Abstract
Background
The site-distribution pattern and relative risk of subsequent primary malignancies (SPMs) in colorectal cancer (CRC) patients remains to be determined.
Materials and methods
A population-based cohort of 288,390 CRC patients diagnosed between 1973 and 2012 from the Surveillance, Epidemiology, and End Results database was retrospectively reviewed. Standardized incidence ratios were calculated to estimate the relative risk for SPMs.
Results
The overall risk of SPMs increased in CRC patients (standardized incidence ratio 1.02) in the first 5 years after CRC diagnosis compared with that in the general population, and was negatively related to age at diagnosis. Risk increased significantly for cancers of the small intestine, ureter, colorectum, renal pelvis, endocrine system, and stomach, and decreased significantly for cancers of the gallbladder, liver, myeloma, and brain, as well as lymphoma. Patients with different prior CRC subsites showed specific sites at high risk of SPM. Prior right-sided colon cancer was associated with cancers of the small intestine, ureter, renal pelvis, thyroid, stomach, pancreas, and breast and prior left-sided colon cancer associated with secondary CRC, whereas rectal cancer was associated with cancers of the vagina, urinary bladder, and lung.
Conclusion
Risk of SPMs increases in CRC survivors, especially in the first 5 years after prior diagnosis. Intensive surveillance should be advocated among young patients, with specific attention to the small intestine, colorectum, renal pelvis, and ureter. The common sites at high risk of SPM originate from the embryonic endoderm. Genetic susceptibility may act as the main mechanism underlying the risk of multiple cancers.
Introduction
Colorectal cancer (CRC) is the third-commonest cancer worldwide and the second-commonest cause of cancer-related death in Western countries.Citation1 Since the widespread adoption of screening methods, including colonoscopy and fecal occult blood test, an increasing number of CRC patients are being diagnosed with localized disease.Citation2 Curative surgery for CRC patients with limited metastases in the liver or with solitary metastasis in the lung can be successfully performed by multidisciplinary teams.Citation3 Targeted treatment also considerably improves the survival of CRC patients with distant metastases.Citation4 A long life exposes these survivors to the possibility of developing secondary or additional primary malignancies.
Studies have found that patients previously diagnosed with CRC are at higher risk of second primary CRCs than the general population.Citation5–Citation7 Synchronous colorectal adenoma and a family history of CRC are risk factors for developing a second primary CRC.Citation8–Citation10 From a clinical perspective, subsequent primary malignancies (SPMs) may occur in any organ of the human body besides the part of the colorectum resected by prior surgery. The aim of the present study was to clarify the unique site-distribution pattern and relative risk of SPMs among CRC survivors and to identify patients who are at increased risk of developing SPMs.
Materials and methods
Data source
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program database was used to identify the cohort of patients analyzed in the present study. SEER currently collects cancer-incidence and -survival data from population-based cancer registries covering approximately 30% of the US population (http://seer.cancer.gov). The SEER data contain no identifiers, and are publicly available for cancer-epidemiology and health-service research studies. The population-based SEER database has a well-defined data collection and extensive quality standards to ensure that the information is accurate. Permission to access the research data was obtained. The study was approved by the review board of the First Affiliated Hospital of Xi’an Jiao Tong University. The SEER 9 registry data, including individual data from 1973 to 2012 from nine registries (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco–Oakland, Seattle–Puget Sound, and Utah), were used by the SEER Stat software to estimate the standardized incidence of SPMs in CRC cases.
Study population
The specific inclusion criteria were as follows: patients diagnosed with prior cancer between 1973 and 2012; site record ICD-O-3/WHO 2008 limited to colon and rectum; and histological type ICD-O-3 limited to adenocarcinoma (8140). Patients were excluded if documentation of age at diagnosis was lacking or if it was reported only on the death certificate or autopsy. Patients diagnosed with CRC were followed until their death or the 2012 year’s end. Information on SPM development was documented. SPM required a minimum latency period of 6 months after the prior CRC diagnosis to exclude synchronous primary malignancies (PMs). Therefore, participants who were followed <6 months or had SPMs diagnosed within the first 6 months after the diagnosis of CRC were not enrolled. Information on age of diagnosis with prior CRC, sex, latency period, sites of SPM, and location of index CRC was extracted from the database. The cohort consisted of 233,890 patients who were diagnosed with primary pathologically confirmed adenocarcinoma of the colorectum.
Statistical analysis
Multiple primary standardized incidence ratios (SIRs) were calculated using SEER Stat software version 8.2.1 to determine the relative SPM risk among this cohort. The SIR represents the ratio of the observed to the expected number of incident-cancer cases based on the corresponding segment of the US general population according to age in 5-year intervals, sex, and calendar year by the specific stratified person-time variable accrued from the CRC cohort. Data on cancer incidence among the general population were retrieved from SEER 9 Regs Research Data Nov 2014 Sub. More detailed information on both SEER Stat software and the method the software uses to derive the SIRs is available on the SEER-registry website (http://seer.cancer.gov/seer-stat). SIRs were determined for subgroups defined by age, latency, and sex. Additionally, cases were stratified according to the anatomic subsite of prior CRC into three groups: right-sided colon cancer, left-sided colon cancer, and rectal cancer. The right-sided colon cancer group included cancers emerging at bowel segments between the cecum and the transverse colon, whereas the left-sided colon cancer group included cancers occurring at bowel segments between the splenic flexure and the rectosigmoid junction. In further stratified analyses according to tumor location, sex, and age, all patients were grouped into two groups according to age: <60 and ≥60 years. Evaluation of the confidence intervals (CIs) of SIRs was used to determine any overlap.
Results
Based on the aforementioned criteria, 33,047 SPMs were identified in 28,863 CRC patients from the SEER database, including 16,618 (57.6%) men and 12,245 (42.4%) women, with a median age of 68 years at the time of diagnosis (inter-quartile range 60–75 years) (). A total follow-up time of 1,628,144 cumulative person-years at risk was calculated, with a mean of 6.97 person-years at risk.
Table 1 Baseline characteristics of patients with subsequent primary malignancies development after colorectal cancer
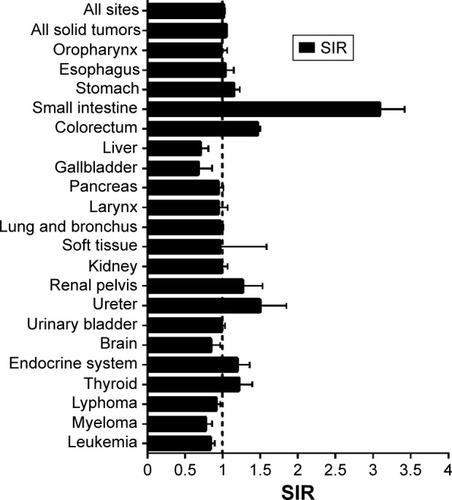
Compared with the incidence in the general population, CRC patients had a higher overall risk of SPM (SIR 1.02, 95% CI 1.01–1.03), especially for solid tumors (SIR 1.05, 95% CI 1.04–1.06) (). The risk increased mainly for cancers of the small intestine (SIR 3.09), ureter (SIR 1.5), colorectum (SIR 1.47), renal pelvis (SIR 1.27), endocrine system (SIR 1.2), and stomach (SIR 1.15). Conversely, risk decreased significantly for the gallbladder (SIR 0.68), liver (SIR 0.71), myeloma (SIR 0.78), brain (SIR 0.85), and lymphoma (SIR 0.92) compared with the general population.
Figure 1 Site distribution of subsequent primary malignancies.
Abbreviation: SIR, standardized incidence interval.
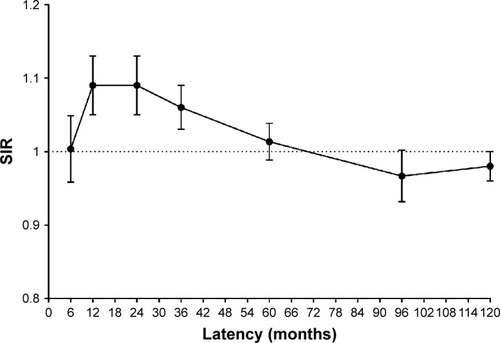
Most of the increased risk was concentrated in the initial 5 years after primary diagnosis (). The risk increased progressively for all solid tumors in the second 5 years. After 10 years, the risk decreased for SPMs of all sites, becoming similar to that in the general population for all solid tumors.
Figure 2 Risk varied by latency for subsequent primary malignancies in all sites.
Abbreviation: SIR, standardized incidence interval.
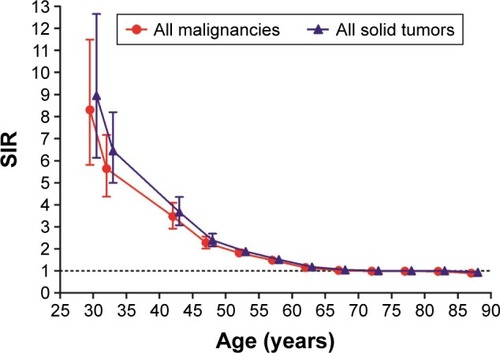
A strong inverse relationship was observed between SIRs and age at primary CRC diagnosis (). SIRs for all SPMs according to age-group were 8.3 for <35 years, 3.47 for 40–44 years, 2.28 for 45–49 years, 1.81 for 50–54 years, 1.49 for 55–59 years, 1.15 for 60–64 years, 1.02 for 65–69 years, 0.99 for 70–74 and 75–79 years, 0.98 for 80–84 years, and 0.9 for patients ≥85 years.
Figure 3 Risk varied by age for subsequent primary malignancies of all sites and all solid tumors.
Abbreviation: SIR, standardized incidence interval.
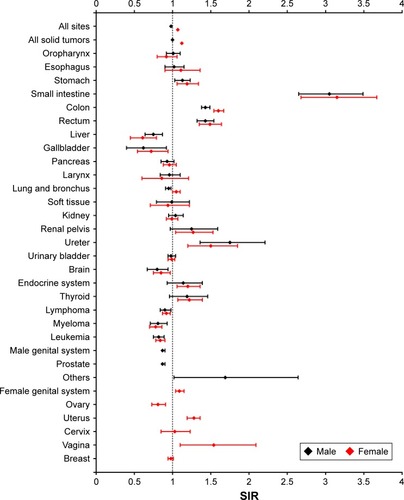
The overall risk for SPMs was higher in women with CRC (SIR 1.07, 95% CI 1.06–1.09) and lower in men with CRC (SIR 0.98, 95% CI 0.97–0.99) than in the general population (). The risk increased consistently for the stomach, small intestine, colorectum, ureter, and the endocrine system, and decreased consistently for the liver, gallbladder, brain, and hematological malignancies in women and men. The relative risks for several cancers differed between men and women. Compared with the general population, lung cancer was more frequent in women with CRC and less frequent in men with CRC. The risk of prostate cancer increased in men, whereas in women the risk of uterine and vaginal cancer increased and that of ovarian cancer decreased.
Figure 4 Risk varied by sex for subsequent primary malignancies in different sites.
Abbreviation: SIR, standardized incidence interval.
There was an inverse relationship between the overall SIRs and the subsites of primary diagnosis: 1.09 in right-sided colon cancer, 1 in left-sided colon cancer, and 0.93 in rectal cancer. There were significant differences in the risk estimates for various malignancies among the three subsite groups (). For right-sided colon cancer survivors, cancer risk was highest for the small intestine, ureter, renal pelvis, thyroid, stomach, pancreas, and breast; in left-sided colon cancer patients, risk was highest for the colorectum; and in rectal cancer patients risk was highest for the vagina, urinary bladder, and lung. On the other hand, cancer risk decreased for the liver, ovary, prostate, pancreas, leukemia, and myeloma in rectal cancer survivors; and decreased for the brain, gallbladder, lymphoma, lung, urinary bladder, and breast in left-sided colon cancer survivors. Stratified analyses () indicated that the risk of all SPMs increased (SIR >1) in young patients, women, or patients with right-sided colon cancer within each subgroup.
Figure 5 Risk varied by subsites of prior colorectal cancer for subsequent primary malignancies in different sites.
Abbreviation: SIR, standardized incidence interval.
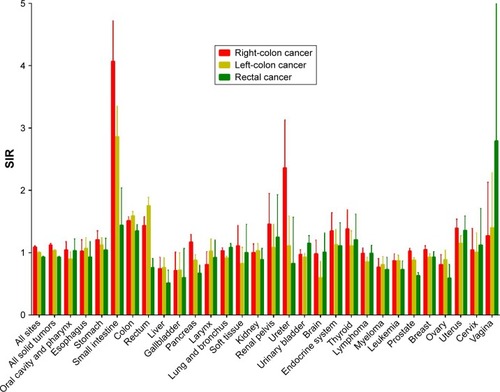
Table 2 Risk of subsequent primary malignancies among colorectal cancer survivors stratified by sex, age, and prior tumor location
Discussion
Improvements in the survival of CRC patients have led to several studies investigating second primary tumors in the survivors;Citation11–Citation13 however, there are few studies focusing on all SPMs in all possible sites. The results of the current study indicated that CRC survivors have a 2% higher overall risk of developing SPMs than the general US population. Lee et al found a 13% higher risk for secondary primary cancer in CRC survivors in Taiwan.Citation11 Although the reasons for the differences in relative risk are not clear, they may be attributed in part to the different populations included in the SEER and the Taiwanese cohort. Different incidence rates were reported for each PM in different populations.Citation14,Citation15 Regarding secondary primary CRC, the cumulative incidence rate of 12% observed in the present study was considerably higher than the 3%–6.3% rate reported in previous studies.Citation16,Citation17 The difference could be attributed to the fact that the current study involved all the SPMs related to all body sites besides secondary cancer located at the residual part of the colorectum, as well as a much longer follow-up duration. The risk of SPMs increased with time from the diagnosis of primary CRC, mostly in the first 5 years. According to the current National Comprehensive Cancer Network guidelines, CRC patients should be screened regularly for disease recurrence or metastasis. The results of the present study support an intensive follow-up strategy in the first 5 years for CRC patients and highlight the possibility of SPM occurrence. In addition, the risk of subsequent CRC increased more obviously in patients with left-sided colon cancer, followed by those with right-sided colon cancer and rectal cancer; therefore, colonoscopy surveillance is especially important in these patients during their follow-up visits.
The lower the age of patients at the time of diagnosis of primary CRC, the higher the risk of developing SPMs, with an SIR >5 in patients 40 years or younger and an SIR of 2.6 in patients <50 years. In an earlier study, old patients were found to be at higher risk than young patients.Citation11 Age ≥70 years was even considered an independent predictor for second primary cancer. However, the present study indicated high risk in young patients, in agreement with most previous studies,Citation12,Citation18,Citation19 and this risk existed regardless of sex and prior tumor location. CRC patients aged ≤50 years, especially those with a positive family history, were at higher risk of secondary primary cancers, suggesting that they should be screened for hereditary CRC.
Lynch syndrome was associated with an increased risk of certain malignancies in the colorectum, uterus, ovary, stomach, small intestine, urine epithelial tissues, central neural system, pancreas, and breast, whereas familial adenomatous polyposis was associated with a high tendency to develop cancers derived from the thyroid and duodenum.Citation20–Citation24 The current study indicated that the risk of cancer was high for the small intestine, colorectum, uterus, renal pelvis, thyroid, and stomach in CRC survivors, especially in young patients, whereas it was low for cancers of the gallbladder, liver, and brain, as well as myeloma and lymphoma, especially in old patients. The sites with increased cancer risk identified in the present study are included in the screening strategy for hereditary CRC. This relationship indicates that multiple malignancies subsequent to CRC and hereditary CRC may be derived from a common entity. Evidence for such a hypothesis was provided by a recent study based on the long-term follow-up of twins, in which the heritability risk was reported to be 15% and 14% for colon and rectal cancer, respectively, consistent with the cumulative incidence of multiple PMs.Citation25 Most previous studies report a low incidence of hereditary CRC. Lynch syndrome, the most common hereditary malignancy, has an incidence of 1% and accounts for 2%–5% of all CRC cases,Citation26,Citation27 which is less than the cumulative incidence of multiple PMs. Hereditary CRC may be recognized as a typical representative group for multiple primary cancers. However, most patients with multiple primary cancers other than hereditary CRC differ from sporadic CRC patients regarding age. Further investigation is necessary to identify additional clinicopathological characteristics for differentiating these patients from all CRC patients.
A strong relation between CRC and urologic cancers was observed in a previous study.Citation28 Among CRC patients, the risk was reported to increase for prostate and esophagus cancers in one study and for lung cancer in another study.Citation13,Citation29 The current study indicated an increased risk of cancers of the digestive system, urinary tract, and endocrine system in overall CRC patients, specifically for cancers of the respiratory system and genital system in women, whereas a similar or even reduced risk of malignancies of the nervous system and hematologic system was observed. Regarding cell derivation, epithelial tissues from all the systems at increased cancer risk were traced to stem cells from the embryonic endoderm, whereas systems with unchanged or reduced cancer risk stemmed from the ectoderm and mesoderm. This indicated a high concordance between the origin of tissue and sites where multiple PMs occurred. Because of this phenomenon, genetic susceptibility, a known risk factor for cancer, was considered the main pathogenetic factor in multiple PMs, especially genes with a high incidence of germ-line mutations, which play an important role in hereditary CRC.
CRC patients with genetic variations are at high risk of relative malignancies. Deficient mismatch repair or BRCA1 and BRCA2 variations were associated with increased risk of cancers of the colorectum, uterus, ovary, breast, stomach, and pancreas. MUTYH gene variation was associated with increased risk of colorectum, stomach, uterus, and breast cancers. EPCAM gene variation was associated with increased risk of gastric cancer. Many other genes, including SMAD4, STK11, BMPR1A, TPX, AURKA, and SEMA4A, were also reported to play important roles in the pathogenesis of multiple PMs.Citation30–Citation33 In addition to the genes listed, genome-sequencing technology has shown that germ-line mutations of POLE and POLD1 are centralized in patients with multiple CRC and associated with endometrial cancer predisposition.Citation34
The association between the risk of multiple PMs and the primary CRC subsite was reported previously in a study by Lee et al.Citation11 These authors showed that the risk of thyroid, prostate, ovary, and hematological system cancers increased in patients with colon cancer, whereas patients with rectal cancer showed an increased risk of bone and soft-tissue cancers and a decreased risk of liver and gallbladder cancers. The present study confirmed the difference between patients with colon cancer and rectal cancer, and identified a difference between patients with right-sided and left-sided colon cancer. Studies have indicated that the risk for metachronous colorectal tumors is higher in patients with right-sided colon cancer than in those with left-sided colon cancer;Citation16,Citation35,Citation36 how-ever, other studies have indicated that patients with left-sided colon cancer have higher risk.Citation37,Citation38 Based on a detailed classification of primary CRC subsites in the present study, the risk of multiple primary cancers gradually decreased from patients with right-sided colon cancer to those with left-sided colon cancer and to rectal cancer. The overall risk of all SPMs increased in patients regardless of age and sex.
Moreover, patients with different prior CRC subsites had specific high-risk sites. Patients with right-sided colon cancer were at high risk of developing cancers of the small intestine, ureter, renal pelvis, colorectum, uterus, and thyroid, whereas patients with rectal cancer had higher risk of cancers of the vagina, bladder, and lung. In patients with left-sided colon cancer, the risk decreased for most cancers, except secondary primary CRC. These results suggest that attention should be paid to the specific sites at high cancer risk in each patient during follow-up. Cancers located in the right-sided colon have a high frequency of genetic variation in certain genes, including mismatch repair and BRAF, and these genes are associated with germ-line mutations. The high occurrence of multiple primary cancers in patients with right-sided colon cancer may be supported by the existence of such molecular changes.
A recent study found that cumulative radioactive iodine dose is predictive of all second PMs combined among thyroid cancer survivors.Citation39 Davis et al also found that patients with prostate cancer who receive radiation are at high risk of developing bladder (SIR 1.42) and rectum (SIR 1.7) cancers.Citation15 Consistent with these studies, the present study showed that radiotherapy was associated with high cancer risk, especially for uterus and urinary bladder cancers (). This underscores the importance of protecting the pelvic organs during radiotherapy in patients with rectal cancer. In addition, risk of SPM was analyzed on year of diagnosis, race, SEER summary stage, and tumor grade (–).
The present study had several limitations. First, detailed information on patient follow-up or specific recommendations for CRC surveillance was lacking, as well as specific advice regarding surveillance methods. Second, information on family history could not be obtained from the database, which made it difficult to screen patients with hereditary CRC. However, the incidence of hereditary CRC is low. The large sample size of the present study makes it representative of the entire population. Third, data on genetic variation was lacking, and direct comparisons at the molecular level were not possible. Detailed data on genetic variation are necessary to identify patients at high risk to perform individual surveillance in future studies.
In conclusion, based on a large sample size and long follow-up duration, the present population-based study showed that CRC survivors have an increased risk of developing multiple PMs, especially in the first 5 years after the initial CRC diagnosis and in young patients. The common sites at high risk of multiple PMs were primarily associated with tissues derived from the embryonic endoderm, including epithelial tissues of the digestive, urologic, endocrine, and female genital systems. Patients with different primary CRC subsites showed specific high-cancer-risk sites. These results suggest that genetic susceptibility is the main mechanism underlying multiple-cancer risk. Detailed information on genetic variation is necessary to identify patients at high risk of developing multiple primary cancers and to design individual surveillance programs.
Supplementary materials
Table S1 Risk of subsequent primary malignancies grouped by radiotherapy among patients with rectal cancer
Table S2 Risk of subsequent primary malignancies grouped by year of prior colorectal cancer diagnosis
Table S3 Risk of subsequent primary malignancies grouped by race among patients with prior colorectal cancer
Table S4 Risk of subsequent primary malignancies grouped by SEER summary stage of prior colorectal cancer
Table S5 Risk of subsequent primary malignancies grouped by grade of prior colorectal cancer
Disclosure
The authors report no conflicts of interest in this work.
References
- ChenWZhengRBaadePDCancer statistics in China, 2015CA Cancer J Clin201666211513226808342
- KimBChoiARParkSJLong-term outcome and surveillance colonoscopy after successful endoscopic treatment of large sessile colorectal polypsYonsei Med J20165751106111427401640
- MoorcraftSYLadasGBowcockAChauIManagement of resectable colorectal lung metastasesClin Exp Metastasis201633328529626659389
- ElezEArgilésGTaberneroJFirst-line treatment of metastatic colorectal cancer: interpreting FIRE-3, PEAK, and CALGB/SWOG 80405Curr Treat Options Oncol201516115226374340
- DasAChakACooperGSTemporal trend in relative risk of second primary colorectal cancerAm J Gastroenterol200610161342134716771959
- GreenRJMetlayJPPropertKSurveillance for second primary colorectal cancer after adjuvant chemotherapy: an analysis of Intergroup 0089Ann Intern Med2002136426126911848723
- ParkIJYuCSKimHCJungYHHanKRKimJCMetachronous colorectal cancerColorectal Dis20068432332716630238
- BallestéBBessaXPiñolVDetection of metachronous neoplasms in colorectal cancer patients: identification of risk factorsDis Colon Rectum200750797198017468913
- NewtonKFGreenKWalshSLallooFHillJEvansDGMetachronous colorectal cancer risk in patients with a moderate family historyColorectal Dis201315330931622943508
- CirilloLUrsoEDParrinelloGHigh risk of rectal cancer and of metachronous colorectal cancer in probands of families fulfilling the Amsterdam criteriaAnn Surg2013257590090422968081
- LeeYTLiuCJHuYWIncidence of second primary malignancies following colorectal cancer: a distinct pattern of occurrence between colon and rectal cancers and association of co-morbidity with second primary malignancies in a population-based cohort of 98,876 patients in TaiwanMedicine (Baltimore)20159426e107926131831
- LiangYHShaoYYChenHMYoung patients with colorectal cancer have increased risk of second primary cancersJpn J Clin Oncol201545111029103526386042
- LeeJWKimJWKimNKClinical characteristics of colorectal cancer patients with a second primary cancerAnn Coloproctol2014301182224639966
- KatoTSuzukiKMutoYMultiple primary malignancies involving primary sporadic colorectal cancer in Japan: incidence of gastric cancer with colorectal cancer patients may be higher than previously recognizedWorld J Surg Oncol2015132325889477
- DavisEJBeebe-DimmerJLYeeCLCooneyKARisk of second primary tumors in men diagnosed with prostate cancer: a population-based cohort studyCancer2014120172735274124842808
- GervazPBucherPNeyroud-CasparISoraviaCMorelPProximal location of colon cancer is a risk factor for development of metachronous colorectal cancer: a population-based studyDis Colon Rectum200548222723215711864
- CaliRLPitschRMThorsonAGCumulative incidence of metachronous colorectal cancerDis Colon Rectum19933643883938458267
- NelsonRMetachronous colorectal cancer and ageColorectal Dis200689812
- ShureiqiICooksleyCDMorrisJSolimanASLevinBLippmanSMEffect of age on risk of second primary colorectal cancerJ Natl Cancer Inst200193161264126611504772
- HemminkiKLiXDongCSecond primary cancers after sporadic and familial colorectal cancerCancer Epidemiol Biomarkers Prev200110779379811440965
- StoffelEMManguPBGruberSBHereditary colorectal cancer syndromes: American Society of Clinical Oncology clinical practice guideline endorsement of the familial risk – colorectal cancer: European Society for Medical Oncology clinical practice guidelinesJ Clin Oncol201533220921725452455
- MøllerPSeppäläTBernsteinICancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome databaseGut Epub2015129
- GerminiDEMaderAMGomesLGTeodoroTRFrancoMIWaisbergJDetection of DNA repair protein in colorectal cancer of patients up to 50 years old can increase the identification of Lynch syndrome?Tumour Biol20163722757276426408182
- Casellas-CabreraNDíaz-AlgorriYCarlo-ChévereVJRisk of thyroid cancer among Caribbean Hispanic patients with familial adenomatous polyposisFam Cancer201615226727426690363
- MucciLAHjelmborgJBHarrisJRFamilial risk and heritability of cancer among twins in Nordic countriesJAMA20163151687626746459
- SirajAKPrabhakaranSBaviPPrevalence of Lynch syndrome in a Middle Eastern population with colorectal cancerCancer2015121111762177125712738
- LynchHTBolandCRGongGPhenotypic and genotypic heterogeneity in the Lynch syndrome: diagnostic, surveillance and management implicationsEur J Hum Genet200614439040216479259
- CalderwoodAHHuoDRubinDTAssociation between colorectal cancer and urologic cancersArch Intern Med200816891003100918474765
- BirgissonHWallinUHolmbergLGlimeliusBSurvival endpoints in colorectal cancer and the effect of second primary other cancer on disease free survivalBMC Cancer20111143821989154
- LuCXieMWendlMCPatterns and functional implications of rare germline variants across 12 cancer typesNature Commun201561008626689913
- SussweinLRMarshallMLPathogenic and likely pathogenic variant prevalence among the first 10,000 patients referred for next-generation cancer panel testingGenet Med201618882383226681312
- SchulzEKlampflPHolzapfelSGermline variants in the SEMA4A gene predispose to familial colorectal cancer type XNature Commun20145519125307848
- YunHRYiLJChoYKDouble primary malignancy in colorectal cancer patients: MSI is the useful marker for predicting double primary tumorsInt J Colorectal Dis200924436937518797888
- PallesCCazierJBHowarthKMGermline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomasNat Genet201345213614423263490
- LeeTJReesCJBlanksRGColonoscopic factors associated with adenoma detection in a national colorectal cancer screening programEndoscopy201446320321124473907
- YoshidaNNaitoYHoKTHigh incidence of metachronous advanced adenoma and cancer after endoscopic resection of colon polyps larger than or equal to 20 mm in sizeDig Endosc201528219
- RennertGRobinsonERennertHSNeugutAIClinical characteristics of metachronous colorectal tumorsInt J Cancer19956067437477896438
- BordaAMartínez-PeñuelaJMBordaFMuñoz-NavasMJiménezFJCarreteroCDrawing up an individual risk index for development of metachronous neoplastic lesions in resected colorectal cancerRev Esp Enferm Dig2012104629129722738698
- TengCJHuYWChenSCUse of radioactive iodine for thyroid cancer and risk of second primary malignancy: a nationwide population-based studyJ Natl Cancer Inst20161082djv31426538627
