the LEHD-CMK group. It indicated that caspase-8 activity may play a crucial role in SiDcR3/TRAIL-induced caspase cascade activation. In addition, Western blot () also showed that the antiapoptotic proteins, Bcl-2, Bcl-xl, Mcl-1, IAP-2, and survivin were downregulated and that the apoptosis-promoting protein Bax was upregulated (P<0.05).
Figure 4 SiDcR3-combined TRAIL activated extrinsic and intrinsic apoptotic signaling pathways.
Abbreviations: DcR, decoy receptor; h, hours; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
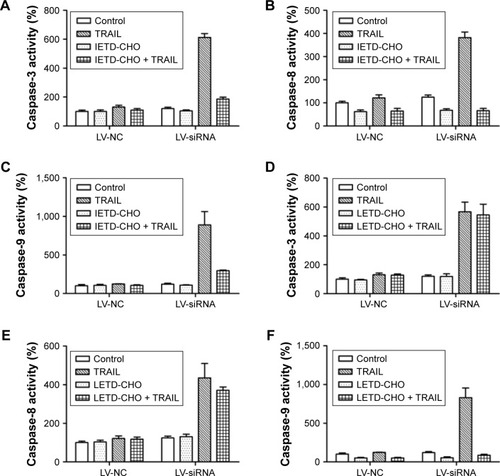
Figure 5 SiDcR3 sensitizes hepatocellular carcinoma cells through Bcl-2 family members.
Abbreviations: DcR, decoy receptor; h, hours; SD, standard deviation; CV, variable coefficient; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
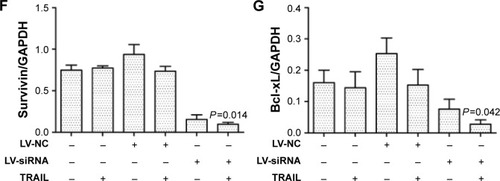
Downregulation of DcR3 combined with TRAIL increased the level of DR5 (TRAIL-R2)
Previous studies showed that TRAIL receptors DR4 (TRAIL-R1) and DR5 (TRAIL-R2) play a key role in TRAIL resistance in cancer cells.Citation27 Therefore, levels of DR4 and DR5 were examined after treatment with TRAIL (100 ng/mL) for 24 h in HepG2, HepG2-LV-NC, and HepG2-LV-SiDcR3; DMSO was used as control. As shown in , the level of DR5 was upregulated in HepG2-LV-SiDcR3, while the level of DR4 showed no significant change. In addition, SiDcR3/TRAIL caused a greater increase of DR5 than SiDcR3 or TRAIL alone. Next, we explored whether the upregulation of DR5 was essential. HepG2-LV-SiDcR3 cells were pretreated with neutralizing antibodies to neutralize DR4 and DR5 before treatment with TRAIL (100 ng/mL, incubated for 24 h). We found that () neutralizing DR5 could decrease the apoptosis of HepG2 measured by Annexin-V/PI assay, but neutralizing DR4 had no obvious effect. In addition, neutralizing DR5 pretreatment could also suppress caspase-8 activation induced by SiDcR3/TRAIL. These results indicated that downregulation of DcR3 could enhance TRAIL-mediated apoptosis through upregulation of DR5 (TRAIL-R2), and SiDcR3/TRAIL might be a potential method to treat HCC.
Figure 6 Silencing DcR3 combined TRAIL (100 ng/mL) can increase the expression of DR5.
Abbreviations: DcR, decoy receptor; h, hours; PI, propidium iodide; SD, standard deviation; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
Discussion
DcR3 is expressed highly in a wide variety of tumors such as gastric cancer,Citation28 colon cancer,Citation20 breast cancer,Citation29 bladder cancer,Citation30 and liver cancer.Citation31 It has been demonstrated that overexpression of DcR3 was correlated with tumor differentiation, lymph node metastasis, as well as TNM stage and prognosis,Citation32 while downregulation of DcR3 could inhibit the proliferation and migration of tumor cells and increase the apoptosis of tumor cells. Our previous studyCitation33 found that the expression of DcR3 in colon cancer cell line SW480 was significantly higher than in normal cells and that the proliferation and migration of colon cancer cells were found to be inhibited when DcR3 was downregulated by siDcR3. In the present study, we demonstrated that the expression of DcR3 in HCC cells was overexpressed compared with normal liver cells. DcR3 has been proven to be one of the ligands binding to FasL.Citation34 Further, it was demonstrated that DcR3 regulated the sensitivity of Fas-mediated apoptosis. Zhou et al,Citation24 in the study of pancreatic cancer cells, found that the proliferation of pancreatic cell lines Panc-1 and SW1990 was significantly inhibited by SiDcR3 transfection, the sensitivity of cancer cells to Fas-mediated apoptosis was upregulated compared with the control group, and the tumor cells in G0/G1 phase were significantly increased. TRAIL and FasL showed similar findings; some studiesCitation25 have proven that downregulation of DcR3 in pancreatic cancer cell lines AsPC-1 and MiaPaCa can upregulate the sensitivity of TRAIL-mediated apoptosis and upregulate the apoptotic protein cleaved-PARP. Immunoprecipitation studies have indicated that DcR3 might be a ligand of TRAIL,Citation25 and so silencing of DcR3 can increase the binding of TRAIL, DR4 (TRAIL-R1), and DR5 (TRAIL-DR5), so as to induce apoptosis. Based on this, we demonstrated that downregulation of DcR3 could sensitize liver cancer cells HepG2 and BEL-7402 to TRAIL-mediated apoptosis, increase HepG2 cells in G0/G1 phase, and inhibit cell proliferation.
TRAIL, a newly discovered member of the TNF superfamily, is different from TNF and Fas. It only has effects on tumor cell apoptosis and is nontoxic for normal cells. TRAIL can produce strong apoptosis in a wide variety of tumor cells, but Wang et alCitation26 found that almost all of the liver cancer cell lines were resistant to TRAIL. Our findings confirmed this as we demonstrated that HepG2 and BEL-7402 were tolerant to TRAIL. Further studies on TRAIL resistance have shown that the Bcl-2 family play a crucial role.Citation35 Bcl-2 family include three classes: apoptotic proteins Bax and Bak; prosurvival protein Bcl-2, Bcl-xl, and Mcl-1; and proapoptotic proteins Bid, Bim, Puma, and Noxa. The balance between these proapoptotic and prosurvival proteins regulates apoptosis by controlling the permeability of mitochondrial outer membranes.Citation36 Overexpression of the antiapoptotic proteins or inhibition of the proapoptotic proteins of Bcl-2 family can result in tumor cell resistance to TRAIL-mediated apoptosis.Citation37–Citation39 It has also been shown that inhibition of Bcl-2 proapoptotic members can increase the sensitivity of tumor cells to TRAIL. Our findings indicated that SiDcR3/TRAIL could decrease the expression level of Bcl-2, Bcl-xl, and Mcl-1 and upregulate the level of Bax. IAP protein can inhibit apoptosis through downregulating the catalytic activity of caspase-3 and caspase-7 or inhibiting the activity of caspase-9 in apoptotic bodies.Citation40 Overexpression of IAP protein was found in tumor cells that were tolerant to TRAIL-mediated apoptosis, and reduction of IAP-2 could enhance the sensitivity of tumor cells to TRAIL.Citation41,Citation42 We found that SiDcR3/TRAIL could reduce the expression of IAP-2 in HepG2. Similarly, it has been found that overexpression of survivin, a member of the proapoptotic proteins, can also contribute to tumor cell resistance to TRAIL, whereas downregulation of survivinCitation43 may increase the sensitivity of tumor cells to TRAIL, and our study showed the same results.
Apoptosis is also called programmed cell death (), and normal apoptosis contributes to the balance of the organism. Disorder of apoptosis will lead to the occurrence of tumor, and the anticancer effect of anticancer drugs is mainly through inducing tumor cell apoptosis.Citation44 There are two ways to activate apoptosis,Citation45 the extrinsic apoptotic pathway (death receptor pathway) and the intrinsic apoptotic pathways (mitochondrial pathway and endoplasmic reticulum pathway). Both of the extrinsic and intrinsic apoptotic pathways are controlled by the activity of caspase. Extrinsic pathway is activated through the binding of death receptor and ligand on cell surface, which can produce Fas-associated death domain and activate caspase-8, then directly activate caspase-3 or caspase-7, and further activate the intrinsic apoptosis pathway. Studies demonstrated that the activation of caspase-8 is necessary for the activation of apoptosis, and downregulation or missing of caspase-8 will contribute to TRAIL resistance.Citation46,Citation47 We found that SiDcR3/TRAIL can activate caspase-3, caspase-8, and caspase-9, implying activation of the intrinsic and extrinsic apoptotic pathways in HepG2. Interestingly, when caspase-8 inhibitor IETD-CHO was used in the treatment, the apoptosis of HCC decreased significantly, which indicated that this specific apoptosis was mainly through death receptor pathway dependent on caspase-8.
Figure 7 DcR3 and TRAIL-induced apoptosis.
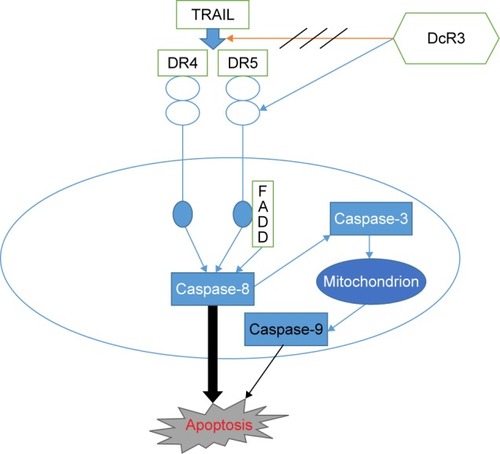
DR4 and DR5 are receptors of TRAIL, and binding of TRAIL and DR4 or DR5 can induce apoptosis by formation of a polymer. Previous studies found that missing DR4 or DR5 in tumor cells contributed to the resistance to TRAIL,Citation48 and increasing the expression of DR4/DR5 could enhance TRAIL-mediated apoptosis in tumor cells.Citation49 Weissinger et alCitation50 found that DcR3 promoted adhesion, migration, and invasiveness in RCC cells, indicating that the expression of DcR3 could be regulated by PI3K/AKT, NFAT, and SP-1, and inhibiting of DFAT and SP-1 could decrease the expression of DcR3. Yoshida and SakaiCitation51 demonstrated that SP1 could increase the expression of DR5 (TRAIL-R2), but that other members of TNFR showed no change, which suggested that DR5 might be related to DcR3. We first found that SiDcR3 in HepG2 could increase the level of DR5; later, when we incubated the cells with DR5 neutralizing antibodies, the apoptosis decreased obviously, and the activity of caspase-8 declined at the same time. These findings confirmed that SiDcR3 enhanced TRAIL-mediated apoptosis by increasing DR5 level.
A previous studyCitation52 has found that upregulation of DcR3 enhances the sensitivity of TRAIL-mediated apoptosis and increases the level of DR5. Unexpectedly, our findings were the opposite. A possible reason is that the findings are associated with cell types, which illustrated the difference between normal cells and tumor cells. Without doubt, this needs further validation.
Conclusion
In summary, SiDcR3 in HepG2 could enhance the sensitivity of TRAIL-mediated apoptosis through upregulation of DR5, and this may provide a theoretical basis for developing new treatment for liver cancer.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (81550033), Beijing Municipal Administration of Hospital Clinical Medicine Development of Special Funding Support (ZYLX201612), Capital Foundation of Medical Development (shoufa2016-2-2053), Beijing Tongren Hospital Funds (TRYY-KYJJ-2015-032).
Disclosure
The authors report no conflicts of interest in this work.
References
- MarquardtJUThorgeirssonSSSnapShot: hepatocellular carcinomaCancer Cell2014254550.e55124735926
- SiegelRNaishadhamDJemalACancer statistics, 2012CA Cancer J Clin2012621102922237781
- LlovetJMBurroughsABruixJHepatocellular carcinomaLancet200336293991907191714667750
- AvilaMABerasainCSangroBPrietoJNew therapies for hepatocellular carcinomaOncogene200625273866388416799628
- Del PozoACLopezPManagement of hepatocellular carcinomaClin Liver Dis200711230532117606209
- WileySRSchooleyKSmolakPJIdentification and characterization of a new member of the TNF family that induces apoptosisImmunity1995366736828777713
- PittiRMMarstersSARuppertSDonahueCJMooreAAshkenaziAInduction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine familyJ Biol Chem19962712212687126908663110
- GolsteinPCell death: TRAIL and its receptorsCurr Biol1997712R750R7539382834
- WuGSBurnsTFMcDonaldER3rdKILLER/DR5 is a DNA damage-inducible p53-regulated death receptor geneNat Genet19971721411439326928
- SheridanJPMarstersSAPittiRMControl of TRAIL-induced apoptosis by a family of signaling and decoy receptorsScience199727753278188219242611
- KischkelFCLawrenceDAChuntharapaiASchowPKimKJAshkenaziAApo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5Immunity200012661162010894161
- SprickMRWeigandMARieserEFADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2Immunity200012659960910894160
- EnariMTalanianRVWongWWNagataSSequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosisNature19963806576727268598910
- HallMAClevelandJLClearing the TRAIL for cancer therapyCancer Cell20071214617613431
- ZhangJSHerreros-VillanuevaMKoenigADifferential activity of GSK-3 isoforms regulates NF-kappaB and TRAIL- or TNFalpha induced apoptosis in pancreatic cancer cellsCell Death Dis20145e114224675460
- OmarHAArafa elSAMaghrabiIAWengJRSensitization of hepatocellular carcinoma cells to Apo2L/TRAIL by a novel Akt/NF- kappaB signalling inhibitorBasic Clin Pharmacol Toxicol2014114646447124401154
- TsujiSHosotaniRYoneharaSEndogenous decoy receptor 3 blocks the growth inhibition signals mediated by Fas ligand in human pancreatic adenocarcinomaInt J Cancer20031061172512794752
- YuKYKwonBNiJZhaiYEbnerRKwonBSA newly identified member of tumor necrosis factor receptor superfamily (TR6) suppresses LIGHT-mediated apoptosisJ Biol Chem199927420137331373610318773
- TakahashiMMiuraYHayashiSTateishiKFukudaKKurosakaMDcR3-TL1A signalling inhibits cytokine-induced proliferation of rheumatoid synovial fibroblastsInt J Mol Med201128342342721537832
- LiangQLWangBRLiGHDcR3 and survivin are highly expressed in colorectal carcinoma and closely correlated to its clinicopathologic parametersJ Zhejiang Univ Sci B200910967568219735100
- WangWLiXSunWTriptolide triggers the apoptosis of pancreatic cancer cells via the downregulation of Decoy receptor 3 expressionJ Cancer Res Clin Oncol201213891597160522581262
- SungHYWuHGAhnJHParkWYDcr3 inhibit p53-dependent apoptosis in gamma-irradiated lung cancer cellsInt J Radiat Biol201086978079020597837
- YangDFanXYinPSignificance of decoy receptor 3 (Dcr3) and external-signal regulated kinase 1/2 (Erk1/2) in gastric cancerBMC Immunol2012132822672288
- ZhouJSongSHeSSilencing of decoy receptor 3 (DcR3) expression by siRNA in pancreatic carcinoma cells induces Fas ligand-mediated apoptosis in vitro and in vivoInt J Mol Med201332365366023846297
- WangWZhangMSunWReduction of decoy receptor 3 enhances TRAIL-mediated apoptosis in pancreatic cancerPLoS One2013810e7427224204567
- WangGZhanYWangHLiWABT-263 sensitizes TRAIL-resistant hepatocarcinoma cells by downregulating the Bcl-2 family of anti-apoptotic proteinCancer Chemother Pharmacol201269379980522037880
- TakedaKStaggJYagitaHOkumuraKSmythMJTargeting death-inducing receptors in cancer therapyOncogene200726253745375717530027
- WuYGuoEYuJXieQHigh DcR3 expression predicts stage pN2-3 in gastric cancerAm J Clin Oncol2008311798318376232
- WuQZhengYChenDLiXLuCZhangZAberrant expression of decoy receptor 3 in human breast cancer: relevance to lymphangiogenesisJ Surg Res2014188245946524612949
- JiangYQZhongTFDangYWOverexpression and clinicopathological contribution of DcR3 in bladder urothelial carcinoma tissuesAsian Pac J Cancer Prev201415219137914225422191
- ChenCZhangCZhuangGDecoy receptor 3 overexpression and immunologic tolerance in hepatocellular carcinoma (HCC) developmentCancer Invest2008261096597419093253
- TongJAoRWangYChangBWangBYPrognostic and clinicopathological differences of DcR3 in gastrointestinal cancer: evidence from meta-analysisInt J Clin Exp Med2014793096310525356187
- YuWXuYCTaoYDcR3 regulates the growth and metastatic potential of SW480 colon cancer cellsOncol Rep20133062741274824101127
- PittiRMMarstersSALawrenceDAGenomic amplification of a decoy receptor for Fas ligand in lung and colon cancerNature199839667126997039872321
- YouleRJStrasserAThe BCL-2 protein family: opposing activities that mediate cell deathNat Rev Mol Cell Biol200891475918097445
- Shamas-DinAKaleJLeberBAndrewsDWMechanisms of action of Bcl-2 family proteinsCold Spring Harb Perspect Biol201354a00871423545417
- IndranIRTufoGPervaizSBrennerCRecent advances in apoptosis, mitochondria and drug resistance in cancer cellsBiochim Biophys Acta20111807673574521453675
- LeBlancHLawrenceDVarfolomeevETumor-cell resistance to death receptor – induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog BaxNat Med20028327428111875499
- HinzSTrauzoldABoenickeLBcl-XL protects pancreatic adenocarcinoma cells against CD95- and TRAIL-receptor-mediated apoptosisOncogene200019485477548611114725
- ZhangLFangBMechanisms of resistance to TRAIL-induced apoptosis in cancerCancer Gene Ther200512322823715550937
- NgCPZismanABonavidaBSynergy is achieved by complementation with Apo2L/TRAIL and actinomycin D in Apo2L/TRAIL-mediated apoptosis of prostate cancer cells: role of XIAP in resistanceProstate200253428629912430140
- JungYHLimEJHeoJKwonTKKimYHTunicamycin sensitizes human prostate cells to TRAIL-induced apoptosis by upregulation of TRAIL receptors and downregulation of cIAP2Int J Oncol20124061941194822426894
- HanZLeeSJeSSurvivin silencing and TRAIL expression using oncolytic adenovirus increase anti-tumorigenic activity in gemcitabine-resistant pancreatic cancer cellsApoptosis201621335136426677013
- FesikSWPromoting apoptosis as a strategy for cancer drug discoveryNat Rev Cancer200551187688516239906
- MoffittKLMartinSLWalkerBFrom sentencing to execution – the processes of apoptosisJ Pharm Pharmacol201062554756220609056
- BodmerJLHollerNReynardSTRAIL receptor-2 signals apoptosis through FADD and caspase-8Nat Cell Biol20002424124310783243
- SeolDWLiJSeolMHParkSYTalanianRVBilliarTRSignaling events triggered by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL): caspase-8 is required for TRAIL-induced apoptosisCancer Res20016131138114311221844
- ZhangYZhangBTRAIL resistance of breast cancer cells is associated with constitutive endocytosis of death receptors 4 and 5Mol Cancer Res20086121861187119074831
- GuptaSCReuterSPhromnoiKNimbolide sensitizes human colon cancer cells to TRAIL through reactive oxygen species- and ERK-dependent up-regulation of death receptors, p53, and BaxJ Biol Chem201128621134114621078664
- WeissingerDTagschererKEMacher-GoppingerSHaferkampAWagenerNRothWThe soluble Decoy Receptor 3 is regulated by a PI3K-dependent mechanism and promotes migration and invasion in renal cell carcinomaMol Cancer201312112024107265
- YoshidaTSakaiTPromoter of TRAIL-R2 geneVitam Horm200467354915110170
- YouRIChangYCChenPMApoptosis of dendritic cells induced by decoy receptor 3 (DcR3)Blood200811131480148818006694
