Abstract
Curcumin was recently discovered to strengthen immune response through multiple mechanisms. Cytotoxic CD8+ T-cells play a critical role in modulating anticancer immune response, but is severely restricted by T-cell exhaustion. Bladder carcinomas express PD-L1 and can abrogate CD8+ T-cell response. Thus, we hypothesized that bisdemethoxycurcumin, a natural dimethoxy derivative of curcumin, may provide a favorable environment for T-cell response against bladder cancer when used in combination with α-PD-L1 antibody. Immunocompetent C56BL/6 mouse models bearing subcutaneous or lung metastasized MB79 bladder cancer were established to validate this conjecture. We found that bisdemethoxycurcumin significantly increased intratumoral CD8+ T-cell infiltration, elevated the level of IFN-γ in the blood, and decreased the number of intratumoral myeloid-derived suppressor cells. Furthermore, α-PD-L1 antibody protected these amplified CD8+ T-cells from exhaustion, and therefore facilitated the secretion of IFN-γ, granzyme B, and perforin through these CD8+ T-cells. As a result, this combination treatment strategy significantly prolonged survival of intraperitoneal metastasized bladder cancer bearing mice, suggesting that bisdemethoxycurcumin in combination with α-PD-L1 antibody may be promising for bladder cancer patients.
Introduction
Bladder cancer, the second most common genitourinary malignancy, is threatening human beings by its invasive and metastatic behavior. Conventional adjuvant chemotherapies such as combinations of platinum, gemcitabine, and ifosfamide usually fail to show survival advantages. Alternative nonsurgical therapeutic strategies are critical to improve patient survival. The immune system plays a critical role in controlling tumor growth, but malignancies, including bladder cancer, can escape immune attack through multiple pathways.Citation1,Citation2 Any method that can improve immune response against bladder cancer will provide opportunities for better outcome, especially for patients in whom first-line chemotherapy failed to control tumor progression.Citation3
In immune cells, CD8+ T-cell is one of the most effective cytotoxic cells in mediating tumor-specific attack. However, there are suffocating restraints in CD8+ T-cell-based immunotherapy, such as immune checkpoint PD-1/PD-L1 axis, and the tumor immunosuppressive microenvironment including local Treg cells. Pathology studies have proven that most bladder cancer cells express PD-L1,Citation4,Citation5 which can profoundly impair the effects of cytotoxic CD8+ T-cells (CTLs). In addition, tumors can develop an immunosuppressive microenvironment via different pathways, and consequently decrease the amount of tumor infiltrating lymphocytes (TILs). These two factors in combination, can result in suppressed CTL homing and weakened CTL function, and finally abrogate the spontaneous immune attack against bladder cancer.
Recently, antibodies for PD-1/PD-L1 pathway displayed favorable results in strengthening the activity of CD8+ T-cells in clinical trials for bladder cancer.Citation6,Citation7 If there is a method to improve the immune response via immune checkpoint independent ways, it can improve the effects of checkpoint inhibitors. Concerning the immunosuppressive environment, intratumoral Treg cells and myeloid-derived suppressor cells (MDSCs) play critical roles. Curcumin, a widely studied component of turmeric, has been proven to facilitate immune response against different solid malignancies via multiple pathways.Citation8–Citation10 However, poor bioavailability has limited the application of curcumin.Citation11 Thus, analogs of curcumin, such as bisdemethoxycurcumin (BDMC), may be promising drugs for clinical application.Citation12 Given the fact that curcumin can mediate immune response, we conjectured that BDMC could promote immune attack against bladder cancer, and the effects could be strengthened when used in combination with PD-1/PD-L1 antibody.
In this study, we tried to validate our hypothesis that BDMC treatment and PD-1/PD-L1 blockade can promote spontaneous immune response against bladder cancer. We established immunocompetent C56BL/6 mouse models bearing subcutaneous (s.c.) or metastatic bladder cancer, and then treated them with BDMC and/or α-PD-L1 antibody. We discovered that low dose BDMC, in combination with α-PD-L1 antibody, displayed favorable effects in facilitating immune response and significantly prolonged mouse survival.
Materials and methods
Ethics approval
The ethics committees from Shanghai Ninth People’s Hospital and Shanghai Yueyang Hospital have approved this animal study, and the approval was obtained prior to the commencement of the study. All animal studies were performed following the guidelines and regulations of the animal care committees of Shanghai Ninth People’s Hospital and Yueyang Hospital.
Animals, cells, and chemicals
Female C57BL/6 mice aged 7–8 weeks were purchased from Shanghai experimental animal center and maintained properly.
The MB49 mouse bladder carcinoma cells were maintained in Dulbecco’s Modified Eagle’s Medium with 10% heat-inactivated fetal bovine serum, 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, and 100 U/mL penicillin-streptomycin.
BDMC (Sigma-Aldrich Co., St Louis, MO, USA) was dissolved in dimethyl sulfoxide at 20 mM stock solution. Working concentrations were freshly prepared before use. Anti-mouse PD-L1 antibody was purchased from Bio X Cell (West Lebanon, NH, USA) and diluted in PBS.
Tumor models
To establish s.c. bladder cancer models, 1×106 MB49 cells in 100 μL PBS were injected in the right shaved flank. For lung metastasis models, 2×105 MB49 cells in 100 μL PBS were administrated intravenously in the tail vein. One week post-tumor inoculation (day 7), the mice were randomized into different groups, each having at least ten mice per group as follows: vehicle control (PBS), BDMC single-drug treatment (BDMC), α-PD-L1 antibody single-drug treatment (α-PD-L1), and combined treatment (combination). All treatments were then started. BDMC was administrated intravenously at 3 mg/kg body weight every 3 days for 2 weeks (metastasis models) or 4 weeks (s.c. models). α-PD-L1 antibody was administrated intraperitoneally three times a week at 200 μg for 2 weeks (metastasis models) or 4 weeks (s.c. models). Tumor volume was measured with caliper and calculated with the following formula: volume = (length × widthCitation2)/2. Survival rate and mouse body weight were monitored.
Sample processing
For mice to be prepared for enzyme-linked immunosorbent assay (ELISA) and flow cytometry analysis, five mice from each group were anesthetized by intraperitoneal administration of ketamine (9 mg/mL in saline) and xylazine (0.9 mg/mL in saline). Peripheral blood was collected from the tail when the mice were warmed up by heating lamp. Then the mice were sacrificed, and the spleens, s.c. tumor tissues, lungs, and TDLNs were harvested. The spleens were mashed, received red blood cell lysis, and passed through 40 μm cell strainers. Also, lymph nodes were mashed and passed through strainers. Tumors and lungs were disaggregated by razors and incubated at 37°C for 1 hour in Roswell Park Memorial Institute 1640 medium with collagenase type IV (2 mg/mL, Sigma-Aldrich Co.), DNase (0.1 mg/mL, Sigma-Aldrich Co.), hyaluronidase (0.1 mg/mL, Sigma-Aldrich Co.), and bovine serum albumin (0.2 mg/mL, Sigma-Aldrich Co.). Cell suspensions were passed through 100 μm cell strainers to remove aggregates. All the cells mentioned previously were then washed with staining buffer (Biolegend, San Diego, CA, USA) and were ready for flow antibody incubation.
ELISA
Serum IFN-γ level was tested by ELISA (R&D Systems, Inc., Minneapolis, MN, USA) following the manufacturer’s instructions.
Flow cytometry
The following antibodies were purchased from Biolegend: anti-CD3 (clone 17A2), anti-CD45 (clone 30-F11), anti-CD4 (clone GK1.5), anti-CD8α (clone 53-6.7), anti-IFN-γ (clone XMG1.2), anti-CD11b (clone M1/70), and anti-Gr-1 (clone RB6-8C5). The following conjugated antibodies were obtained from eBioscience (San Diego, CA, USA): anti-granzyme B (clone NGZB) and anti-PD-1 (clone J43). Live cells were determined by 7AAD viability staining (Biolegend). For cell surface markers, cells were stained with antibodies at room temperature for 30 minutes. For intracellular antigen detection, an intracellular staining kit containing fixation/permeabilization reagents from eBioscience was used. Flow cytometry analyses were performed on BD LSRFortessa X-20 (BD Biosciences, San Jose, CA, USA).
All gating strategies were determined by fluorescence minus one. Flowjo 10 was used to analyze the flow data.
Statistical analysis
Survival curves were illustrated using Kaplan–Meyer method. Statistical analysis was determined by one-way analysis of variance with Dunn’s multiple comparison. All the analysis was performed on GraphPad Prism 5. All column data were presented as mean ± standard error of the mean. P<0.05 was regarded as statistically significant.
Results
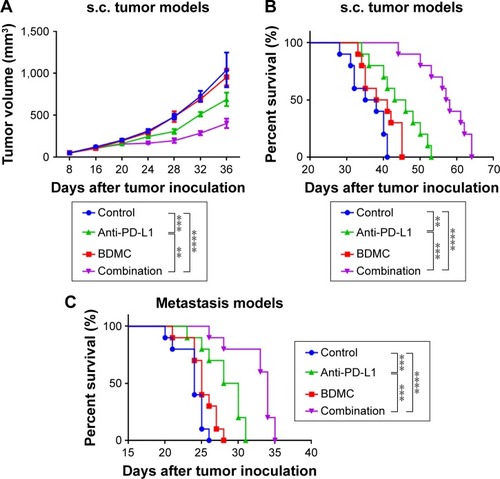
Combination treatment controlled tumor growth and prolonged mouse survival
We evaluated the volumes of s.c. tumors and monitored the survival of mice receiving different treatment regimens in both s.c. tumor and metastasis models. Low dose BDMC alone did not delay tumor growth () in s.c. tumor models, but did have a trend in prolonging survival in both models, although the effect was modest (). In addition, BDMC treatment did not reduce the body weight of mice (data not shown). α-PD-L1 antibody alone, however, not only shrank the tumor, but also prolonged survival of mice. For mice receiving phosphate-buffered saline (PBS), the median survival of s.c. tumor models and lung metastasis models was 36.5 days and 24 days, respectively; while in α-PD-L1 antibody treatment alone groups, the median survival was 44.5 days (P=0.0033) and 29 days (P=0.0007), respectively. Furthermore, combined treatment displayed much stronger benefit both in controlling tumor progression and prolonging survival. On day 36, the median volume of tumors in the control group was 1,040 mm3, while it was 402 mm3 in the combination treatment group (P<0.0001), ie, combined therapy significantly delayed tumor growth. The median survival in combination group in s.c. tumor models and metastasis models was 57.5 days (P<0.0001) and 34 days (P<0.0001), respectively. The combination strategy of BDMC and α-PD-L1 antibody illustrated much more powerful antitumor effects than single drug treatment.
Figure 1 Combined treatment of BDMC and α-PD-L1 antibody inhibited tumor progression and significantly increased survival of mice bearing s.c. and metastatic bladder cancer.
Abbreviations: BDMC, bisdemethoxycurcumin; s.c., subcutaneous; SEM, standard error of the mean.
BDMC increased the number and activity of CD8+ T-cells
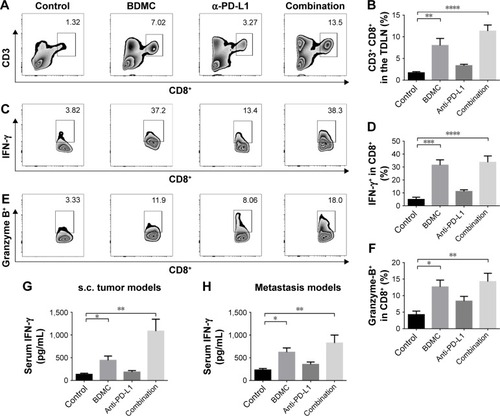
To reveal the effects of BDMC on lymphocytes, we analyzed the phenotypes of splenic lymphocytes and cells in tumor-draining lymph nodes (TDLNs) from different groups in both models. In splenocytes, BDMC significantly increased the proportion of CD8+ T-cells () in both models. This elevated number of CD8+ cells indicated that BDMC helped in CD8+ T-cell survival. In TDLNs from s.c. tumor models, again, an increased number of CD8+ T-cells were discovered in BDMC treated mice, while PD-L1 antibody had no effect on the number of T-cells (). These elevated CD8+ T-cells (by BDMC) expressed a higher level of IFN-γ () and granzyme B () than that in control or α-PD-L1 antibody alone groups, indicating that BDMC not only elevated the number of CD8+ T-cells in the TDLNs, but also strengthened their antitumor activity. Furthermore, serum IFN-γ level was measured. Unsurprisingly, BDMC elevated serum IFN-γ level in both s.c. and lung metastasis models (), demonstrating that BDMC significantly strengthened immune response.
Figure 2 Combination treatment of BDMC and α-PD-L1 antibody increased the proportion of CD8+ T-cells in the spleen of mice bearing s.c. and metastatic bladder cancer.
Abbreviations: BDMC, bisdemethoxycurcumin; s.c., subcutaneous; SEM, standard error of the mean.
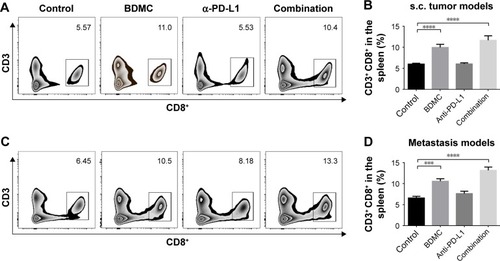
Figure 3 Combined treatment significantly strengthened CD8+ T-cells in TDLNs.
Abbreviations: TDLNs, tumor-draining lymph nodes; ELISA, enzyme-linked immunosorbent assay; BDMC, bisdemethoxycurcumin; s.c., subcutaneous; SEM, standard error of the mean.
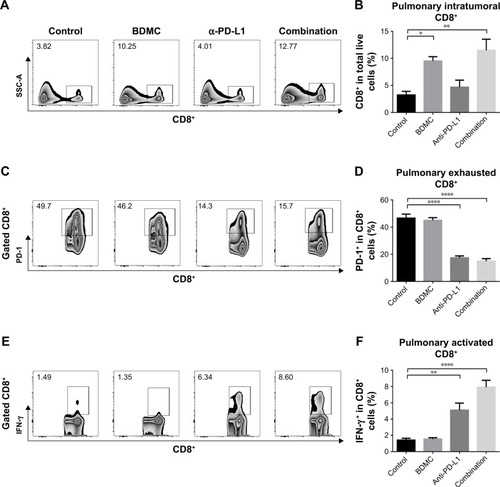
CD8+ T cells were more infiltrated but restricted to exhaustion after BDMC treatment
In both models, flow cytometry results showed a significantly larger number of CD8+ TILs in BDMC treated mice when compared with PBS control mice. BDMC alone resulted in a four-fold (s.c. model) () or three-fold (lung metastasis model) () increase of intratumoral CD8+ T-cells. However, in both models, most of these intratumoral CD8+ T-cells were PD-1 positive (; ), while very few of them expressed IFN-γ (; ). This status was also seen in PBS control mice, which indicated that although more CD8+ CTLs infiltrated the tumor after low dose BDMC treatment, these cells failed to present their antitumor ability. The exhausted status of intratumoral CD8+ T-cells may account for the limited anticancer effect of BDMC treatment alone.
Figure 4 Combination treatment elevated the number and activity of intratumoral CD8+ T-cells in s.c. tumors.
Abbreviations: s.c., subcutaneous; SEM, standard error of the mean.
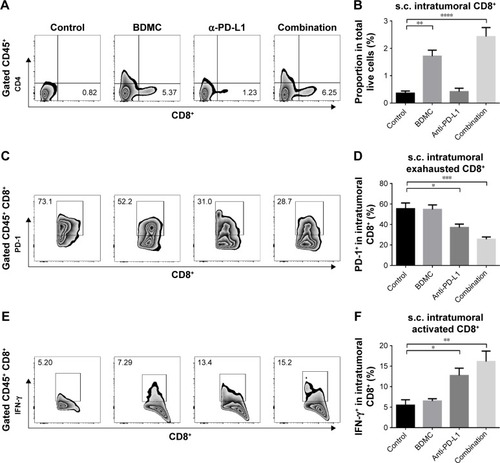
Figure 5 Combination treatment boosted immune response in pulmonary metastasized bladder cancer.
Abbreviation: SEM, standard error of the mean.
α-PD-L1 antibody boosted T-cell response
We validated the role α-PD-L1 antibody played in both s.c. and lung metastasis models. Although α-PD-L1 antibody alone did not display a statistically significant benefit for CD8+ T-cells in the spleen, it did have favorable effects in CTLs in TDLNs. α-PD-L1 antibody elevated the secretion of IFN-γ () and granzyme B () by CD8+ T-cells. In tumor tissues from both models, although there was no statistically significant elevation in the number of tumor infiltrated CD8+ T-cells, α-PD-L1 antibody down-regulated the expression of PD-1 on intratumoral CD8+ T-cells (; ) and elevated the level of IFN-γ in these cells (; ), indicating that α-PD-L1 antibody alone protected the function of intratumoral effector T-cells.
In mice receiving combined treatment of BDMC and α-PD-L1 antibody, the immune response was markedly triggered. As mentioned above, BDMC had positive effects on CD8+ T-cell and activation, and α-PD-L1 antibody protected the intratumoral CD8+ T-cells from exhaustion. In the combination group, the number of CD8+ T-cells in TDLNs from s.c. models was significantly improved (), along with improved functioning of CD8+ T-cells (). In addition, a profoundly elevated secretion of IFN-γ by intratumoral CD8+ T-cells was discovered, which was much higher than that in BDMC or α-PD-L1 antibody treatment alone group (; ). Furthermore, the proportion of exhausted T-cells in the combination group was much lower than in BDMC alone group (; ). Combination treatment increased the number of intratumoral CTLs, facilitated immune response, and protected effector T-cells from exhaustion.
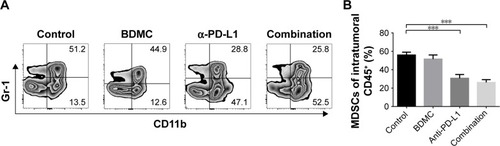
Combination treatment reduced the proportion of MDSCs in s.c. models
MDSCs are highly immune suppressive in the tumor microenvironment. We analyzed the intratumoral MDSCs in both models. BDMC treatment alone had a trend in controlling the number of MDSCs, but α-PD-L1 antibody treatment and combined treatment significantly decreased the number of MDSCs in the tumor () in s.c. models. In lung metastasis models, however, comparatively fewer MDSCs were discovered in the lung. No statistically significant difference was seen among groups (data not shown).
Figure 6 Combination treatment inhibited MDSC proliferation in s.c. tumor models.
Abbreviations: MDSC, myeloid-derived suppressor cell; s.c., subcutaneous; SEM, standard error of the mean.
Discussion
CD8+ T-cells play critical roles in immune attack against tumors. However, the underlying mechanisms affecting T-cell activation, differentiation, function, and survival are still largely unknown. Any method which can facilitate T-cell activity will undoubtedly help in cancer immunotherapy.
Curcumin, and its analogs, were recently validated as a potential immune stimulator.Citation8,Citation10 However, the underlying mechanisms by which curcumin modulates immune response are still elusive. It has been demonstrated that curcumin could facilitate immune response by preventing T-cell apoptosis and altering the tumor microenvironment.Citation13–Citation15 Besides, the clinical application of curcumin has been restricted because of its poor bioavailability.Citation11 In addition, malignancies, especially solid tumors, can weaken immune attack by cytotoxic T-cells through multiple pathways. PD-1/PD-L1 pathway plays one of the most important roles in inhibiting immune response.Citation16,Citation17 Without controlling the immune checkpoint modulators, T-cell-mediated antitumor immune response will be severely impaired.
In the current study, we evaluated the combination treatment of BDMC, an analog of curcumin which has elevated bioavailability and stability, and α-PD-L1 antibody for bladder cancer in immunocompetent mouse models. We demonstrated that mice receiving low-dose BDMC treatment had strengthened T-cell response. Elevated levels of IFN-γ and granzyme B secretion were monitored. However, although experiments on peripheral lymphocytes and the number of intratumoral T-cells revealed favorable results, single-agent treatment of BDMC still had modest effects in prolonging mouse survival and intratumoral T-cell activity. In tumor tissues, most of the infiltrated T-cells displayed an exhausted phenotype, which may be correlated with the expression of PD-L1 on bladder cancer cells. On the other hand, the single-agent administration of α-PD-L1 antibody maintained the activity of CD8+ T-cells, while the number of infiltrated T-cells was still far from satisfactory. Thus, the combination treatment of both BDMC and α-PD-L1 antibody successfully boosted immune response against bladder cancer, and significantly prolonged survival. CTLs, regardless of whether in the TDLNs or in the tumors, secreted significantly higher levels of IFN-γ and granzyme B, and consequently controlled tumor progression. Besides, potent immune suppressor cells, MDSCs, were also inhibited after BDMC treatment. We observed decreased expression of PD-1 on intratumoral CD8+ T-cells, which was not seen in single-agent treated mice. We conjectured that it might be associated with the decreased number of intratumoral MDSCs.Citation18
Clinical trials for bladder cancer using different kinds of immunotherapies such as adoptive cell transfer and PD-1/PD-L1 antibodies are ongoing. However, solid tumors escape immune response through different mechanisms, including immune suppressive local environment and immune checkpoint ligands/receptors. Any method which can facilitate immune response will be valuable for cancer immunotherapy. BDMC can be used in combination with other therapies, and theoretically reach at least additive, or even synergistic effects. The authors are looking forward to the future, in which cocktail immunotherapy, combing adoptive cell transfer, adjuvants such as BDMC, and checkpoint inhibitors, can achieve the most powerful anticancer effects. Of note, the potential side effects of BDMC and related combination treatment are not negligible. As an analog of curcumin, BDMC shares most of the side effects of curcumin, including allergies, gastrointestinal problems such as nausea and diarrhea, etc.Citation11,Citation12 However, these side effects are usually mild and subside fast.Citation12
In conclusion, this is the first study demonstrating that BDMC treatment in combination with α-PD-L1 antibody can suppress bladder cancer progression in vivo and prolong mouse survival. Combination treatment boosted immune response by stimulating CD8+ T-cell activity and suppressing MDSCs. Our results suggest that the combination of BDMC and α-PD-L1 antibody may be a promising therapeutic regimen for treatment of bladder cancer patients.
Disclosure
The authors report no conflicts of interest in this work.
References
- VannemanMDranoffGCombining immunotherapy and targeted therapies in cancer treatmentNat Rev Cancer201212423725122437869
- KamatAMHahnNMEfstathiouJABladder cancerLancet2016388100612796281027345655
- DoninNMLenisATHoldenSImmunotherapy for the treatment of urothelial carcinomaJ Urol20171971142227460757
- WangXTengFKongLYuJPD-L1 expression in human cancers and its association with clinical outcomesOnco Targets Ther201695023503927574444
- InmanBASeboTJFrigolaXPD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progressionCancer200710981499150517340590
- PowlesTEderJPFineGDMPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancerNature2014515752855856225428503
- RosenbergJEHoffman-CensitsJPowlesTAtezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trialLancet2016387100311909192026952546
- LuYMiaoLWangYCurcumin micelles remodel tumor microenvironment and enhance vaccine activity in an advanced melanoma modelMol Ther201624236437426334519
- ZhouJDonatelliSSGilvaryDLTherapeutic targeting of myeloid-derived suppressor cells involves a novel mechanism mediated by clusterinSci Rep201662952127405665
- BillMABakanCBensonDJJrFuchsJYoungGLesinskiGBCurcumin induces proapoptotic effects against human melanoma cells and modulates the cellular response to immunotherapeutic cytokinesMol Cancer Ther2009892726273519723881
- LiuHXuHJiangYPreparation, characterization, in vivo pharmacokinetics, and biodistribution of polymeric micellar dimethoxycurcumin for tumor targetingInt J Nanomedicine2015106395641026504386
- BasileVFerrariELazzariSBellutiSPignedoliFImbrianoCCurcumin derivatives: molecular basis of their anti-cancer activityBiochem Pharmacol200978101305131519580791
- BhattacharyyaSMandalDSahaBSenGSDasTSaGCurcumin prevents tumor-induced T cell apoptosis through Stat-5a-mediated Bcl-2 inductionJ Biol Chem200728222159541596417392282
- BaoBAhmadALiYTargeting CSCs within the tumor microenvironment for cancer therapy: a potential role of mesenchymal stem cellsExpert Opin Ther Targets201216101041105422877147
- JiangGMXieWYWangHSCurcumin combined with FAPalphac vaccine elicits effective antitumor response by targeting indolamine-2,3-dioxygenase and inhibiting EMT induced by TNF-alpha in melanomaOncotarget2015628259322594226305550
- ZouWWolchokJDChenLPD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinationsSci Transl Med20168328324r328r
- LiBZhuXSunLInduction of a specific CD8+ T-cell response to cancer/testis antigens by demethylating pre-treatment against osteosarcomaOncotarget2014521107911080225301731
- DufaitISchwarzeJKLiechtensteinTEx vivo generation of myeloid-derived suppressor cells that model the tumor immunosuppressive environment in colorectal cancerOncotarget2015614123691238225869209
