Abstract
Background
Epidermal growth factor receptor (EGFR) is a commonly altered gene that is identified in various cancers, including head and neck squamous cell carcinoma (HNSCC). Therefore, EGFR is a promising molecular marker targeted by monoclonal antibodies and small molecule inhibitors targeting the tyrosine kinase (TK) domain.
Objective
The objective of this study was to investigate the spectrum of mutations in exons 18, 19, 20, and 21 of the EGFR gene in HNSCC patients.
Materials and methods
This retrospective study included 47 confirmed HNSCC cases. Mutations in the TK domain, exons 18, 19, 20, and 21 of the EGFR gene, were detected by Scorpion® chemistry and ARMS® technologies on Rotor-Gene Q real-time polymerase chain reaction.
Results
The tumors exhibited EGFR-TK domain mutations in 57% of cases. Four cases of T790M mutations were reported for the first time among HNSCC patients. Out of the total mutations, L861Q (exon 21), exon 20 insertions and deletions of exon 19 accounted for the majority of mutations (21%, 19%, and 17%, respectively). EGFR mutation status was correlated with the higher grade (P=0.026) and advanced stage (P=0.034) of HNSCC tumors.
Conclusion
Higher frequency of EGFR-TK domain mutations together with the presence of the T790M mutation suggests that identification of these mutations might streamline the therapy and provide a better prognosis in HNSCC cases.
Introduction
Head and neck carcinoma represents one of the 10 most common cancers worldwide.Citation1 More than 95% of head and neck cancers are squamous cell carcinoma (head and neck squamous cell carcinoma [HNSCC]), indicating that it is relatively a homogeneous disease. However, the wide spectrum of unexpected genetic aberrations that have been observed in recent genomic studiesCitation2,Citation3 highlighted the molecular heterogeneity of the disease. Despite aggressive and diverse therapies, the survival rate of these patients has not markedly improved because of the frequent development of locoregional recurrences, distant metastases, and second primary tumors.Citation4
Epidermal growth factor receptor (EGFR) protein overexpression is seen in >80% of HNSCC casesCitation5 and is associated with a poor prognosis.Citation6 The overexpression of EGFR may apparently be triggered by numerous mechanisms such as epigenetic changes, gene amplification, and oncogenic viruses.Citation7 Hypoxic tumor microenvironment increases the EGFR mRNA translation, thereby inducing EGFR overexpression.Citation8 EGFR is a founding member of a HER (erbB) family, which consists of four closely related transmembrane receptors.Citation9 EGFR has a single hydrophobic transmembrane domain, a cytoplasmic tyrosine kinase (TK)-containing domain, and an extracellular ligand-binding domain.Citation10 The cascade on binding of a ligand to the latter domain initiates the EGFR oligomerization followed by autophosphorylation based on the activation of TK residue at C terminus. The phosphorylated tyrosine becomes a binding site for SH2 (Src homology 2) or PTB (phosphotyrosine Binding) domain-containing signaling molecules, including growth factor receptor-bound protein (GRB2) and phosphatidylinositol-3,4,5-triphosphate (PIP3). This initiates a cascade of downstream intracellular signal transduction pathways, namely, mitogen-activated protein kinase (MAPK), phosphatidylinositol-3-kinase (PIK3)-AKT, and signal transducer and activator of transcription (STAT),Citation11 which regulate cell proliferation, differentiation, invasion, angiogenesis, and metastasis.Citation12 The EGFR gene is frequently overexpressed and mutated in human cancers, which is one of the driving forces behind the development of drugs to target the EGFR in general and TK in particular.
The TK domain mutations mostly occur between exons 18 and 21, which constitute single-base substitutions, insertions, and deletions. Alterations in exons 19 and 21 are most common in non-small cell lung carcinoma (NSCLC), which is the reason that this carcinoma responds to EGFR-tyrosine kinase inhibitors (TKIs).Citation13,Citation14 However, theoretically, most of the mutations in the TK domain result in conformational change, which regulates the sensitivity of TKI therapy.Citation15
These hot spot mutations (exons 18–21) of the EGFR gene were mostly studied in NSCLC, and the mutation frequency was ethnically specific.Citation16 Sparse data are available on EGFR-TK domain mutations in HNSCC globally and in the Saudi population. Previously, the PIK3CA mutation pattern in HNSCC patients of Saudi origin was studied, and a high frequency of PIK3CA mutations and five novel mutations were reported.Citation17 The PI3K signaling molecules upon binding to the phosphorylated TK initiate a cascade of intracellular signal transduction pathways.
Therefore, the study of the prevalence of mutations in EGFR-TK in our population may be used as a predictive biomarker resulting in the provision of personalized treatment and shed some light on furthering the understanding of the genetic nature of the EGFR-TK domain. Molecular profiling of tumors, with a particular focus on genetic alterations, is essential to reduce the disease burden and increase the survival rate through improved therapies and more accurate prognosis. To explore the aforementioned hypothesis, the spectrum of mutations in exons 18–21 of the EGFR gene was investigated in 47 HNSCC cases.
Materials and methods
Tissue samples
This retrospective study was conducted on a total of 47 formalin-fixed paraffin-embedded tissue samples from HNSCC tumors from Saudi patients with HNSCC who attended from the King Fahd Hospital of the university. Of the 47 samples, 20 tumors were in the nasopharynx, 13 in the larynx, 4 in the hypopharynx, and 5 each in the oropharynx and the oral cavity. None of the patients underwent any pre-surgical intervention, including radiotherapy and/or chemotherapy. This study was approved by the Institutional Review Board of University of Dammam (#IRB-2014-08-044). All the participants in the research study gave written informed consent. Demographical and clinical parameter details were collected from the hospital records.
Microdissection
Each tumor tissue block with >70% tumor content was cut into four 10 µm sections using a microtome (SLEE Medical, Mainz, Germany).
Genomic DNA isolation
Genomic DNA was isolated by using QIAamp DNA FFPE tissue kit (Qiagen, Manchester, UK) as per the manufacturer’s instructions.
Quantification of DNA
The concentration of the extracted DNA was determined by using Nanodrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The average DNA quantity observed was 95.1 ng/µL and the purity was 2.1.
Control assay
As per the Therascreen® EGFR-Rotor-Gene Q (RGQ) polymerase chain reaction (PCR) kit (Qiagen, Hilden, Germany) mandate, the total amplifiable DNA for each sample was assessed on RGQ real-time PCR (Qiagen) using the control reaction mix. Control assay amplifies the exon 2 region of the EGFR gene. All the samples fulfilled the criteria of CT value between 23 and 30.69 in the green channel.
Mutation assay
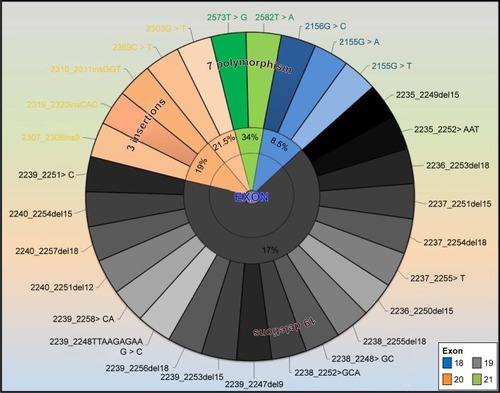
The Therascreen EGFR RGQ PCR kit (Qiagen) detects a total of 29 () specific somatic mutations in exons 18–21 of the EGFR gene utilizing both ARMS® and Scorpion® technologies. Data analysis was performed by using Rotor gene proprietary software, and the mutation status was categorized qualitatively as positive and negative based on the Therascreen EGFR RGQ PCR kit guidelines.
Figure 1 The somatic mutations of EGFR detected by Therascreen® EGFR RGQ PCR kit.
Abbreviations: EGFR, epidermal growth factor receptor; RGQ, Rotor-Gene Q; PCR, polymerase chain reaction.
Statistical analysis
The results were categorized as positive or negative for each mutation of the EGFR gene, and the Fisher exact test was used to test the proportions across the cohort with variables such as age, gender, smoking status, tumor grade, stage, and prognosis was analyzed by using Statistical Package for the Social Sciences (SPSS) version 18 software (SPSS Inc., Chicago, IL, USA).
Results
The patient cohort comprised Saudi nationals with a median age of 52 years. The gender ratio skewed toward males (66%). The majority of tumors were nasopharyngeal squamous cell carcinoma tumors (42.5%), followed by laryngeal tumors (27.6%), with the remainder mainly comprising hypopharyngeal, oropharyngeal, and oral cavity. Stage 1 and 2 HNSCC represented 40.5% of the cancers, whereas stages 3 and 4 represented 59.5%. In the cohort, both grade 3 and grade 2 tumors were represented equally (40.5% each), followed by grade 1 tumors (19%). Data reflected that 44.7% of patients whose tumor samples were included in the study were smokers ().
Table 1 Patient characteristics
Twenty-seven patients (57%) exhibited at least one of the specific somatic mutation in the EGFR-TK domain spanning exons 18–21. Of the total mutations, L861Q (exon 21), exon 20 insertions, and deletions of exon 19 accounted for the majority of the mutations (21%, 19%, and 17%, respectively). The resistant mutation T790M (exon 20) and G719A/S/C (exon 18) were the least observed (8.5%). Grade 3 tumors had a statistically significant higher rate of mutations compared with low-grade tumors (P=0.041) (). Similarly, advanced stage tumors were correlated with a high rate of mutation status (P=0.034). Smoking status, gender, and age did not yield any significant correlation with the mutation status.
Table 2 Effect of parameters on EGFR mutation
Furthermore, each specific mutation was compared with specific categories, namely, gender, age (<50 and >50 years), smoking status, and prognosis (). The male population had a significantly higher mutation rate of exon 20 insertions (P=0.019). Patients aged <50 years had more exon 19 deletions compared with those aged >50 years (P=0.047).
Table 3 Association of age, gender, smoking status, and prognosis with the individual EGFR mutations
Discussion
The overexpression of EGFR is observed in several malignancies, such as NSCLC, renal cancer, colon cancer, breast cancer, ovarian cancer, and HNSCC. The overexpression of this protein is correlated with a decreased occurrence of disease-free survival.Citation18–Citation23 Monoclonal antibodies block the ligand-binding region of EGFR and reduce the TK activation.Citation24,Citation25 Currently, the FDA-approved anti-EGFR monoclonal antibodies are cetuximab and panitumumab, with the former for HNSCC and both for colorectal cancer.Citation16 Small molecule TKIs bind to the intracellular catalytic domain of the EGFR and inhibit their autophosphorylation and downstream signaling.Citation26
Preliminary studies on TKIs such as gefitinib and erlotinib established biological and clinical activity in limited phenotypic and genotypic subclass of lung cancers.Citation27 Further exploration revealed optimum response observed in patients who have TK domain mutations. Those mutations are exon 18 (G719X), exon 19 deletions, and exon 21 (L858R) mutations.Citation28 The acquired resistance was observed in patients having T790M mutation at exon 20.Citation29 Recent studies and clinical trials in NSCLC indicate that patients having EGFR mutations show a promising response to the TKIs. Therefore, the study of EGFR mutations in various cancers has gained a lot of importance.
In HNSCC, EGFR mutation frequency differed in ethnic populations, ranging from 7% in Japanese population,Citation30 7.3% in Asian population,Citation31 15.7% in Korean population,Citation32 and 15.8% in Greek population.Citation33 The highest mutation frequency was 81.39% in southern Indian population.Citation34 No mutations were observed in BelgiumCitation16 and SpanishCitation35 populations. Novel mutations were identified in Japanese and southern Indian populations.Citation30,Citation34 The present study observed a frequency of 57% EGFR mutation among the Saudi population. This EGFR polymorphic variation in HNSCC is ethnically specific, with varying prevalence in different populations.Citation36 The variation may also be due to the method of EGFR mutation detection used, which may include Sanger sequencing, hybridization, immunohistochemistry, and real-time PCR. Each assay varies in its sensitivity and specificity. The present study used the Therascreen EGFR RGQ PCR kit for the detection of specific somatic mutations in the EGFR oncogene. This method detects mutation as low as 0.5% in the background of wild-type DNA.
The major etiological factors for HNSCC are smoking and alcohol consumption, which is why men are at a higher risk than females.Citation30 Our study cohort is in line with this, as 66% of the patients were males and 58% of the male patient group were smokers compared with 18.75% in female patients. It is known that the higher-grade HNSCC tumors are associated with a higher number of mutations.Citation17 This has also been confirmed by the present study, where a higher number of mutations were observed in high pathological grade tumors (P=0.026).
Molecular studies on HNSCC cohorts revealed that 6.9%Citation37 and 10.5%Citation30 of the patients had a deletion in exon 19 and that the former cohort exhibited a poor prognosis when treated with cetuximab and radiotherapy. Hama et al reported that patients with exon 19 deletions and exon 21 point mutations who had been treated with gefitinib had longer progression and survival times (7.7 vs 2.2 months and 11.6 vs 4.6 months, respectively) when compared with patients who had wild-type EGFR mutation. In the present study, 17% of patients exhibited a deletion in exon 19, and it was observed that this mutation had a higher prevalence in younger patients (P=0.047) and also that exon 20 insertions in 19% of the study cohort and all the insertions in exon 20 were only observed in male patients (P=0.019).
The frequency of exon 18 mutation in our population was 8.5% compared with that found in Indian population (58%).Citation34 The T790M mutation in exon 20, which is resistant to TKI therapy, was observed in four patients in the present cohort. The present study seems to be the first study reporting the T790M mutation in a HNSCC cohort. Based on the above data, the high frequency of exon 19 deletions and low frequency of T790M resistant mutation indicate that TKIs may be used as an effective treatment strategy. The coexistence of several mutations in the EGFR gene may shed some light on the HNSCC prognosis.
Conclusion
The higher frequency of EGFR-TK domain mutations together with the presence of the T790M mutation suggests that identification of these mutations might streamline the therapy and provide a better prognosis in HNSCC cases.
Acknowledgments
The current research was supported by The Deanship of Scientific Research, University of Dammam, Dammam (Grant No: 2014064). The authors would like to thank Mr Geoffrey James Tam Moro and Mr Florentino Jr Mata for their technical support.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRNaishadhamDJemalACancer statistics, 2012CA Cancer J Clin2012621102922237781
- AgrawalNFrederickMJPickeringCRExome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1Science201133360461154115721798897
- StranskyNEgloffAMTwardADThe mutational landscape of head and neck squamous cell carcinomaScience201133360461157116021798893
- LeemansCRBraakhuisBJBrakenhoffRHThe molecular biology of head and neck cancerNat Rev Cancer201111192221160525
- YewaleCBaradiaDVhoraIPatilSMisraAEpidermal growth factor receptor targeting in cancer: a review of trends and strategiesBiomaterials201334348690870723953842
- MitsudomiTYatabeYEpidermal growth factor receptor in relation to tumor development: EGFR gene and cancerFEBS J2010277230130819922469
- HirschFRVarella-GarciaMCappuzzoFPredictive value of egfr and her2 overexpression in advanced non-small-cell lung cancerOncogene200928Suppl 13237
- FranovicAGunaratnamLSmithKRobertIPattenDLeeSTranslational up-regulation of the EGFR by tumor hypoxia provides a nonmutational explanation for its overexpression in human cancerProc Natl Acad Sci U S A200710432130921309717670948
- ZaczekABrandtBBielawskiKPThe diverse signaling network of EGFR, HER2, HER3 and HER4 tyrosine kinase receptors and the consequences for therapeutic approachesHistol Histopathol20052031005101515944951
- OlayioyeMANeveRMLaneHAHynesNEThe ErbB signaling network: receptor heterodimerization in development and cancerEMBO J200019133159316710880430
- HanCBMaJTLiFZouHWMolecular markers for the prediction of anti-EGFR monoclonal antibody treatment efficacy in metastatic colorectal cancerJ Cancer Ther20112675682
- BabaYFujiiMTokumaruYKatoYPresent and future of EGFR inhibitors for head and neck squamous cell cancerJ Oncol2012201298672522545054
- SahooRHariniVVBabuVCScreening for EGFR mutations in lung cancer, a report from IndiaLung Cancer201173331631921315473
- WykoskyJMukasaAFurnariFCaveneeWKEscape from targeted inhibition: the dark side of kinase inhibitor therapyCell Cycle2010991661166220404502
- GreulichHChenTHFengWOncogenic transformation by inhibitor-sensitive and -resistant EGFR mutantsPLoS Med2005211e31316187797
- BoeckxCWeynCVanden BemptIMutation analysis of genes in the EGFR pathway in head and neck cancer patients: implications for anti-EGFR treatment responseBMC Res Notes2014733724899223
- Al-AmriAMVatteCCyrusCNovel mutations of PIK3CA gene in head and neck squamous cell carcinomaCancer Biomark201616337738326889984
- MellonKWrightCKellyPHorneCHNealDELong-term outcome related to epidermal growth factor receptor status in bladder cancerJ Urol19951533 Pt 29199257853575
- TannerMHollménMJunttilaTTAmplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumabAnn Oncol200516227327815668283
- LoHWHsuSCHungMCEGFR signaling pathway in breast cancers: from traditional signal transduction to direct nuclear translocalizationBreast Cancer Res Treat200695321121816261406
- AraújoARibeiroRAzevedoIGenetic polymorphisms of the epidermal growth factor and related receptor in non-small cell lung cancer – a review of the literatureOncologist200712220121017296816
- FarneboLJedlinskiAAnsellAProteins and single nucleotide polymorphisms involved in apoptosis, growth control, and DNA repair predict cisplatin sensitivity in head and neck cancer cell linesInt J Mol Med200924454955619724896
- HsiehYYTzengCHChenMHChenPMWangWSEpidermal growth factor receptor R521K polymorphism shows favorable outcomes in KRAS wild-type colorectal cancer patients treated with cetuximab-based chemotherapyCancer Sci2012103479179622321154
- GarrettTPMcKernNMLouMCrystal structure of a truncated epidermal growth factor receptor extracellular domain bound to transforming growth factor alphaCell2002110676377312297049
- OgisoHIshitaniRNurekiOCrystal structure of the complex of human epidermal growth factor and receptor extracellular domainsCell2002110677578712297050
- AzemarMSchmidtMArltFRecombinant antibody toxins specific for ErbB2 and EGF receptor inhibit the in vitro growth of human head and neck cancer cells and cause rapid tumor regression in vivoInt J Cancer200086226927510738256
- FukuokaMYanoSGiacconeGMulti-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (the IDEAL 1 trial)J Clin Oncol200321122237224612748244
- LynchTJBellDWSordellaRActivating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinibN Engl J Med2004350212129213915118073
- PaoWMillerVAPolitiKAAcquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domainPLoS Med200523e7315737014
- HamaTYuzaYSaitoYPrognostic significance of epidermal growth factor receptor phosphorylation and mutation in head and neck squamous cell carcinomaOncologist200914990090819726454
- LeeJWSoungYHKimSYSomatic mutations of EGFR gene in squamous cell carcinoma of the head and neckClin Cancer Res20051182879288215837736
- NaIIKangHJChoSYEGFR mutations and human papilloma-virus in squamous cell carcinoma of tongue and tonsilEur J Cancer200743352052617224267
- MurraySBobosMAngouridakisNScreening for EGFR mutations in patients with head and neck cancer treated with gefitinib on a compassionate-use program: A Hellenic Cooperative Oncology Group StudyJ Oncol2010201070967821274259
- NagalakshmiKJamilKPingaliUReddyMVAttiliSEpidermal growth factor receptor (EGFR) mutations as biomarker for head and neck squamous cell carcinomas (HNSCC)Biomarkers201419319820624712396
- Lemos-GonzálezYPáez de la CadenaMRodríguez-BerrocalFJRodríguez-PiñeiroAMPallasEValverdeDAbsence of activating mutations in the EGFR kinase domain in Spanish head and neck cancer patientsTumour Biol200728527327917962724
- Loeffler-RaggJWitsch-BaumgartnerMTzankovALow incidence of mutations in EGFR kinase domain in Caucasian patients with head and neck squamous cell carcinomaEur J Cancer200642110911116324836
- SmilekPNeuwirthovaJJarkovskyJEpidermal growth factor receptor (EGFR) expression and mutations in the EGFR signaling pathway in correlation with anti-EGFR therapy in head and neck squamous cell carcinomasNeoplasma201259550851522668015
