Abstract
Excessive chronic alcohol consumption has become a worldwide health problem. The oncogenic effect of chronic alcohol consumption is one of the leading concerns. The mechanisms of alcohol-induced tumorigenesis and tumor progression are largely unknown, although many factors have been implicated in the process. This review discusses the recent progress in this research area with concentration on alcohol-induced dysregulation of cytokines and chemokines. Based on the available evidence, we propose that alcohol promotes tumor progression by the dysregulation of the cytokine/chemokine system. In addition, we discuss specific transcription factors and signaling pathways that are involved in the action of these cytokines/chemokines and the oncogenic effect of alcohol. This review provides novel insight into the mechanisms of alcohol-induced tumor promotion.
Introduction
Alcohol is a tumor promoter.Citation1 Alcohol is involved in tumor promotion by not only alcohol itself but also the derivatives of alcohol, such as acetaldehyde and free radicals that are generated in the metabolic process. For example, acetaldehyde, proteins, and DNA may form adduct, which leads to genetic mutations that induce tumorigenesis. Alcohol abuse is defined as intake of alcohol that exceeds 21 units weekly or 3 units per day. Alcohol abuse is a major risk factor for tumors, especially the upper gastrointestinal tract (oral cavity, pharynx, larynx, and esophagus) tumors, hepatocellular carcinoma (HCC), colorectal cancer, and breast cancer.Citation2–Citation6 Furthermore, in many types of tumors, including gastrointestinal tract, gynecological, brain, and renal tumors, the activity of alcohol dehydrogenase is significantly increased.Citation7
Alcohol exposure also enhances the progression and metastasis of a variety of tumors, such as skin, liver, lung, breast, and colon cancers.Citation8–Citation13 Chronic alcohol consumption is often associated with poor clinical prognosis.Citation9 However, the underlying cellular and molecular mechanisms are complex.
Alcohol consumption is one of the risk factors that correlate with high cancer mortality rates,Citation14 and this is mainly because chronic alcohol consumption often leads to advanced TNM stages, greater vessel invasion, and poorer prognosis.Citation9 The underlying mechanism may be that alcohol consumption activates the expression of some cytokines, such as VEGF, MCP-1, and NF-κB, which are associated with tumor progression and metastasis.Citation8–Citation10
This review discusses the current research progress in the cellular and molecular mechanisms underlying alcohol-induced tumor promotion. We specifically focus on the role of cytokines and chemokines in alcohol-promoted tumor progression and aggressiveness.
Alcohol-induced dysregulation of cytokines and chemokines
Cytokines are small molecule proteins with a broad range of biological activities. They are normally synthesized and secreted by the immune cells, such as monocytes, macrophages, T cells, B cells, and natural killer cells, as well as some nonimmune cells, such as endothelial cells and fibroblasts. Cytokines regulate cell growth and differentiation by binding to the specific receptors and mediate the immune response.Citation15,Citation16 Chemokines are chemotactic cytokines that mediate the migration and positioning of the target cells.Citation17 In addition to mediating immune response, chemokines are also involved in regulating the embryonic development, angiogenesis, and apoptosis. More importantly, chemokines are now known to play a vital role in tumorigenesis, tumor progression, and metastasis.Citation18,Citation19 The most well-investigated cytokines and chemokines include VEGF, MCP-1, TLR, and TNF-α. Alcohol exposure affects the cytokine and chemokine systems.Citation20–Citation22 Particularly, the effects of alcohol on VEGF and MCP-1 receive great attention due to their roles in tumorigenesis and progression.
VEGF
VEGF is a highly conservative glycoproteinCitation23,Citation24 and plays an important role in angiogenesis, invasion of tumors,Citation25,Citation26 cell proliferation, and vascular permeability.Citation27–Citation29 VEGF is significantly upregulated in tumor tissues and positively correlated with malignancy.Citation30,Citation31 It is proposed that alcohol promotes tumor growth, angiogenesis, and metastasis in skin cancer, liver cancer, and breast cancer by increasing the VEGF levels.Citation8,Citation32 In addition, oxidative stress and NF-κB mediate the alcohol-induced VEGF upregulation.Citation9 However, the exact signaling mechanisms are still largely unknown.
MCP-1
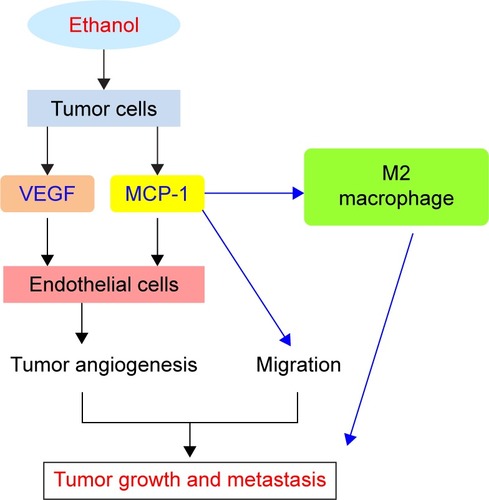
MCP-1, also called CCL2, activates the mononuclear cells and macrophages by increasing the intracytoplasmic Ca2+ concentration and releasing the superoxide anion and lysozyme.Citation33 Moreover, many studies also demonstrated that MCP-1 plays essential roles in tumor microenvironment by regulating tumor angiogenesis, tumor growth, and metastasis.Citation34–Citation36 Alcohol exposure upregulates MCP-1 and recruits M2 macrophages in tumor microenvironment, which, in turn, promotes the growth and metastasis of lung cancer ().Citation10 More importantly, it is reported that alcohol promotes the growth and angiogenesis of breast cancer through activation of MCP-1 and its receptor CCR2 ().Citation11 Consistent with this idea, antagonists of CCR2 can inhibit tumor angiogenesis induced by alcohol.Citation11 Alcohol can also promote the migration and invasion of colorectal cancer cells (CSCs) by regulating the GSK3β/β-catenin/MCP-1 signaling pathway.Citation12 However, the exact mechanisms of how MCP-1 mediated the effects, ie, alcohol stress-induced tumorigenesis and tumor growth, are still unclear.
Figure 1 Role of VEGF and MCP-1 in alcohol-induced tumor growth and metastasis.
TLR
TLR is a type I transmembrane receptor and pathogenic pattern recognition receptor, which participates in natural immunity. TLR plays an important role in cellular immune responses.Citation37 Furthermore, some cytokines also regulate stem cells. For example, TLR induces NANOG gene expression, which is involved in the regulation of tumor-initiating stem-like cells (TICs).Citation38
Recent studies found that TLR may play an important role in alcohol-induced liver cancer by regulating the TICs,Citation38 at least partially through Nanog, one of the embryonic stem cell markers, which, in turn, regulates the proliferation and differentiation of liver stem/progenitor cells.Citation39 Once activated, the proportion of CD133/Nanog-positive cells increased in liver cancer.Citation40 Conversely, knocking off TLR genes can prevent tumor growth to a certain extent.Citation41 Interestingly, studies found that ethanol-treated malignant cells fused to dendritic cells (DCs) exposed to TLR stimulation, thus promoting abnormal cellular immune response, which is thought to be an important mechanism of tumor promotion.Citation42
Overall, these data suggested that chronic alcohol stress may play an important role in tumor promotion at least partially through the TLR/Nanog pathway.
TNF-α
TNF-α is a type II membrane protein, which is mainly secreted by the activated mononuclear macrophages and could act either as a tumor suppressor or as an oncogene. TNF-α plays an antitumor role by inducing apoptosis, inhibiting tumor angiogenesis, and enhancing host immunity.Citation43,Citation44 TNF-α also acts as an oncogene by upregulating inflammatory cytokines, inhibiting apoptosis, and activating multiple signaling pathways, causing tumor-associated gene mutation.Citation45,Citation46 The increased TNF-α in tumor microenvironment can promote tumor cell migration and invasion,Citation47 which is associated with metastasis, recurrence, and poor prognosis of cancer patients.Citation48 More importantly, chronic ethanol exposure can upregulate TNF-α, which, in turn, facilities infiltration of monocytes/macrophages in liver and, therefore, promotes liver lesions. For instance, TNF increased significantly in the liver tissue of alcohol-fed mice.Citation49 However, the underlying mechanisms of TNF-α variation in alcohol-induced tumor promotion need to be further explored.
Alcohol-induced dysregulation of transcription factors mediated by cytokines and chemokines
Cytokines and chemokines directly or indirectly regulate transcription factors, which play key roles in integrating the multiple extracellular signaling pathways and regulating gene expression. The transcription factors known to be regulated by cytokines and chemokines include Egr-1, AP-1, and STAT.
Alcohol exposure affects the activity of transcription factors. Some of these transcription factors are known to be involved in the action of cytokines and chemokines as well as tumorigenesis/progression.Citation50–Citation53 In the present study, we focus on the three transcription factors that are affected by alcohol exposure, namely, Egr-1, STAT, and AP-1.
Egr-1
Egr-1 is a nuclear transcription factor that is regulated by the mitogen-activated protein kinase (MAPK) signaling pathway.Citation54 Egr-1 binds to the promoter region of TNF-α and increases the sensitivity of liver macrophages.Citation55 Egr-1 is upregulated in the process of alcoholic cirrhosis.Citation50 Lipopolysaccharide (LPS)-induced activation of Egr-1 in the liver Kupffer cells contributes to the pathogenesis of alcoholic liver disease.Citation51 In addition, chronic alcohol exposure also induces Egr-1 to bind to the promoter region of the TNF-α and increases the sensitivity of liver macrophages.Citation55 Alcohol-induced liver cirrhosis may in the long run increase the risk of liver carcinogenesis.
Egr-1 also plays an important role in the process of tumorigenesis.Citation56 Egr-1 has been found highly expressed in gastric cancer and bladder cancer,Citation57,Citation58 and it may upregulate invasion and metastasis-related genes, such as matrix metalloproteinases (MMP3).Citation59 However, whether alcohol promotes tumor metastasis by activating Egr-1 is largely unknown.
STAT
The activity of STAT is regulated by JAK-mediated signal transduction, which can be activated by cytokines and chemokines.Citation60 MAPK/STAT3 signaling pathway increases the DNA binding ability of AP-1 through the activation of Src kinases, thereby inducing the production of IL-10.Citation61,Citation62
During alcoholic liver injury, STAT3 modulates the liver immune response in a cell-type-dependent manner. For example, in liver cells, STAT3 exerts a pro-inflammatory role, whereas in mononuclear cells and Kupffer cells, STAT3 plays an anti-inflammatory role.Citation52 In addition, the decreased liver regeneration capacity of patients with alcoholic cirrhosis is related to the decreased activity of STAT3, revealing a positive correlation between STAT3 and liver regeneration ability.Citation53 Nevertheless, it is still unclear how ethanol exposure regulates STAT3 in liver cancer.
AP-1
AP-1 is a transcription factor that belongs to the leucine zipper protein family.Citation63 AP-1 responds to cytokines by regulating the expression of a number of genes related to carcinogenesis and tumor progression. AP-1 and other transcription factors, including STAT3, SP-1, and SP-3, are all involved in LPS-induced production of IL-10 in monocytes and T cells.Citation64–Citation67 Meanwhile, it has been demonstrated that alcohol exposure activates the Src/MAPK/STAT3 signaling pathway and increases the DNA binding ability of AP-1, thereby inducing the production of IL-10.Citation61 In the context of liver injury, alcohol exposure enhances the sensitivity of liver macrophages via upregulating AP-1 and increasing the expression of CD14.Citation68 Alcohol exposure can also activate AP-1 in a protein kinase C (PKC)-dependent manner and enhance proliferation ability of liver cells by increasing the expression of c-Jun and c-Fos in the liver cells.Citation69 Alcohol-induced liver injury may also in the long run increase the risk of liver carcinogenesis.
Alcohol-induced dysregulation of signaling pathways in the action of cytokines and chemokines
There are a number of signaling pathways that are regulated by cytokines and chemokines and play an important role in tumorigenesis and progression. On the other hand, these signaling pathways can also modulate the expression of cytokines and chemokines. The most important signaling pathways involved in the action of cytokines and chemokines are NF-κB-TNF-α signaling pathway, MAPK signaling pathway, and Wnt/β-catenin signaling pathway.
Alcohol can cause the dysregulation of some signaling pathways that are involved in the action of cytokines, chemokines, tumorigenesis, and progression. In the present review, we discuss the three most important signaling pathways affected by alcohol exposure.
NF-κB-TNF-α signaling pathway
TNF-α is involved in multiple signaling pathways and can activate NF-κB which, in turn, also participates in regulating TNF-α.Citation70 LPS-induced oxidative stress activates NF-κB, increasing TNF-α production in Kupffer cells. LPS activates Kupffer cells and induces the production of superoxides that activate NF-κB. Once activated, NF-κB is translocated to the nucleus and regulates the expression of several critical inflammatory cytokines, particularly TNF-α.Citation71 Blocking the activation of NF-κB markedly decreases cytokine production and ameliorates inflammation and necrosis.Citation72 It has been reported that alcohol-induced liver damage is associated with the activation of the NF-κB signaling pathway as well as the high expression of pro-inflammatory cytokines and upregulation of TLR3 and TLR7.Citation73 It is believed that alcohol exposure regulates the inflammatory response of hepatic macrophages at least partially through the NF-κB signaling pathway. Alcohol can activate the NF-κB signaling pathway by affecting its nuclear localization, formation of NF-κB p65/p50 dimmers, and DNA binding activity.Citation71,Citation73,Citation74 In the mouse model of alcohol-induced liver injury, alcohol consumption causes a significant increase in DNA binding activity of NF-κB in the liver.Citation73
Our recent study demonstrated that alcohol exposure activates the NF-κB signaling pathway and upregulates its target cytokines and chemokines, VEGF and MCP-1, which promote angiogenesis, growth, and metastasis of liver cancer cells.Citation9
MAPK signaling pathway
MAPK signaling pathway is involved in mediating the tumorigenesis and metastasis.Citation75 Cytokines and chemokines can activate three members of the MAPK family, namely, ERK1/2, p38 MAPK, and JNK/SAPK.Citation76,Citation77 Among them, the p38 MAPK pathway is required for the induction of TNF-α-RNA-binding activity and mediates stabilization of TNF-α mRNA in myeloid cells stimulated with bacterial LPS.Citation78 Alcohol affects the MAPK family and activates the three members of this family, namely ERK, p38 MAPK, and JNKs. A recent study showed that alcohol promotes malignant progression of breast cancer by activating the p38γ MAPK/RhoC signaling pathway.Citation79 Alcohol activates p38 MAPK in mononuclear cells, which generates anti-inflammatory cytokines such as IL-10.Citation80 Similarly, it is reported that alcohol stimulates the liver macrophages to produce TNF-α through the activation of ERK1/2.Citation81 Alcohol exposure also activates Src/MAPK/STAT3 and increases the binding ability of AP-1.Citation61 Meanwhile, JNK was activated by alcohol in breast cancer cells and required for alcohol-induced cell invasion.Citation82 Since MAPKs play an important role in tumorigenesis and progression, the effect of alcohol on MAPKs has a significant impact on tumor promotion.
Wnt/β-catenin signaling pathway
Wnt/β-catenin signaling pathway regulates tumor growth and metastasisCitation83 as well as self-renewal and differentiation of cancer stem cells.Citation84 Some cytokines are implicated in the regulation of Wnt/β-catenin signaling. On the one hand cytokine such as TNF-α activates Wnt/β-catenin signaling, on the other hand Wnt/β-catenin signaling also affects specific cytokines.Citation34,Citation85,Citation86 For example, IL-1β and TNF-α induced rat nucleus pulposus cells apoptosis by activating the caveolin-1/Wnt/β-catenin signaling pathway.Citation85 TNF-α-induced NF-κB activation inhibits osteogenesis of mesenchymal stem cells by targeting Wnt/β-catenin signaling.Citation86 Furthermore, MCP-1 is a target of the β-catenin/TCF/LEF pathway in breast tumor cells and plays a key role in breast tumor progression.Citation34
Recent studies demonstrated that alcohol activates the Wnt/β-catenin signaling pathway and promotes malignant proliferation of cancer cells and tumor formation in the liver.Citation87,Citation88 Our previous study found that the Wnt/β-catenin pathway mediates alcohol-stimulated MCP-1 gene transcription and enhances CSC metastasis.Citation12 Whether the Wnt/β-catenin signaling pathway is activated by alcohol stress in other tumors, such as breast cancer, colon cancer, and the underlying molecular mechanisms are undiscovered.
Cytokines and chemokines as potential therapeutic targets
Cytokines and chemokines are potential therapeutic targets for the treatment of alcohol-induced tumor promotion as well as other alcohol-related diseases. They may be used as biomarkers for alcohol-induced organ damage. For example, MCP-1 levels in cerebrospinal fluid, together with gamma glutamyltransferase (GGT) and aspartate aminotransferase/glutamic oxaloacetic transaminase (AST/GOT) in peripheral blood, are indicators of clinical alcoholic hepatitis in alcoholics.Citation89 Recent studies showed that blocking NF-κB-regulated MCP-1 and VEGF expression inhibits alcohol-induced tumor formation ability of liver cancer cells.Citation9 Similarly, blocking Wnt/β-catenin signaling inhibits alcohol-stimulated MCP-1 expression and alleviates alcohol-induced invasion of colon cancer cells.Citation12 These studies suggest that targeting cytokines and chemokines may be a potential therapeutic strategy for cancer treatment, particularly in the context of alcohol-induced tumor promotion.
Conclusion
Excessive alcohol consumption is a risk factor for cancer. Alcohol promotes tumorigenesis, progression, and metastasis.
Alcohol causes dysregulation of cytokines and chemokines, which may mediate alcohol-induced tumor promotion. Alcohol may also affect transcription factors and signaling pathways that regulate the expression/function of cytokines and chemokines.
Systematic screening of cytokines and chemokines that are affected by alcohol during tumorigenesis and cancer progression is necessary for providing more insight into the role of cytokines and chemokines in alcohol-induced tumor promotion. Targeting cytokines and chemokines could be a potential therapeutic strategy for cancer treatment, particularly in the context of alcohol-induced tumor promotion/progression.
Acknowledgments
This work was supported by the Project of the National Natural Sciences Foundation of China (81272258, 81572749).
Disclosure
The authors report no conflicts of interest in this work.
References
- PöschlGStickelFWangXDSeitzHKAlcohol and cancer: genetic and nutritional aspectsProc Nutr Soc2004631657115070439
- ScocciantiCCecchiniMAndersonASEuropean code against cancer 4th edition: alcohol drinking and cancerCancer Epidemio201539suppl 1S67S74
- PöschlGSeitzHKAlcohol and cancerAlcohol Alcohol200439315516515082451
- BaanRStraifKGrosseYWHO International Agency for Research on Cancer Monograph Working GroupCarcinogenicity of alcoholic beveragesLancet Oncol20078429229317431955
- RehmJBaliunasDBorgesGLThe relation between different dimensions of alcohol consumption and burden of disease: an overviewAddiction2010105581784320331573
- SuzukiRYeWRylander-RudqvistTSajiSColditzGAWolkAAlcohol and postmenopausal breast cancer risk defined by estrogen and progesterone receptor status: a prospective cohort studyJ Natl Cancer Inst200597211601160816264180
- OrywalKSzmitkowskiMAlcohol dehydrogenase and aldehyde dehydrogenase in malignant neoplasmsClin Exp Med Epub2016217
- TanWBaileyAPShparagoMChronic alcohol consumption stimulates VEGF expression, tumor angiogenesis and progression of melanoma in miceCancer Biol Ther2007681211121717660711
- WangFYangJLYuKKActivation of the NF-κB pathway as a mechanism of alcohol enhanced progression and metastasis of human hepatocellular carcinomaMol Cancer2015141025622857
- YuKYangJWangFAlcohol supports macrophage recruitment and reinforces invasion and migration of lewis lung carcinomaAlcohol Clin Exp Res201438102597260625346504
- WangSXuMLiFAlcohol promotes mammary tumor growth and angiogenesis: the involvement of chemoattractant factor MCP-1Breast Cancer Res Treat201213331037104822160640
- XuMWangSQiYRole of MCP-1 in alcohol-induced aggressiveness of colorectal cancer cellsMol Carcinog20165551002101126014148
- ImHJKimHGLeeJSA preclinical model of chronic alcohol consumption reveals increased metastatic seeding of colon cancer cells in the liverCancer Res20167671698170426857263
- LicajISandinSSkeieGAlcohol consumption over time and mortality in the Swedish Women’s Lifestyle and Health cohortBMJ Open2016611e012862
- RamjiDPDaviesTSCytokines in atherosclerosis: key players in all stages of disease and promising therapeutic targetsCytokine Growth Factor Rev201526667368526005197
- MossJWRamjiDPCytokines: roles in atherosclerosis disease progression and potential therapeutic targetsFuture Med Chem20168111317133027357616
- Van der VorstEPDöringYWeberCChemokinesArterioscler Thromb Vasc Biol201535115256
- AryaMPatelHRWilliamsonMChemokines: key players in cancerCurr Med Res Opin200319655756414594528
- BalkwillFChemokine biology in cancerSemin Immunol2003151495512495640
- DanilukJKandefer-SzerszeńMThe effect of alcohol on the immune system and cytokinesPostepy Hig Med Dosw199852149659608231
- KawarataniHTsujimotoTDouharaAThe effect of inflammatory cytokines in alcoholic liver diseaseMediators Inflamm2013201349515624385684
- AnLWangXCederbaumAICytokines in alcoholic liver diseaseArch Toxicol20128691337134822367091
- TischerEMitchellRHartmanTThe human gene for vascular endothelial growth factor, multiple protein forms are encoded through alternative exon splicingJ Biol Chem19912661811947119541711045
- FerraraNVascular endothelial growth factor: molecular and biological aspectsCurr Top Microbiol Immunol19992371309893343
- YancopoulosGDDavisSGaleNWRudgeJSWiegandSJHolashJVascular-specific growth factors and blood vessel formationNature2000407680124224811001067
- TsaiMSChangCCKuoMLWuYTVascular endothelial growth factor-A and changes in a tumor-bearing mouse model with Lewis lung cancerOncol Lett2011261143114722848279
- Claesson-WelshLWelshMVEGFA and tumour angiogenesisJ Intern Med2013273211412723216836
- FerraraNGerberHPLeCouterJThe biology of VEGF and its receptorsNat Med20039666967612778165
- HicklinDJEllisLMRole of the vascular endothelial growth factor pathway in tumor growth and angiogenesisJ Clin Oncol20052351011102715585754
- El-AssalONYamanoiASodaYClinical significance of microvessel density and vascular endothelial growth factor expression in hepatocellular carcinoma and surrounding liver: possible involvement of vascular endothelial growth factor in the angiogenesis of cirrhotic liverHepatology1998276155415629620326
- MiseMAriiSHigashitujiHClinical significance of vascular endothelial growth factor gene expression in liver tumorHepatology19965455464
- LuYNiFXuMAlcohol promotes mammary tumor growth through activation of VEGF-dependent tumor angiogenesisOncol Lett20148267367825009649
- McClellanJLDavisJMSteinerJLLinking tumor-associated macrophages, inflammation, and intestinal tumorigenesis: role of MCP-1Am J Physiol Gastrointest Liver Physiol20123031010871095
- MestdagtMPoletteMButticeGTransactivation of MCP-1/CCL2 by β-catenin/TCF-4 in human breast cancer cellsInt J Cancer20061181354216003740
- YoshidomeHKohnoHShidaTSignificance of monocyte chemoattractant protein-1 in angiogenesis and survival in colorectal liver metastasesInt J Oncol200934492393019287949
- Armaiz-PenaGNGonzalez-VillasanaVNagarajaASAdrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growthOncotarget2015664266427325738355
- MatsuokaYTakagiHYamataniMKurodaYSatoKKojimaNRequirement of TLR4 signaling for the induction of a Th1 immune response elicited by oligomannose-coated liposomesImmunol Lett2016163013130136
- MachidaKFeldmanDETsukamotoHTLR4-dependent tumor-initiating stem cell-like cells (TICs) in alcohol-associated hepatocellular carcinogenesisAdv Exp Med Biol201581513114425427905
- MachidaKTsukamotoHMkrtchyanHToll-like receptor 4 mediates synergism between alcohol and HCV in hepatic oncogenesis involving stem cell marker NanogProc Natl Acad Sci U S A200910651548155319171902
- MachidaKTLRs, alcohol, HCV, and tumorigenesisGastroenterol Res Pract2010201051867421331379
- FrenchSWOlivaJFrenchBALiJBardag-GorceFAlcohol, nutrition and liver cancer: role of Toll-like receptor signalingWorld J Gastroenterol201016111344134820238401
- KoidoSHommaSOkamotoMImproved immunogenicity of fusions between ethanol-treated cancer cells and dendritic cells exposed to dual TLR stimulationOncoimmunology201328e2537524167764
- KossMPfeifferGRWangYEzrin/radixin/moesin proteins are phosphorylated by TNF-α and modulate permeability increases in human pulmonary microvascular endothelial cellsJ Immunol200617621218122716394012
- ZhangWChenZLiFTumour necrosis factor-alpha (TNF-alpha) transgene-expressing dendritic cells (DCs) undergo augmented cellular maturation and induce more robust T-cell activation and anti-tumour immunity than DCs generated in recombinant TNF-alphaImmunology2003108217718812562326
- AkiyamaMHideshimaTHayashiTNuclear factor-kappaB p65 mediates tumor necrosis factor alpha-induced nuclear translocation of telomerase reverse transcriptase proteinCancer Res2003631182112517770
- SoriaGOfri-ShahakMHaasIInflammatory mediators in breast cancer: coordinated expression of TNF-α & IL-β with CCL2 & CCL5 and effects on epithelial-to-mesenchymal transitionBMC Cancer20111114711491
- RadhakrishnanPChachadiVLinMFSinghRKannagiRChengPWTNFα enhances the motility and invasiveness of prostatic cancer cells by stimulating the expression of selective glycosyl and sulfotransferase genes involved in the synthesis of selectin ligandsBiochem Biophys Res Commun2011409343644121596021
- FerrajoliAKeatingMJManshouriTThe clinical significance of tumor necrosis factor-α plasma level in patients having chronic lymphocytic leukemiaBlood200210041215121912149200
- OllerosMLMartinMLVesinDFat diet and alcohol-induced steatohepatitis after LPS challenge in mice: role of bioactive TNF and Th1 type cytokinesCytokine200844111812518722787
- DonohueTMJrOsnaNATramblyCSEarly growth response-1 contributes to steatosis development after acute ethanol administrationAlcohol Clin Exp Res201236575976722141421
- KishoreRHillJRMcMullenMRFrenkelJNagyLEERK1/2 and Egr-1 contribute to increased TNF-α production in rat Kupffer cells after chronic alcohol feedingAm J Physiol Gastrointest Liver Physiol20022821G6G1511751152
- HoriguchiNWangLMukhopadhyayPCell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injuryGastroenterology200813441148115818395093
- ChenJBaoHSawyerSKunosGGaoBEffects of short and long term ethanol on the activation of signal transducer and activator transcription factor 3 in normal and regenerating liverBiochem Biophys Res Commun199723936666699367825
- GashlerASukhatmeVPEarly growth response protein 1 (Egr-1) prototype of a zinc-finger family of transcription factorsProg Nucleic Acid Res Mol Biol1995501912247754034
- PritchardMTNagyLEAlcohol-induced liver injury: potential roles for egr-1Alcohol Clin Exp Res20052911 suppl146S150S16344600
- MitchellACrispinRDassLThe neuroplasticity-associated gene is a direct transcriptional target of early growth response (Egr) transcription factorsMol Cell Biol200525102810300
- KobayashiDYamadaMKamagataCOverexpression of early growth response-1 as a metastasis-regulatory factor in gastric cancerAnticancer Res2002226C3963397012553019
- EgerodFLNielsenHSIversenLThorupIStorgaardTOleksiewiczMBBiomarkers for early effects of carcinogenic dual-acting PPAR agonists in rat urinary bladder urothelium in vivoBiomarkers200510429530916240504
- KatoNKobayashiTHondaHScreening of stress enhancer based on analysis of gene expression profiles: enhancement of hyperthermia-induced tumor necrosis by an MMP-3 inhibitorCancer Sci200394764464912841876
- KisselevaTBhattacharyaSBraunsteinJSchindlerCWSignaling through the JAK/STAT pathway, recent advances and future challengesGene20022851–212412039028
- NorkinaODolganiucAShapiroTKodysKMandrekarPSzaboGAcute alcohol activates STAT3, AP-1, and Sp-1 transcription factors via the family of Src kinases to promote IL-10 production in human monocytesJ Leukoc Biol200782375276217575268
- NorkinaODolganiucACatalanoDAcute alcohol intake induces SOCS1 and SOCS3 and inhibits cytokine-induced STAT1 and STAT3 signaling in human monocytesAlcohol Clin Exp Res20083291565157318616672
- AngelPKarinMThe role of Jun, Fos and the AP-1 complex in cell proliferation and transformationBiochem Biophys Acta199110722–31291571751545
- WangZYSatoHKusamSSehraSToneyIDentARegulation of IL-10 gene expression in Th2 cells by Jun proteinsJ Immunol200517442098210515699140
- MaWLimWGeeKThe p38 mitogen-activated kinase pathway regulates the human interleukin-10 promoter via the activation of Sp1 transcription factor in lipopolysaccharide-stimulated human macrophagesJ Biol Chem200127617136641367411278848
- ToneMPowellMToneYThompsonSWaldmannHIL-10 gene expression is controlled by the transcription factors Sp1 and Sp3J Immunol2000165128629110861063
- BenkhartEMSiedlarMWedelAWernerTZiegler-HeitbrockHRole of Stat3 in lipopolysaccharide-induced IL-10 gene expressionJ Immunol200016531612161710903771
- WheelerMDThurmanRGUpregulation of CD14 in liver due to acute alcohol involves oxidant-dependent AP-1 pathwayJ Biol Chem20032781083458351
- CasiniAGalliGSalzanoRAcetaldehyde induces c-Fos and c-Jun proto-oncogenes in fat-storing cell cultures through protein kinase C activationAlcohol Alcohol19942933033147945571
- DempseyPWDoyleSEHeJQChengGThe signaling adaptors and pathways activated by TNF superfamilyCytokine Growth Factor Rev2003143–419320912787559
- WheelerMDYamashinaSFrohMRusynIThurmanRGAdenoviral gene delivery can inactivate Kupffer cells: role of oxidants in NF-kappaB activation and cytokine productionJ Leukoc Biol200169462263011310849
- NanjiAAJokelainenKRahemtullaAActivation of nuclear factor kappa B and cytokine imbalance in experimental alcoholic liver disease in the ratHepatology199930493494310498645
- StärkelPDe SaegerCStrainAJLeclercqIHorsmansYNFkappaB, cytokines, TLR 3 and 7 expression in human end-stage HCV and alcoholic liver diseaseEur J Clin Invest201040757558420658750
- SzaboGMandrekarPAlcohol-mediated regulation of transcription factors in immunocompetent cellsFront Biosci200278089
- LiuYWZuoPYZhaXNOctacosanol enhances the proliferation and migration of human umbilical vein endothelial cells via activation of the PI3K/Akt and MAPK/Erk pathwaysLipids201550324125125638063
- PetecchiaLSabatiniFUsaiCCaciEVaresioLRossiGACytokines induce tight junction disassembly in airway cells via an EGFR-dependent MAPK/ERK1/2-pathwayLab Invest20129281140114822584669
- KorobowiczABiology of tumor necrosis factor type alpha (TNF-alpha)Pol Merkur Lekarski20062112435836117205778
- MahtaniKRBrookMDeanJLSullyGSaklatvalaJClarkARMitogen-activated protein kinase p38 controls the expression and posttranslational modification of tristetraprolin, a regulator of tumor necrosis factor alpha mRNA stabilityMol Cell Biol200121196461646911533235
- XuMWangSRenZChronic alcohol exposure enhances the aggressiveness of breast cancer: the role of p38γOncotarget2016733489350526655092
- DrechslerYDolganiucANorkinaOHeme oxygenase-1 mediates the anti-inflammatory effects of acute alcohol on IL-10 induction involving p38MAPK activation in monocytesJ Immunol200617742592260016888021
- AgogliaAESharkoACPsilosKEHolsteinSEReidGTHodgeCWAlcohol alters the activation of ERK1/2, a functional regulator of binge alcohol drinking in adult C57BL/6J miceAlcohol Clin Exp Res201539346347525703719
- MaCLinHLeonardSSShiXYeJLuoJOverexpression of erbb2 enhances ethanol-stimulated intracellular signaling and invasion of human mammary epithelial and breast cancer cells in vitroOncogene200322345281529012917629
- RattisFMVoermansCReyaTWnt signaling in the stem cell nicheCurr Opin Hematol2004112889415257024
- MikiTYasudaSYKahnMWnt/β-catenin signaling in embryonic stem cell self-renewal and somatic cell reprogrammingStem Cell Rev20117483684621603945
- WangJChenHCaoPInflammatory cytokines induce caveolin-1/β-catenin signalling in rat nucleus pulposus cell apoptosis through the p38 MAPK pathwayCell Prolif201649336237227125453
- WangNZhouZWuTTNF-α-induced NF-κB activation upregulates microRNA-150-3p and inhibits osteogenesis of mesenchymal stem cells by targeting β-cateninOpen Biol20166315025826935950
- MercerKEHenningsLSharmaNAlcohol consumption promotes diethylnitrosamine-induced hepatocarcinogenesis in male mice through activation of the Wnt/β-catenin signaling pathwayCancer Prev Res (Phila)20147767568524778325
- MercerKEHenningsLRonisMJAlcohol consumption, Wnt/β-catenin signaling, and hepatocarcinogenesisAdv Exp Med Biol201581518519525427908
- UmhauJCSchwandtMSolomonMGCerebrospinal fluid monocyte chemoattractant protein-1 in alcoholics: support for a neuroinflammatory model of chronic alcoholismAlcohol Clin Exp Res20143851301130624689518
