Abstract
Objective
To compare the radiobiological response between simultaneously dose-escalated and non-escalated intensity-modulated radiation therapy (DE-IMRT and NE-IMRT) for patients with upper thoracic esophageal cancer (UTEC) using radiobiological evaluation.
Methods
Computed tomography simulation data sets for 25 patients pathologically diagnosed with primary UTEC were used in this study. DE-IMRT plan with an escalated dose of 64.8 Gy/28 fractions to the gross tumor volume (GTV) and involved lymph nodes from 25 patients pathologically diagnosed with primary UTEC, was compared to an NE-IMRT plan of 50.4 Gy/28 fractions. Dose-volume metrics, tumor control probability (TCP), and normal tissue complication probability for the lung and spinal cord were compared. In addition, the risk of acute esophageal toxicity (AET) and late esophageal toxicity (LET) were also analyzed.
Results
Compared with NE-IMRT plan, we found the DE-IMRT plan resulted in a 14.6 Gy dose escalation to the GTV. The tumor control was predicted to increase by 31.8%, 39.1%, and 40.9% for three independent TCP models. The predicted incidence of radiation pneumonitis was similar (3.9% versus 3.6%), and the estimated risk of radiation-induced spinal cord injury was extremely low (<0.13%) in both groups. Regarding the esophageal toxicities, the estimated grade ≥2 and grade ≥3 AET predicted by the Kwint model were increased by 2.5% and 3.8%. Grade ≥2 AET predicted using the Wijsman model was increased by 14.9%. The predicted incidence of LET was low (<0.51%) in both groups.
Conclusion
Radiobiological evaluation reveals that the DE-IMRT dosing strategy is feasible for patients with UTEC, with significant gains in tumor control and minor or clinically acceptable increases in radiation-induced toxicities.
Introduction
Esophageal cancer (EC) is the eighth most common cancer and the sixth leading cause of cancer-related mortality worldwide.Citation1,Citation2 Concurrent chemoradiotherapy (CCRT) has been established as the standard definitive treatment for locally advanced EC,Citation3,Citation4 with a total dose of 50 to 50.4 Gy delivered in a standard dose of 1.8 to 2 Gy per fraction.Citation5,Citation6 Unfortunately, the standard dose strategy is clinically disappointing because 90% of local failures still occur in the gross tumor volume (GTV),Citation7 suggesting that a higher radiation dose to GTV might be more beneficial for improving local control.
The implementation of intensity-modulated radiation therapy (IMRT) can modify dose distribution and improve normal tissue sparing.Citation8,Citation9 Dose escalation using a simultaneous integrated boost (SIB) is a novel technique in which an accelerated higher dose, and a conventional lower dose can be delivered to the primary tumor and the subclinical disease simultaneously.Citation10 Recently, an increasing number of studies have demonstrated its feasibility, efficacy, and safety for the treatment of EC patients with the combination of IMRT and dose escalation techniques.Citation11–Citation13 However, to the best of our knowledge, only several studies investigated the potential benefits of dose-escalated IMRT (DE-IMRT) to offer a dose escalation to the primary tumor, and improve the sparing of normal tissues compared with non-escalated IMRT (NE-IMRT) for EC patients.Citation14–Citation16 Unfortunately, the studies failed to provide further information on esophageal toxicity, which is a common radiation-induced side effect in the treatment of EC patients.Citation11,Citation12,Citation17,Citation18
Radiobiological evaluation is a method to predict the outcome for the tumor and normal tissue using mathematical analysis with parameters derived from clinical trials. The method has an advantage in translating the dosimetric changes into radiobiological responses, and thus has been widely used to predict the feasibility of dose escalation for EC and primary prostate cancer.Citation16,Citation19
In this study, we aimed to compare the radiobiological dose response between the DE-IMRT and NE-IMRT strategies across a group of representative patients suffering from EC, using radiobiological evaluation. We sought to evaluate the feasibility of DE-IMRT treatment for EC patients.
Materials and methods
Ethics statement
A total of 25 patients with pathologically confirmed upper thoracic esophageal cancer (UTEC) receiving CCRT were used in the study (ClinicalTrial.gov number, NCT01670409, and Chinese Clinical Research Registry number, ChiCTR-ONC-12002356). The Clinical Research Ethics Review Committee of Cancer Hospital of Shantou University Medical College approved this study. Only the computed tomography (CT) images of these patients were used in this study. All the patients have given their written informed consent for their CT images to be used before the study started. The patients’ information in the CT images was de-identified to avoid any ethical concerns.
Immobilization and CT simulation
Patients were immobilized in the supine position with the head and shoulders wrapped in a thermoplastic mask restricting system (Guangzhou Klarity Medical & Equipment Co., Ltd, Guangzhou, People’s Republic of China). A contrast-enhanced CT scan with 5 mm slice thickness from the neck to the upper abdomen was obtained using a 16-slice CT scanner (Philips Brilliance CT Big Bore Oncology Configuration, Cleveland, OH, USA). Afterwards, CT images were delivered to the Eclipse treatment planning system (Version 10.0; Varian Medical Systems, Palo Alto, CA, USA) by Digital Imaging and Communications in Medicine 3.0 for target volume contouring, organs at risk (OAR) contouring, and treatment planning.
Target volume and OAR delineation
We have previously reported the approaches for target volume and OAR contouring in EC patients.Citation14 Briefly, the GTV consisted of the primary tumor (GTVP) and the positive regional lymph nodes (GTVLN). The contouring of GTV was identified using CT images, endoscopic reports or barium swallow fluoroscopy. GTVLN included mediastinal or supra-clavicular lymph nodes if the shortest axis was ≥10 mm. The clinical target volume (CTV) was delineated with 20 mm longitudinal and 5–10 mm radial margins with respect to the GTVP and a 5 mm uniform margin from GTVLN. Paraesophageal or tracheoesophageal groove lymph nodes that did not meet the criteria of positive lymph nodes, but with shortest axis ≥5 mm, were also encompassed in CTV. Two planning target volumes (PTVs) PTV64.8 and PTV50.4 were generated with a uniform 5 mm margin expansion from CTV and GTVLN. OAR contours, including the spinal cord and lung, were created as previously described.Citation20 Briefly, the lung contour was limited to the air-inflated lung parenchyma without inclusion of fluid and atelectasis visible on the CT images, excluding the proximal bronchial tree. The contour of the spinal cord started at the same cranial level as the esophagus to the bottom of L2 or at the level in which the cord ended. The planning OAR volume (PRV) for the spinal cord was generated by expanding the spinal cord to a 5 mm margin. The esophagus contour began from the level of cricoid cartilage on every CT image to the gastroesophageal junction.Citation21–Citation23
Planning objectives
The dose constraints for OAR were as follows: spinal cord, maximum dose (Dmax) <45 Gy; PRV for spinal cord, V50 ≤1 cc; lung, V5 <60%, V10 <50%, V20 <30% and mean lung dose <15 Gy, where Vx is percentage of the target volume receiving ≥ x Gy. The dose was normalized to ensure that 95% of the PTV received 100% of the prescribed dose.
Treatment planning
The prescribed dose for the DE-IMRT plan was 64.8 Gy in 28 fractions for PTV64.8 (delivered in 2.31 Gy/fraction), and 50.4 Gy in 28 fractions for PTV50.4 (delivered in 1.8 Gy/fraction) according to Welsh et al.Citation15 The prescribed dose for the NE-IMRT plan was set to 50.4 Gy in 28 fractions (delivered in 1.8 Gy/fraction) for the target of PTV50.4. Plans with different prescribed doses were generated using five coplanar sliding-window based IMRT fields with beam arrangements of 210°, 300°, 0°, 60°, and 150°. All plans were designed using a 6 MV photon beam from a TrueBeam linear accelerator (Varian Medical Systems). Optimization was performed using the dose volume optimizer (version 10.0.28) algorithm. Dose calculation was performed using the anisotropic analytical algorithm (version 10.0.28), considering the heterogeneity correction. Several dose-limiting structures were created to make the dose more conformal to the target. The method termed base dose function (BDF) described in our previous study was used to acquire more homogeneous dose distribution.Citation24 Briefly, the fractions of the original plan were modified to half (from 28 to 14 fractions in the study), and then the half-prescribed plan was copied and re-optimized using the BDF incorporated in Eclipse with the half-prescribed plan as the base dose plan. When the dose was finally calculated, the fractions of the plan were doubled to generate the target plan.
Radiobiological evaluation
Both the tumor control probability (TCP) and normal tissue complication probability (NTCP) calculations were performed using in-house developed programs designed with MATLAB 7.0 (MathWorks, Natick, MA, USA). Three radiobiological models, including the Geh model, Webb-Nahum model (WN model), and equivalent uniform dose-based model (EUD model) were used to predict the TCP value. We emphasize that the Geh is a multivariate logistic regression model fitted to the data obtained from 26 chemo-radiotherapy trials for preoperative EC, and the analysis is based on the total radiotherapy dose, dose per fraction, radiotherapy duration, chemotherapy, and patient’s age. The values of the covariates and coefficients were obtained from the original paper.Citation25 The chemotherapy regimen in our study consisted of two cycles of CCRT and two cycles of adjuvant chemotherapy with 75 mg/m2 cisplatin administered intravenously on day 1 and 500 mg/m2 5-fluorouracil (5-FU) intravenously from day 1 to 4 as in our previous study.Citation13 The WN model assumes a normal distribution of radiosensitivity α values among a cohort of patients.Citation26 The αm and σα represent the mean and standard deviation of the values of α, respectively. The values used were αm =0.40 Gy−1, σα=0.08 Gy−1 and ρ=107/cm3, respectively, which were chosen to fit the observed local control rates for EC patients.Citation27 The EUD model used the following values obtained from studies performed by Okunieff et al: doses required to achieve 50% tumor control (TCD50)=49.09 Gy, γ50=2.16 and α=0.40 Gy−1.Citation28
We utilized the Lyman-Kutcher-Burman (LKB) predicting model to estimate the NTCP values for the lung and spinal cord. The tolerance dose for a 50% complication (TD50), n and m values in the LKB model for predicting grade ≥2 radiation pneumonitis and radiation-induced myelitis/necrosis were obtained from studies performed by Seppenwoolde et al and Burman et al.Citation29,Citation30
In addition, we used the Kwint model to predict grade ≥2 and grade ≥3 acute esophageal toxicity (AET).Citation22 The Kwint model, derived from 139 patients after CCRT treatment for non-small cell lung cancer (NSCLC), demonstrated a sigmoid-shaped relationship between grade ≥2 AET and V50. Moreover, the Wijsman model, which is an LKB-based predicting model generated from 149 advanced stage NSCLC patients undergoing CCRT, was also established to estimate grade ≥2 AET.Citation23 The parameters used were n=1.04, m=0.65, and D50=32.84 Gy in the Wijsman model. The Chen model is also an LKB-based model derived from 171 NSCLC patients treated with CCRT, and we used this model to predict the incidence of late esophageal toxicity (LET).Citation31 The parameters used were n=0.03, m=0.03, and TD50=76.1 Gy in this model. All physical doses were converted to a biologically equivalent dose in 2 Gy fraction (EQD2) before modeling. An α/β value of 4.9 Gy was used for TCP prediction. The α/β values of 2 Gy and 3 Gy were used for NTCP calculation for the spinal cord and lung, respectively. With regard to the esophageal toxicity estimation, the α/β of 10 Gy and 3 Gy were employed for AET and LET prediction, respectively. A detailed procedure of modeling has been published in our previous work.Citation32
Statistical analysis
A comparison of the data was performed using a paired two-tailed Student’s t-test. A Wilcoxon matched-pair signed-rank test was alternatively used when the data did not follow a normal distribution. These results were considered statistically significant when P-value <0.05.
Results
Patient characteristics
CT simulation data sets of 25 UTEC patients, treated in our department from August 2012 to March 2014, were used in this study. The patients were between 49 and 73 years old. The median volumes of GTV, PTV64.8, and PTV50.4 were 25.1±15.4 cc, 72.9±36.0 cc, and 201.3±62.5 cc, respectively. The patients’ characteristics are listed in .
Table 1 Characteristics of 25 patients with UTEC
Dose difference between the DE-IMRT and NE-IMRT strategies
The mean dose-volume parameters averaging from 25 patients for the two dosing strategies are listed in . The DE-IMRT plan resulted in a 28.4% dose escalation to the GTV on average compared with the NE-IMRT plan (65.9 Gy versus 51.3 Gy). The V10, V20, and mean dose (Dmean) of the lung were increased by 1.0%, 1.3%, and 0.4 Gy, respectively (P<0.05). Dmax of the spinal cord was 2 Gy higher in the DE-IMRT group. With regard to the esophageal dose, the V30, V40, and V50 of the esophagus were comparable between the DE-IMRT and NE-IMRT groups (P>0.05). However, the V60, Dmean, and Dmax were dramatically increased in the DE-IMRT group (P<0.001). The dose volume histogram of the GTV, lung, spinal cord, and esophagus is presented in . The dose distribution in the transversal, coronal, and sagittal views from one representative case is shown in .
Figure 1 DVH of the GTV, lung, spinal cord, and esophagus.
Abbreviations: DVH, dose volume histogram; GTV, gross tumor volume; DE-IMRT, dose-escalated intensity-modulated radiation therapy; NE-IMRT, non-escalated intensity-modulated radiation therapy.
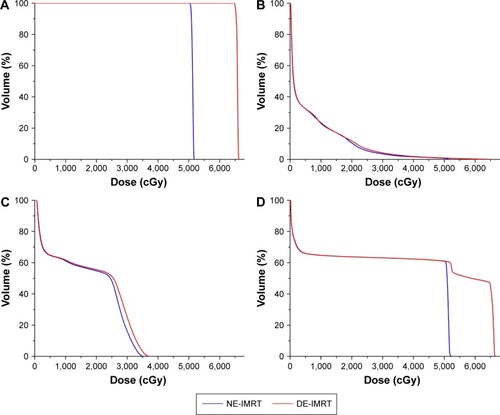
Figure 2 Dose distributions in the transversal, coronal, and sagittal views from one representative case.
Abbreviations: PTV, planning target volume; GTV, gross tumor volume; DE-IMRT, dose-escalated intensity-modulated radiation therapy; NE-IMRT, non-escalated intensity-modulated radiation therapy.
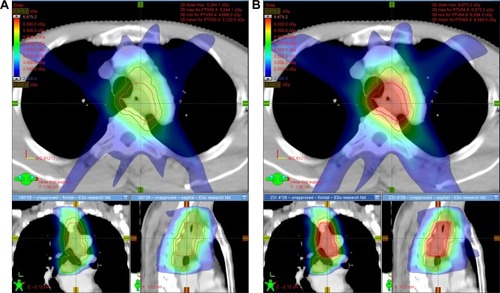
Table 2 Physical dose difference between the DE-IMRT and NE-IMRT plans
Radiobiological evaluation of the DE-IMRT and NE-IMRT plans
The results of the radiobiological evaluation are shown in . TCP in the DE-IMRT plan was increased by 31.8% (increased from 35.8% to 67.6% on average), 39.1% (increased from 52.5% to 91.6% on average), and 40.9% (increased from 54.2% to 95.1% on average) with the Geh model, WN model, and EUD model, respectively. The risk of grade ≥2 radiation pneumonitis predicted by the LKB model was similar between the two plans (3.9% versus 3.6% on average), although the difference was significantly different. The predicted incidence of radiation-induced myelitis/necrosis was extremely low (<0.13%) in both groups. Regarding the esophageal toxicity, the grade ≥2 and grade ≥3 AET predicted using the Kwint model in the DE-IMRT group was increased by 2.5% and 3.8%, respectively. However, the risk of grade ≥2 AET calculated using the Wijsman model was increased by 14.9% in the DE-IMRT group. The prediction of LET using the Chen model was increased notably in the NE-IMRT compared to the DE-IMRT strategy; however, the prediction was extremely low in absolute value for both groups (<0.51%).
Table 3 Prediction of tumor control and toxicities in the DE-IMRT and NE-IMRT plans
Discussion
After utilizing a standard dose of 50.4 Gy for definitive chemoradiation treatment for EC patients, 90% of local failures still occurred in the GTV.Citation7 Therefore, the question was raised whether it is feasible to escalate the dose to the tumor to improve local control, while maintaining clinically acceptable side effects to normal tissues. Increasing evidence has demonstrated that the SIB technique is more clinically beneficial for EC patients;Citation11–Citation13 however, the difference in the radiobiological response, particularly for esophageal toxicity between the DE-IMRT and NE-IMRT strategies for UTEC patients, remains unknown. To the best of our knowledge, this is the first study to compare tumor control and esophageal toxicity between two dosing strategies using radiobiological evaluation. We found that it is feasible to apply the DE-IMRT technique for UTEC patients, with significant gains (31.8%–40.9%) for tumor control, while minor increased toxicity was observed for the lung and spinal cord, and acceptable toxicities were found for the esophagus.
The results of this study are consistent with previously reported dosimetric data, which identified an improvement in dose escalation to the tumor using the DE-IMRT strategy. Welsh et al reported a planning study in distal EC patients using the SIB technique to deliver a boosted dose to the GTV, using the same dose prescription employed in our study. They found that the use of DE-IMRT produced a 28% dose escalation (from 52 Gy to 66.9 Gy) to the GTV, while providing a comparable dose to the lungs and a slightly higher dose to the spinal cord compared with the conventional dose plan.Citation15 Warren et al administered a boosted dose (62.5 Gy in 25 fractions) to the tumor in mid-EC patients, and found that significant gains (>18%) in tumor control were achieved compared with the non-escalated plan using radiobiological evaluation.Citation16 Similar to patients with middle and distal EC, our results demonstrated that the DE-IMRT technique was also applicable for UTEC patients. Apart from dosimetric investigations, an increase in the number of clinical trials has also demonstrated the feasibility and efficacy of the DE-IMRT technique for EC patients, with encouraging improvement in local control and well tolerated acute toxicities, although the escalated dose was slightly different. Both Yu et al and Chen et al initiated a phase II study to investigate the efficacy of radiation dose escalation using the DE-IMRT technique combined with chemotherapy, for the treatment of EC patients.Citation11,Citation13 The results showed a similar trend of improvement in local-regional control and overall survival, indicating that the method of theoretical evaluation could obtain results that were similar to those obtained in clinical practices. Taken together, these described studies theoretically and practically validate the efficacy of dose escalation for EC patients.
Our analysis was partly dependent on the choice of radio-biological models and the parameters used. To strengthen the reliability of our data, we used three TCP predicting models and two esophageal toxicity models from the literature, to predict the tumor control and likelihood of grade ≥2 AET. Interestingly, three independent models exhibited a similar trend of TCP increase using the DE-IMRT method, although the changes were different among the models. Given that the Geh model is a multivariate logistic regression model fitted to the data from 26 chemoradiotherapy trials that considers many factors, such as the dose of radiotherapy, duration, chemotherapy (cisplatin and 5-FU) dose, and age,Citation25 we believe that our data in TCP prediction is reliable. Regarding esophageal toxicity, both the Kwint and Wijsman models displayed an increase in grade ≥2 AET using the DE-IMRT technique. However, we found that when using the Wijsman model, this increase was much more significant than that obtained in the Kwint model. This finding might be partly due to the different chemotherapy administration and radiation techniques in the two independent investigations: CCRT was used for all the patients in the Kwint model, while only selective patients with good performance status received CCRT and most patients underwent sequential treatment or radiotherapy alone in the Wijsman model.Citation22,Citation23 Moreover, the concurrent chemotherapy regimen consisted of low-dose intravenous cisplatin in the Kwint model, whereas gemcit-abine combined with cisplatin or etoposide combined with cisplatin were delivered in sequential chemotherapy in the Wijsman model. Since CCRT has been widely reported to be associated with the incidence of AET in many studies, the difference in chemotherapy practice might greatly have affected our esophageal toxicity prediction.Citation23,Citation33,Citation34 On the other hand, all of the patients were treated with IMRT in the Kwint model, whereas both IMRT and volumetric-modulated arc therapy were used in the Wijsman model. Radiotherapy technique was reported be significantly correlated with grade ≥2 AET and it might partly influence the esophageal toxicity prediction.Citation23 We preferred the prediction obtained from the Kwint model, because CCRT with IMRT administration is the recommended strategy for the definitive treatment of EC patients in most publications,Citation11–Citation13,Citation18 however, further clinical data are needed to validate the model.
AET and LET characterized by dysphagia, odynophagia, stenosis, and perforation are common radiation-induced adverse events that significantly affect quality-of-life, and negatively affect long-term survival when receiving thoracic irradiation.Citation31,Citation34,Citation35 In the definitive treatment of EC patients, the esophagus is more prone to developing these symptoms because a part of the esophagus is located in the radiation field, resulting in high-dose exposure during treatment. It was reported that approximately 40% of patients developed grade 3 AET after SIB radiation dose treatment.Citation12 Unfortunately, mod-eling of the incidence of esophageal toxicity in EC patients is challenging due to the following two reasons. 1) Extensive publications have developed dosimetric predictors or predicting models to estimate the incidence of AET or LET in NSCLC patients; however, none of these studies have yet developed models derived from EC cases. Our group completed a phase II study, and the establishment of an esophagitis-predicting model first derived from EC patients is still ongoing.Citation13 2) There is no consensus on the contouring of the esophagus in EC patients. Whether the tumor and the esophagus outside the treatment field should be included, still remains unclear. Caglar et al found that the esophagus in-field is a new predictor for esophagitis in NSCLC patients.Citation21 However, other studies have used the entire esophagus as the predictor.Citation22,Citation23,Citation36,Citation37 Given that both the Kwint and Wijsman models were generated from the entire esophagus, our study used this information to maintain consistency with the original work. However, whether different definitions of the esophagus will affect the evaluation of esophageal toxicity remains an important issue that needs to be addressed in our future work.
In the past few years, many studies have proposed dosimetric predictors to correlate with the occurrence of grade ≥2 or grade ≥3 AET.Citation36–Citation38 Unfortunately, only several studies have developed models to predict their incidence. Furthermore, most of the developed models were generated from the 3-dimensional conformal radiation therapy (3DCRT) technique.Citation39–Citation42 Because IMRT has been reported to be more superior in delivering a conformal dose and improving normal tissue sparing than 3DCRT,Citation8,Citation9 the models generated from 3DCRT might potentially limit the evaluation of AET for IMRT-treated patients. To date, AET predicting models derived from patients undergoing IMRT and chemotherapy are scarce, except for the Kwint and Wijsman models. Consistent with this information, we had to use these models for the prediction of esophageal toxicity in the study.
Several SIB dosing regimens, including 62.5 Gy/25 fractions, 63 Gy/28 fractions, 64.8 Gy/28 fractions, and 66 Gy/30 fractions, have been demonstrated to provide improved local control and clinically acceptable side effects for EC patients.Citation11,Citation13,Citation15,Citation16 However, whether a variety of dosing regimens may translate into dose-response differences, is still questionable. Consequently, we calculated the EQD2 for the tumor and esophagus from different dosing regimens. We found that the EQD2 for the tumor was 67 Gy, 65.3 Gy, 67.7 Gy, and 67.9 Gy, respectively, using an α/β value of 4.9 Gy. Similarly, the calculated EQD2 for the esophagus was 65.1, 64.3, 66.5, and 67.1 Gy, respectively, using an α/β value of 10 Gy. This result suggests that comparable EQD2 is acquired for both the tumor and the esophagus among the four dosing regimens. Thus, the difference in dosing regimens may have had a slight impact on our results.
Although our study has demonstrated the feasibility of DE-IMRT for the treatment of UTEC patients, it does exhibit some limitations, including the following. 1) It should be noted that we employed the esophagitis-predicting models from lung cancer patients, but their applicability and feasibility need to be further validated. 2) We only used the Kwint model to predict grade ≥3 AET, and this might have partly weakened our result. 3) The sample size of our study is a bit small to fully interpret the benefits and disadvantages of the DE-IMRT technique for UTEC patients. Thus, a larger patient cohort is needed for further validation in a clinical setting.
Conclusion
In summary, our study has demonstrated the benefits of using the DE-IMRT strategy to significantly improve tumor control with minor side effects, or clinically acceptable toxicities for UTEC patients. These findings require further validation in clinical samples.
Author contributions
Each author had participated sufficiently in the work to take public responsibility for appropriate portions of the content. BTH and CZC conceived and designed the experiments. LLW, LJG, LYX, and JZC performed the experiments. BTH, LLW, LJG, LYX, and RHH collected the data. PXL analyzed the data. BTH wrote the paper. DRL and CZC revised the paper. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This work was sponsored by National Natural Science Foundation of China (81602667), Science and Technology Planning Project of Guangdong Province (2016ZC0166), Medical Scientific Research Foundation of Guangdong Province (A2015534), Shantou University Medical College Clinical Research Enhancement Initiative (201424), Collaborative and Creative Center, Molecular Diagnosis and Personal-ized Medicine, Shantou University, Guangdong Province, People’s Republic of China.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRNaishadhamDJemalACancer statistics, 2013CA Cancer J Clin2013631113023335087
- PennathurAGibsonMKJobeBALuketichJDOesophageal carcinomaLancet2013381986440041223374478
- CooperJSGuoMDHerskovicAChemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology GroupJAMA1999281171623162710235156
- HerskovicAMartzKal-SarrafMCombined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagusN Engl J Med199232624159315981584260
- MinskyBDPajakTFGinsbergRJINT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapyJ Clin Oncol20022051167117411870157
- National Comprehensive Cancer NetworkEsophageal and Esoph-agogastric Junction CancersNational Comprehensive Cancer Network2015 Available from: https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf
- WelshJSettleSHAminiAFailure patterns in patients with esophageal cancer treated with definitive chemoradiationCancer2012118102632264022565611
- ChandraAGuerreroTMLiuHHFeasibility of using intensity-modulated radiotherapy to improve lung sparing in treatment planning for distal esophageal cancerRadiother Oncol200577324725316298001
- FenkellLKaminskyIBreenSHuangSVan ProoijenMRingashJDosimetric comparison of IMRT vs. 3D conformal radiotherapy in the treatment of cancer of the cervical esophagusRadiother Oncol200889328729118789828
- LeclercMMaingonPHamoirMA dose escalation study with intensity modulated radiation therapy (IMRT) in T2N0, T2N1, T3N0 squamous cell carcinomas (SCC) of the oropharynx, larynx and hypopharynx using a simultaneous integrated boost (SIB) approachRadiother Oncol2013106333334023541643
- YuWWZhuZFFuXLSimultaneous integrated boost intensity-modulated radiotherapy in esophageal carcinoma: early results of a phase II studyStrahlenther Onkol20141901197998624609941
- YuWCaiXWLiuQSafety of dose escalation by simultaneous integrated boosting radiation dose within the primary tumor guided by (18)FDG-PET/CT for esophageal cancerRadiother Oncol2015114219520025586952
- ChenJGuoHZhaiTRadiation dose escalation by simultaneous modulated accelerated radiotherapy combined with chemotherapy for esophageal cancer: a phase II studyOncotarget2016716227112271926992206
- ZhangWZChenJZLiDRSimultaneous modulated accelerated radiation therapy for esophageal cancer: a feasibility studyWorld J Gastroenterol20142038139731398025320535
- WelshJPalmerMBAjaniJAEsophageal cancer dose escalation using a simultaneous integrated boost techniqueInt J Radiat Oncol Biol Phys201282146847421123005
- WarrenSPartridgeMCarringtonRHurtCCrosbyTHawkinsMARadiobiological determination of dose escalation and normal tissue toxicity in definitive chemoradiation therapy for esophageal cancerInt J Radiat Oncol Biol Phys201490242342925304796
- HiguchiKKomoriSTanabeSDefinitive chemoradiation therapy with docetaxel, cisplatin, and 5-fluorouracil (DCF-R) in advanced esophageal cancer: a phase 2 trial (KDOG 0501-P2)Int J Radiat Oncol Biol Phys201489487287924867539
- RoederFNicolayNHNguyenTIntensity modulated radiotherapy (IMRT) with concurrent chemotherapy as definitive treatment of locally advanced esophageal cancerRadiat Oncol2014919125175056
- KuangYWuLHirataEMiyazakiKSatoMKweeSAVolumetric modulated arc therapy planning for primary prostate cancer with selective intraprostatic boost determined by 18F-choline PET/CTInt J Radiat Oncol Biol Phys20159151017102525832692
- KongFMRitterTQuintDJConsideration of dose limits for organs at risk of thoracic radiotherapy: atlas for lung, proximal bronchial tree, esophagus, spinal cord, ribs, and brachial plexusInt J Radiat Oncol Biol Phys20118151442145720934273
- CaglarHBOthusMAllenAMEsophagus in-field: a new predictor for esophagitisRadiother Oncol2010971485320832884
- KwintMUyterlindeWNijkampJAcute esophagus toxicity in lung cancer patients after intensity modulated radiation therapy and concurrent chemotherapyInt J Radiat Oncol Biol Phys2012842e223e22822560551
- WijsmanRDankersFTroostEGMultivariable normal-tissue complication modeling of acute esophageal toxicity in advanced stage non-small cell lung cancer patients treated with intensity-modulated (chemo-)radiotherapyRadiother Oncol20151171495426341608
- LuJYCheungMLHuangBTImproving target coverage and organ-at-risk sparing in intensity-modulated radiotherapy for cervical oesophageal cancer using a simple optimisation methodPLoS One2015103e012167925768733
- GehJIBondSJBentzenSMGlynne-JonesRSystematic overview of preoperative (neoadjuvant) chemoradiotherapy trials in oesophageal cancer: evidence of a radiation and chemotherapy dose responseRadiother Oncol200678323624416545878
- WebbSNahumAEA model for calculating tumour control probability in radiotherapy including the effects of inhomogeneous distributions of dose and clonogenic cell densityPhys Med Biol19933866536668346278
- BedfordJLViviersLGuzelZChildsPJWebbSTaitDMA quantitative treatment planning study evaluating the potential of dose escalation in conformal radiotherapy of the oesophagusRadiother Oncol200057218319311054522
- OkunieffPMorganDNiemierkoASuitHDRadiation dose-response of human tumorsInt J Radiat Oncol Biol Phys1995324122712377607946
- SeppenwooldeYLebesqueJVde JaegerKComparing different NTCP models that predict the incidence of radiation pneumonitis. Normal tissue complication probabilityInt J Radiat Oncol Biol Phys200355372473512573760
- BurmanCKutcherGJEmamiBGoiteinMFitting of normal tissue tolerance data to an analytic functionInt J Radiat Oncol Biol Phys19912111231352032883
- ChenCUyterlindeWSonkeJJde BoisJvan den HeuvelMBelderbosJSevere late esophagus toxicity in NSCLC patients treated with IMRT and concurrent chemotherapyRadiother Oncol2013108233734124074814
- HuangBTLuJYLinPXChenJZLiDRChenCZRadiobiologi-cal modeling analysis of the optimal fraction scheme in patients with peripheral non-small cell lung cancer undergoing stereotactic body radiotherapySci Rep201551801026657569
- AuperinALe PechouxCRollandEMeta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancerJ Clin Oncol201028132181219020351327
- RoseJRodriguesGYaremkoBLockMD’SouzaDSystematic review of dose-volume parameters in the prediction of esophagitis in thoracic radiotherapyRadiother Oncol200991328228718950881
- Bar-AdVOhriNWerner-WasikMEsophagitis, treatment-related toxicity in non-small cell lung cancerRev Recent Clin Trials201271313521864251
- UyterlindeWChenCKwintMPrognostic parameters for acute esophagus toxicity in intensity modulated radiotherapy and concurrent chemotherapy for locally advanced non-small cell lung cancerRadiother Oncol2013107339239723647749
- PalmaDASenanSOberijeCPredicting esophagitis after chemoradiation therapy for non-small cell lung cancer: an individual patient data meta-analysisInt J Radiat Oncol Biol Phys201387469069624035329
- ZhangZXuJZhouTRisk factors of radiation-induced acute esophagitis in non-small cell lung cancer patients treated with concomitant chemoradiotherapyRadiat Oncol201495424528546
- ZehentmayrFSohnMExeliAKNormal tissue complication models for clinically relevant acute esophagitis (≥ grade 2) in patients treated with dose differentiated accelerated radiotherapy (DART-bid)Radiat Oncol20151012126018527
- GomezDRTuckerSLMartelMKPredictors of high-grade esophagitis after definitive three-dimensional conformal therapy, intensity-modulated radiation therapy, or proton beam therapy for non-small cell lung cancerInt J Radiat Oncol Biol Phys20128441010101622920974
- HuangEXBradleyJDEl NaqaIModeling the risk of radiation-induced acute esophagitis for combined Washington University and RTOG trial 93-11 lung cancer patientsInt J Radiat Oncol Biol Phys20128251674167921658856
- ZhuJZhangZCLiBSAnalysis of acute radiation-induced esophagitis in non-small-cell lung cancer patients using the Lyman NTCP modelRadiother Oncol201097344945421067834
