?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
Impaired immunonutritional status has disadvantageous effects on outcomes for cancer patients. Preoperative albumin-to-globulin ratio (AGR) and the prognostic nutrition index (PNI) have been used as prognostic factors in various cancers. We aimed to evaluate the clinical significance of the AGR and PNI in glioblastoma.
Materials and methods
This retrospective analysis involved 166 patients. Demographic, clinical, and laboratory data were collected. AGR and the PNI were calculated as AGR = albumin/(total serum protein − albumin) and PNI = albumin (g/L) + 5 × total lymphocyte count (109/L). Overall survival (OS) was estimated by Kaplan–Meier analysis. Receiver-operating characteristic analysis was used to assess the predictive ability of AGR and the PNI. Cox proportional-hazard models estimating hazard ratios (HRs) and 95% confidence intervals (CIs) were used for univariable and multivariable survival analyses.
Results
The cutoff values of AGR and PNI were 1.75 and 48. OS was enhanced, with high AGR (>1.75) and the PNI (>48) (P<0.001 for both). Areas under the receiver-operating characteristic curve for AGR and the PNI were 0.68 and 0.631 for 1-year survival and 0.651 and 0.656 for 2-year survival (P<0.05 for all), respectively. On multivariable analyses, both AGR and the PNI were independent predictors of OS (AGR, HR 0.785, 95% CI 0.357–0.979 [P=0.04]; PNI, HR 0.757, 95% CI 0.378–0.985 [P=0.039]). On subgroup analysis, AGR and the PNI were significant prognostic factors for OS in patients with adjuvant therapy (AGR P<0.001; PNI P=0.001).
Conclusion
Preoperative AGR and the PNI may be easy-to-perform and inexpensive indices for predicting OS with glioblastoma. AGR and the PNI could also help in developing good adjuvant-therapy schedules.
Introduction
The 5-year relative survival for glioblastoma (GB), the most commonly occurring glioma histological type (~45% of all gliomas), is ~5%.Citation1 The widely accepted therapy regimen for newly diagnosed GB includes maximal safe resection and concurrent chemotherapy with temozolomide and radiotherapy. The median survival of GB is only about 15 months, even with recent diagnostic and therapeutic advances.Citation2 Because of molecular and cellular heterogeneity, together with individual differences, the clinical outcomes of individual patients with similar diagnosis and treatment remain diverse.Citation3 Moreover, a high incidence of local recurrence (~90%) is an important cause of low survival.Citation4 Consequently, identifying prognostic biomarkers, such as in peripheral blood, could help in designing treatment strategies and stratifying patients with poor overall survival (OS) after surgery.
Understanding the crucial biomarkers that play a part in GB malignant behavior might help clinicians educate patients about prognosis and monitor therapeutic efficacy. Laboratory markers found as prognostic factors for GB include MGMT, IDH1, IDH2, EGFR, telomerase reverse transcriptase, p53, PDGF and PTEN.Citation5–Citation9 Some clinical markers, such as histopathologic transformation, as well as geometric heterogeneity and/or spherical rim widths seen on magnetic resonance imaging, show high individual and combined prognostic value.Citation10,Citation11 However, the high cost of testing and the analytic subjectivity and instability of results limit the application of these markers in clinical practice. Novel and practical biomarkers for predicting outcomes with GB are needed.
Preoperative albumin-to-globulin ratio (AGR) could reflect both malnutrition and systemic inflammation in cancer patients.Citation12 As a major component in serum protein, serum albumin is used to measure long-standing malnutrition and is also associated with systemic inflammation.Citation13 Survival seems longer for patients with high than low preoperative serum albumin levels in adenocarcinoma of the gastric cardia and ovarian cancer.Citation14,Citation15 Globulin functions as a carrier of hormones, and plays an important role in immunity and inflammation.Citation16 Recently, AGR was used to predict outcome with bladder urothelial carcinoma, small-cell lung cancer, and natural killer/T-cell lymphoma.Citation12,Citation17,Citation18
The prognostic nutrition index (PNI), based on albumin level and lymphocyte count, could reflect immunonutritional status and systemic inflammatory reaction with more accuracy than with each variable alone.Citation19,Citation20 Lymphocytes are key components of the immune system and are essential effector cells in antitumor immunity.Citation21,Citation22 Lymphocyte count (particularly CD4 cells) has been found to be decreased in GB and associated with poor survival time.Citation23,Citation24 The value of the PNI in predicting clinical outcomes has been verified in colorectal cancer, gastric cancer, and renal cell carcinoma, for example.Citation25–Citation27 However, no study has examined the prognostic role of AGR and the PNI in GB. In this study, we examined clinical data to analyze the prognostic value of the two immunonutritional factors AGR and PNI for OS in GB patients.
Materials and methods
Patients
We examined clinical data for 243 patients with a diagnosis of GB confirmed by postoperative pathological examination at Qilu Hospital of Shandong University from January 2010 to January 2015. Patients were followed every month for the first few years after surgery and thereafter every 2 months until death. The median follow-up time was 14 months (range 1–52 months). We excluded patients with metabolic syndrome, diabetes, heart disease, hypertension, autoimmune disease, and infection within 3 months.
We collected data on age, sex, smoking and drinking status, body mass index, Karnofsky performance status (KPS), tumor size, tumor localization (region of brain), extent of resection, preoperative lymphocyte count, preoperative albumin level, adjuvant radiotherapy and chemotherapy, and death. Tumor size was calculated from magnetic resonance-imaging data: longest × widest diameter × thickness (section thickness × number of layers) × 0.5.Citation28
Data for hematological variables were obtained for all enrolled patients within 1 week before surgery. The time from surgery to death was defined as OS. Data for patients alive at last follow-up were considered censored. The study protocol was approved by the ethics committee of Qilu Hospital. Patients all granted written informed consent for surgery and the use of clinical records.
Evaluation of AGR and PNI
We used the following formulas for calculating AGR and PNI:Citation29
(1)
(2)
Statistical analysis
To determine differences between groups, continuous variables were analyzed by unpaired t-tests and categorical variables by χ2 test. Data are shown as means (standard deviation) or median (interquartile range). We used the Kaplan–Meier method for estimating OS. Receiver-operating characteristic (ROC) curve analysis was applied to determine the best cutoff values for AGR and PNI, and area under the ROC curve estimation was used to assess the predictive ability of AGR and PNI. Univariable Cox analysis was used to analyze the prognostic significance of the variables. Multivariable analysis by Cox proportional-hazard model was used to determine the independence of the prognostic factors, estimating hazard ratios (HRs) and 95% confidence intervals (CIs). All statistical analyses involved the use of Statistical Package for the Social Sciences (SPSS) version 23.0 (IBM, Armonk, NY, USA). P<0.05 was considered statistically significant and the inclusion criterion to use a variable from univariable analysis in the multivariable analysis.
Results
Clinical characteristics of patients
After excluding data for 65 patients with the aforementioned diseases and 12 patients lost to follow-up, we examined data for 166 patients. Baseline characteristics are in . The median age at diagnosis was 50 (6–83) years, and 84 (50.6%) were male. The median body mass index was 22 (15.57–34.38). KPS was 80–100 in 91 patients (54.8%) and ≤70 in 75 patients (45.2%). The median tumor size was 40.1 (6.35–245.52) cm3. Most tumors were located in the frontal (38.6%), temporal (33.7%), and parieto-occipital (20.5%) regions. Other locations, including ventricles, cerebellum, insular lobe, thalamus, and callosum, represented 7.2% of tumors. Total resection was performed to treat 109 patients (65.7%). The median preoperative albumin level, lymphocyte count, AGR, and PNI were 41.61 (33.91–52.11) g/L, 1.89×109/L (0.73–3.67), 1.66 (1.19–2.58), and 51.1 (41.15–68.95), respectively. In all, 105 patients (63.3%) received adjuvant radiotherapy and 42 (25.3%) adjuvant chemotherapy.
Table 1 Clinical characteristics of 166 patients with glioblastoma
Association of clinical characteristics with preoperative AGR and PNI
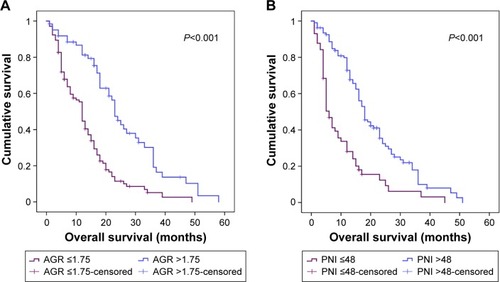
On ROC analysis, the cutoff values for AGR and PNI were 1.75 (sensitivity 0.554 and specificity 0.806) and 48 (sensitivity 0.782 and specificity 0.575). The high-AGR and -PNI groups had higher preoperative albumin levels than the low-AGR and -PNI groups (P<0.001 for both) (). Similarly, preoperative lymphocyte counts in the high-AGR and -PNI groups were higher than those in the low-AGR and -PNI groups (P=0.013 and P<0.001).
Table 2 Clinical characteristics of patients by AGR and PNI cutoffs
Prognostic value of AGR and PNI
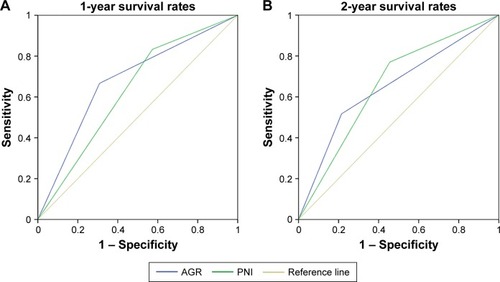
The median OS was 13 (range 1–52) months. Kaplan–Meier curves for OS based on preoperative AGR and PNI demonstrated that patients with AGR ≤1.75 or PNI ≤48 had significantly poorer OS than those with AGR >1.75 or PNI >48 (P<0.001 for both) (). For 1-year survival, the area under the ROC curve for AGR and PNI was 0.68 (sensitivity 0.67, specificity 0.774, 95% CI 0.577–0.783; P=0.038), and 0.631 (sensitivity 0.834, specificity 0.429, 95% CI 0.523–0.739; P=0.045), and for 2-year survival was 0.651 (sensitivity 0.524, specificity 0.786, 95% CI 0.55–0.752; P=0.024) and 0.656 (sensitivity 0.766, specificity 0.55, 95% CI 0.548–0.765; P=0.041) ().
Univariable and multivariable analyses of prognostic factors for OS
We examined the functions of preoperative AGR and PNI in survival in our cohort (). On univariable Cox analysis, OS was associated with age (P=0.045), KPS (P=0.009), tumor size (P=0.022), extent of resection (P=0.017), preoperative albumin level (P=0.035), lymphocyte count (P=0.028), AGR (P=0.003), PNI (P<0.001), and adjuvant radiotherapy (P=0.004) and chemotherapy (P=0.002). On multivariable analysis, predictors of OS were KPS (HR 0.528, 95% CI 0.341–0.851; P=0.032), tumor size (HR 1.343, 95% CI 1.014–1.726; P=0.037), extent of resection (HR 0.416, 95% CI 0.334–0.885; P=0.026), preoperative AGR (HR 0.785, 95% CI 0.357–0.979; P=0.04), PNI (HR 0.757, 95% CI 0.378–0.985; P=0.039), and adjuvant radiotherapy (HR 0.599, 95% CI 0.32–0.983; P=0.031).
Table 3 Prognostic factors for overall survival for 166 patients with glioblastoma
Prognostic impact of AGR and PNI with adjuvant therapy
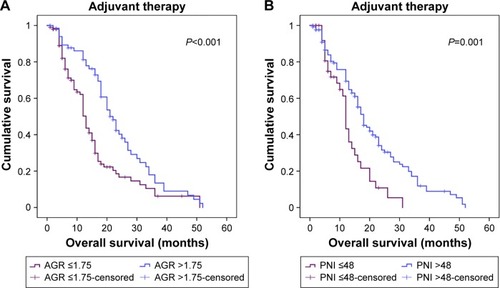
Adjuvant therapy was defined as chemotherapy and/or radiotherapy. In our cohort, 123 (74.1%) patients received adjuvant therapy. On subgroup analyses of patients with adjuvant therapy, survival differed with AGR ≤1.75 and >1.75 (P<0.001) () and PNI ≤48 and >48 (P=0.001) ().
Discussion
Impaired immunonutritional status has disadvantageous effects on outcomes for cancer patients. AGR and the PNI have been used as prognostic factors in various cancers. We evaluated the clinical significance of AGR and the PNI in 166 patients with GB. OS was enhanced with high AGR (>1.75) and PNI (>48). Both were independent predictors of OS, and significant prognostic factors for OS in patients with adjuvant therapy. Preoperative AGR and PNI may be easy-to-perform and inexpensive indices for predicting OS in GB patients.
Cancer patients frequently show malnutrition, which is responsible for decreased capacity for treatment tolerance, increased mortality, and worse quality of life in the postoperative period.Citation30 Moreover, malnutrition has been found to be associated with an immunosuppressed condition,Citation31 which suggests inadequate antitumor immunity.Citation21 Chronic inflammation has been identified as a hallmark of cancer, and most neoplastic tissues contain an inflammatory component, even those not causally associated with an obvious inflammatory reaction.Citation32 Cell inflammatory mediators promote tumorigenesis by increasing tumor proliferation and metastasis, and can also exert an immunosuppressive effect, with resulting impaired lymphocyte function and reduced lymphocyte count, which enables the escape of cancer cells from immunosurveillance.Citation33–Citation35 Meanwhile, inflammation was found to be closely related to the genesis and development of GB.Citation23,Citation24,Citation28,Citation36 In view of the relationship between nutrition and immunocompetence, as well as the influence of inflammation on GB, patients’ nutritional status and immunocompetence should be evaluated in the management of GB.
In this study, we evaluated AGR and the PNI, two integrated immunonutritional indices, for their prognostic values for GB patients, and found cutoff values of 1.75 and 48, respectively. Preoperative AGR and the PNI were significantly associated with OS in GB, which agrees with findings for other malignancies.Citation12,Citation17,Citation18,Citation25–Citation27 Although AGR and PNI were associated with preoperative albumin level and lymphocyte count, the predictive value of both remained significant after adjustment for other clinical characteristics on multivariable analysis. According to the ROC analysis, AGR had better specificity than the PNI, and the PNI had better sensitivity than AGR. The two factors may accurately predict OS with GB. Based on indicators of preoperative inflammation, immunity, and nutritional status, AGR and the PNI could provide important prognostic information in GB.
The good prognosis with high AGR and PNI values might be explained by the functions of albumin, globulin, and lymphocytes. Albumin has antioxidative effects against some of the most common carcinogens, including nitrosamines and aflatoxins. Also, it can stabilize cell growth and DNA replication and buffer sex-hormone homeostasis to prevent sex hormone-induced cancers.Citation37 Albumin can inhibit the proliferation of human breast cancer cells by regulating the activities of autocrine growth-regulatory factors in vitro.Citation38 However, the inflammatory response was reported to play an independent role in decreasing albumin level apart from malnutrition.Citation13 In cancer-related inflammation, the proinflammatory cytokines released by tumor and immune cells modulate albumin production and help in cancer progression, angiogenesis, and malignant transformation.Citation32,Citation39 Serum globulin, including immunoglobulins, CRP, complements, and other acute-phase proteins are upregulated with inflammation and play a crucial role in immunity and inflammation.Citation40,Citation41 As a component of the innate immune system, high CRP levels can induce the production of inflammatory cytokines and tissue factor in monocytes.Citation42 Complement activation can initiate or sustain the inflammatory process by leading to chemotaxis, plasma protein exudation at inflammatory sites, and opsonization of infectious agents and damaged cells.Citation41 High levels of globulin reflect an inflammatory state, believed to play a major role in cancer progression and metastasis.Citation33 Lymphocytes play a key role in host defense against cancer by inhibiting the proliferation and invasion of tumor cells via inducing cytotoxic cell death and production of cytokines.Citation32 Cytotoxic T lymphocytes inhibit tumor growth by inducing apoptosis of cancer cells, whereas CD8+ T-lymphocyte infiltration is associated with overall good outcome.Citation43 Lymphocytopenia, reflecting insufficient antitumor immunocompetence, can be induced by a systemic inflammatory reaction.Citation20
We performed a subgroup analysis of OS for patients with adjuvant therapy (radiotherapy and/or chemotherapy). For GB patients receiving adjuvant therapy after surgery, low AGR or PNI value was significantly associated with short OS, so these patients acquired less benefit from adjuvant therapy. Patients with poor immune and nutrition status showed inferior tolerance to adjuvant therapy, and the adverse effects of adjuvant therapy were more prominent in patients with immunosuppression and malnutrition, thus affecting the long-term outcomes.Citation30,Citation44 Albumin could protect tissues against most forms of radiation,Citation37 thereby protecting against the adverse effects of radiotherapy. Moreover, systemic inflammation might impair the activity of CYP3A4, a crucial drug-metabolizing enzyme.Citation45 Decreased activity of CYP3A4 has been found to be related to reduced hepatic drug clearance and increased drug toxicity.Citation46 Therefore, it is important to maximize the curative effects of adjuvant therapy and diminish its adverse effects.
AGR and the PNI may be appropriate factors for survival in stratifying patients who acquire more benefit from adjuvant therapy, and immunonutritional intervention and anti-inflammation therapy could be considered efficient therapeutic strategies for GB patients who have received radiotherapy and/or chemotherapy.
The present study has several limitations. First, the retrospective design and limited number of patients might indicate bias. Second, unknown physiological and pathophysiological factors might have had an unavoidable impact on AGR and the PNI. Furthermore, although serum CRP and cytokine levels were important indicators of cancer-related inflammation, these factors were not routinely examined in our hospital, which prevents us from acquiring a better understanding of the mechanism of tumor progression caused by inflammation. In this study, AGR and the PNI were assessed only once before surgery. The predictive significance of the kinetics of AGR and the PNI remains to be investigated.
Conclusion
This study demonstrated that low AGR and PNI values could be promising immunonutritional markers to identify GB patients with poor prognostic potential. The application of AGR and the PNI in evaluating preoperative immunonutritional status might facilitate the formulation of more effective preoperative regulation strategies and better adjuvant-therapy schedules.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81301127 and 81401867), the China Postdoctoral Science Foundation (2014T70661 and 2014M560562), and the Key Research and Development Program of Shandong Province (2015GSF118095).
Disclosure
The authors report no conflicts of interest in this work.
References
- OstromQTBauchetLDavisFGThe epidemiology of glioma in adults: a “state of the science” reviewNeuro Oncol201416789691324842956
- StuppRMasonWPvan den BentMJRadiotherapy plus concomitant and adjuvant temozolomide for glioblastomaN Engl J Med20053521098799615758009
- AumDJKimDHBeaumontTLLeuthardtECDunnGPKimAHMolecular and cellular heterogeneity: the hallmark of glioblastomaNeurosurg Focus2014376E11
- NaborsLBPortnowJAmmiratiMCentral Nervous System Cancers, Version 1.2015J Natl Compr Canc Netw201513101191120226483059
- WickWPlattenMMeisnerCTemozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trialLancet Oncol201213770771522578793
- IdoateMAEchevesteJDiez-ValleRLozanoMDAristuJBiological and clinical significance of the intratumour heterogeneity of PTEN protein expression and the corresponding molecular abnormalities of the PTEN gene in glioblastomasNeuropathol Appl Neurobiol201440673674624417635
- LabussiereMBoisselierBMokhtariKCombined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classesNeurology201483131200120625150284
- MichaletSRohrJWarshanDPhytochemical analysis of mature tree root exudates in situ and their role in shaping soil microbial communities in relation to tree N-acquisition strategyPlant Physiol Biochem20137216917723727287
- PhillipsJJArandaDEllisonDWPDGFRA amplification is common in pediatric and adult high-grade astrocytomas and identifies a poor prognostic group in IDH1 mutant glioblastomaBrain Pathol201323556557323438035
- HoALKochMJTanakaSImpact of histopathological transformation and overall survival in patients with progressive anaplastic gliomaJ Clin Neurosci2016319910527279154
- Perez-BetetaJMartinez-GonzalezAMolinaDGlioblastoma: does the pre-treatment geometry matter? A postcontrast T1 MRI-based studyEur Radiol Epub2016621
- LiuJDaiYZhouFThe prognostic role of preoperative serum albumin/globulin ratio in patients with bladder urothelial carcinoma undergoing radical cystectomyUrol Oncol20163411484.e1484.e8
- McMillanDCWatsonWSO’GormanPPrestonTScottHRMcArdleCSAlbumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight lossNutr Cancer200139221021311759282
- AsherVLeeJBaliAPreoperative serum albumin is an independent prognostic predictor of survival in ovarian cancerMed Oncol20122932005200921735143
- LienYCHsiehCCWuYCPreoperative serum albumin level is a prognostic indicator for adenocarcinoma of the gastric cardiaJ Gastrointest Surg2004881041104815585392
- ZhangBYuWZhouLQPrognostic significance of preoperative albumin-globulin ratio in patients with upper tract urothelial carcinomaPLoS One20151012e014496126681341
- ZhouTHeXFangWPretreatment albumin/globulin ratio predicts the prognosis for small-cell lung cancerMedicine (Baltimore)20169512e309727015181
- BiXWWangLZhangWWThe pretreatment albumin to globulin ratio predicts survival in patients with natural killer/T-cell lymphomaPeerJ20164e174226966671
- OkamuraYAshidaRItoTSugiuraTMoriKUesakaKPreoperative neutrophil to lymphocyte ratio and prognostic nutritional index predict overall survival after hepatectomy for hepatocellular carcinomaWorld J Surg20153961501150925670038
- HongSZhouTFangWThe prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patientsTumour Biol20153653389339725527156
- MellmanICoukosGDranoffGCancer immunotherapy comes of ageNature2011480737848048922193102
- ShibutaniMMaedaKNagaharaHThe prognostic significance of the postoperative prognostic nutritional index in patients with colorectal cancerBMC Cancer20151552126177820
- WaziriAGlioblastoma-derived mechanisms of systemic immunosuppressionNeurosurg Clin N Am2010211314219944964
- GustafsonMPLinYLaPlantBImmune monitoring using the predictive power of immune profilesJ Immunother Cancer20131725512872
- YangYGaoPChenXPrognostic significance of preoperative prognostic nutritional index in colorectal cancer: results from a retrospective cohort study and a meta-analysisOncotarget2016736585435855227344182
- WangSHZhaiSTLinHRole of prognostic nutritional index in patients with gastric cancer: a meta-analysisMinerva Med2016107532232727285120
- GrimesNTysonMHannanCMulhollandCA systematic review of the prognostic role of hematologic scoring systems in patients with renal cell carcinoma undergoing nephrectomy with curative intentClin Genitourin Cancer201614427127626949171
- HanSLiuYLiQLiZHouHWuAPre-treatment neutrophil-to-lymphocyte ratio is associated with neutrophil and T-cell infiltration and predicts clinical outcome in patients with glioblastomaBMC Cancer20151561726341881
- PinatoDJNorthBVSharmaRA novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI)Br J Cancer201210681439144522433965
- KandaMMizunoATanakaCNutritional predictors for postoperative short-term and long-term outcomes of patients with gastric cancerMedicine (Baltimore)20169524e378127310954
- MainousMRDeitchEANutrition and infectionSurg Clin North Am19947436596768197536
- MantovaniAAllavenaPSicaABalkwillFCancer-related inflammationNature2008454720343644418650914
- ColottaFAllavenaPSicaAGarlandaCMantovaniACancer-related inflammation, the seventh hallmark of cancer: links to genetic instabilityCarcinogenesis20093071073108119468060
- KusmartsevSGabrilovichDIImmature myeloid cells and cancer-associated immune suppressionCancer Immunol Immunother200251629329812111117
- Salazar-OnfrayFLopezMNMendoza-NaranjoAParadoxical effects of cytokines in tumor immune surveillance and tumor immune escapeCytokine Growth Factor Rev2007181–217118217329145
- BamburyRMTeoMYPowerDGThe association of pretreatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiformeJ Neurooncol2013114114915423780645
- SeatonKAlbumin concentration controls cancerJ Natl Med Assoc2001931249049311800279
- LaursenIBriandPLykkesfeldtAESerum albumin as a modulator on growth of the human breast cancer cell line, MCF-7Anticancer Res1990102A3433512346307
- RothschildMAOratzMSchreiberSSSerum albuminHepatology1988823854013281888
- LiQMengXLiangLXuYCaiGCaiSHigh preoperative serum globulin in rectal cancer treated with neoadjunctive chemoradiation therapy is a risk factor for poor outcomeAm J Cancer Res2015592856286426609491
- GabayCKushnerIAcute-phase proteins and other systemic responses to inflammationN Engl J Med199934064484549971870
- BallouSPLozanskiGInduction of inflammatory cytokine release from cultured human monocytes by C-reactive proteinCytokine1992453613681420997
- FuYChenSWChenSQA preoperative nutritional index for predicting cancer-specific and overall survival in Chinese patients with laryngeal cancer: a retrospective studyMedicine (Baltimore)20169511e296226986105
- CaiBChenHSunHWanPSunHPanJProduction of immunoregulatory polysaccharides from Crassostrea hongkongensis and their positive effects as a nutrition factor in modulating the effectiveness and toxicity of 5-FU chemotherapy in miceFood Funct20167139039726507007
- CharlesKARivoryLPBrownSLLiddleCClarkeSJRobertsonGRTranscriptional repression of hepatic cytochrome P450 3A4 gene in the presence of cancerClin Cancer Res200612247492749717189422
- RivoryLPSlavieroKAClarkeSJHepatic cytochrome P450 3A drug metabolism is reduced in cancer patients who have an acute-phase responseBr J Cancer200287327728012177794