Abstract
Purpose
Among men, prostate cancer (PCa) is one of the most commonly diagnosed cancers and the leading cause of cancer death worldwide. Phosphatase and tension homolog (PTEN) acts as a negative regulator of the phosphatidylinositol 3-kinase (PIK3)/Akt pathway and suppresses tumor progression. Meanwhile, PTEN is frequently deleted in PCa. Identifying the specific molecular markers of biochemical recurrence (BCR) in PCa patients is critical in clinical practice. Our systematic review summarizes the evidence about the PTEN expression and BCR rate in PCa patients.
Methods
To clarify the impact of PTEN expression on the PCa BCR rate, a systematic review and meta-analysis was performed by searching the PubMed, Embase, and Web of Science databases, to identify the relevant literature. The analysis of pooled data was performed with Stata 12. The combined odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) were evaluated by the fixed-effects or random-effects models. The combined sensitivity and publication bias were also estimated.
Results
In total, nine articles containing ten independent cohort studies, including 2,154 cases with positive expression of PTEN and 1,006 PTEN deletion cases, were deemed eligible for the meta-analysis. Overall, the positive expression of PTEN was associated with a significantly lower BCR rate (OR =0.521, 95% CI: 0.431–0.630). Subgroup analysis stratified by race revealed that in multiple races (OR =0.215, 95% CI: 0.072–0.648) and Caucasian (OR =0.469, 95% CI: 0.373–0.591) races, positive expression of PTEN showed a significant association with lower BCR rate. Subgroup analysis also showed the significant result in different sample sizes.
Conclusion
PTEN deletion has a relationship with a higher BCR rate in PCa compared with positive expression of PTEN.
Keywords:
Introduction
Prostate cancer (PCa), the most commonly diagnosed cancer in men, is one of the most leading causes of male cancer death all over the world.Citation1,Citation2 The mainly established prognostic factors for PCa are the Gleason score (GS), pathological T (pT) stage, and serum level of prostatic-specific antigen (PSA). The increasing serum PSA level relates to biochemical recurrence (BCR).Citation3 BCR is an early indication of clinical progression, distant metastases, and mortality, indicating that the patients will be treated with secondary treatment.Citation3,Citation4 Previous studies showed that increased phosphorylation of the serine threonine kinase Akt is an important predictor of the risk of BCR in PCa.Citation5,Citation6
Phosphatase and tension homolog (PTEN), located on chromosome 10, acts as a tumor suppressor by inhibiting the phosphatidylinositol 3-kinase (PIK3)/Akt pathway, which is a critical tumor-promoting pathway.Citation7–Citation9 PTEN also can initiate numerous signaling events, including cell proliferation, invasion, and metastasis.Citation9–Citation11 PTEN loss is proposed to be a critically important and frequently occurring molecular event in PCa progression.Citation12–Citation15 Some studies indicated that patients with PTEN deletion were associated with higher pathological stage or GS.Citation5,Citation16 PTEN loss also correlates with earlier BCR in some studies.Citation4,Citation5,Citation17
To confirm the association of PTEN expression with BCR in patients with PCa, we combined the results from the latest published literature on this topic to produce a comprehensive meta-analysis.
Materials and methods
Literature search and inclusion criteria
The electronic databases PubMed, Embase, and Web of Science were searched for studies to add to our meta-analysis. The key words included “PTEN”, “phosphatase and tensin homolog on chromosome 10”, “prostate cancer”, “prostate tumor”, “prostate carcinoma”, “PCa”, “prostate adenocarcinomas”, “prostate specific antigen”, “PSA”, and “biochemical recurrence”.
Literature selected for inclusion in the meta-analysis met the following criteria: 1) published in English; 2) measured PTEN expression in PCa tissue with immunohistochemistry (IHC) or other methods; 3) the patients in every study had positive and lost expression of PTEN; 4) provided information about BCR, GS, and pT stage; and 5) were original epidemiological studies on the correlation between PTEN expression and BCR rate of PCa.
Data extraction
Two reviewers independently extracted studies for inclusion based on the Newcastle Ottawa Scale (NOS), which included three aspects of selection, comparability, and exposure. The scores ranged from 0 to 9. When NOS score was >7, it was considered as high quality.
The following data were collected: first author, year of publication, patient’ country of origin and race, total number of patients, median age of patients at diagnosis, test method, median GS, pT stage, number of patients with BCR, and number of patients with positive and lost expression of PTEN.
There were different definitions on positive and lost expression of PTEN in every study. Since it was hard to distinguish positive and lost expression of PTEN on a unified standard, we respectively combined all patients with lost expression of PTEN and all patients with positive expression of PTEN in our meta-analysis according to their original group in each individual study.
Statistical analysis
The association strength between PTEN expression and BCR rate was measured by odds ratios (ORs), and their 95% confidence intervals (CIs) were also combined to give the effective value. The estimates of pooled ORs were achieved by calculating a weighted average of OR from each study. A 95% CI was used for statistical significance test. If the studies were shown to be homogeneous with P>0.1 for the Q-statistics, the OR was calculated by a fixed-effects model. Otherwise, a random-effects model was selected.
Subgroup analyses were also conducted to explore the effects of confounding factors: ethnicities and sample size. Sensitivity analyses, by which a single study in the meta-analysis was deleted each time, were performed to identify individual study’s effect on pooled results and test the reliability of results. Statistical analysis of the association between PTEN expression and PCa BCR rate was performed with a P-value of <0.05, which was considered as statistically significant.
Results
Characteristics of eligible studies
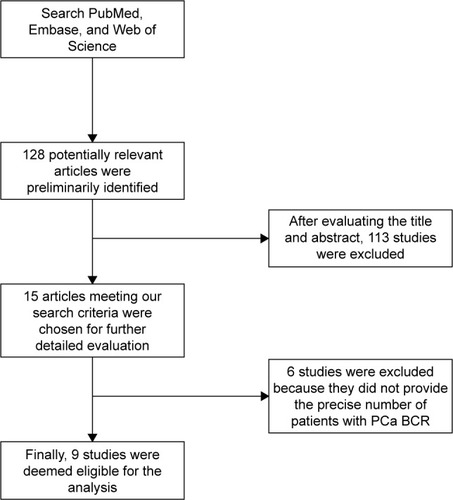
In total, ten retrospective cohort studies from nine articles published between 2007 and 2016 were eligible according to inclusion and exclusion criteria for this systematic review with meta-analysis.Citation4,Citation5,Citation13,Citation17–Citation22 The detailed screening process and the result of each step are shown in . Our search strategy preliminarily identified 128 potentially relevant articles for further evaluation. After further evaluating the title and abstract, a total of 15 articles meeting our search criteria were chosen for further detailed evaluation. After careful, detailed evaluation, we excluded six studies because they did not provide the precise number of patients with PCa BCR. A total of ten studies (nine articles), including a total of 2,154 PTEN positive expression cases and 1,006 PTEN deletion cases, were finally deemed eligible for the analysis.
Figure 1 Flowchart of study selection.
All ten studies were related to clinical research and reported the number of patients with PTEN expression or PCa BCR. All of them were studies of prostatic adenocarcinoma specimens obtained from radical prostatectomy. Among the ten studies, seven studies analyzed PTEN expression quantitatively via IHC and the remaining three studies via fluorescence in situ hybridization. The main characteristics of the ten eligible articles are shown in . The mainly established prognostic factors for PCa, the GS and the pT stage are listed in detail.
Table 1 Characteristics of the included studies
Meta-analysis results
Ten articles that included a total of 2,154 PTEN positive expression cases and 1,006 PTEN deletion cases were used to evaluate the relationship between PTEN expression and PCa BCR rate. All the included studies arrived at a high methodological quality (NOS≥7). All results of this meta-analysis are summarized in .
Table 2 Meta-analyses results overall and by subgroups stratified by race or sample size
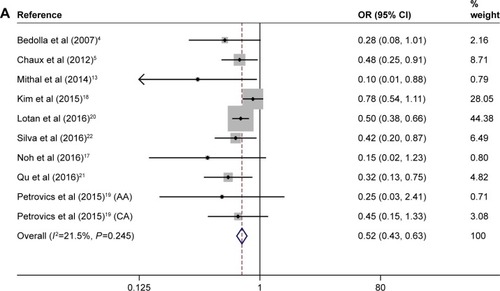
As shown in , a forest plot of meta-analysis for the overall, the OR for all eligible studies evaluating the associations of positive expression of PTEN with BCR rate was 0.521 (95% CI: 0.431–0.630, POR=0.000) calculated by a fixed-effects model (Pheter=0.245), indicating that positive expression of PTEN has a close relationship with lower PCa BCR rate when all eligible studies were pooled into the meta-analysis. In other words, PTEN deletion is associated with a higher BCR rate in PCa.
Figure 2 Forest plots for the association between PTEN expression and BCR rate overall (A) and in subgroups stratified by race (B) and by sample size (C) in PCa.
Abbreviations: AA, African American; BCR, biochemical recurrence; CA, Caucasian American; CI, confidence interval; OR, odds ratio; PCa, prostate cancer; PTEN, phosphatase and tension homolog.
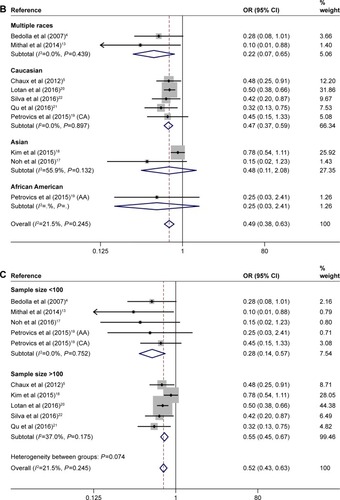
We then performed subgroup analyses to investigate the effect of race and sample size. The subgroup of multiple races consisted of Caucasian, Hispanic, and African American. As shown in , in the subgroups of “Multiple races” (n=2) and “Caucasian” (n=5), there was a statistically lower BCR rate in the PTEN positive expression cases; the ORs of Multiple races and Caucasian were 0.215 (95% CI: 0.072–0.648, POR=0.006) and 0.469 (95% CI: 0.373–0.591, POR=0.000), respectively. However, in the subgroup of “Asian” (n=2), we did not find any significant relationship between positive expression of PTEN and BCR rate, which was analyzed by the random-effects model. The OR of “Asian” was 0.48 (95% CI: 0.111–2.077, POR=0.326). There was only one cohort study evaluating association of positive expression of PTEN with BCR in African American, and the original OR was 0.253 (95% CI: 0.027–2.407, POR=0.232).
There were five studies with a sample size <100, while five studies with a sample size >100. The OR of “<100” sample size was 0.285 (95% CI: 0.143–0.567, POR=0.000), while the OR of “>100” sample size was 0.547 (95% CI: 0.450–0.666, POR=0.000). Subgroup analyses according to sample size suggested that the associations of positive expression of PTEN with BCR rate were significant in both subgroups, as shown in .
In summary, the positive expression of PTEN was associated with lower BCR rate significantly overall and in subgroups according to race or sample size, except in the subgroups of African American and Asian.
Publication bias
Publication bias was assessed by Begg’s test funnel plot and Egger’s test. As shown in , Begg’s test funnel plot was roughly symmetrical (PBegg=0.074). Egger’s test was then performed and showed significant publication bias (PEgger=0.018). The publication bias did exist. Then, we detected which studies were responsible for publication bias by deleting one or some of them.
Figure 3 Funnel plots assessing evidence of publication bias from (A) all ten eligible studies (PBegg =0.074, PEgger =0.018) and (B) the eight studies after excluding Mithal et als’ studyCitation13 and the African American cohort of Petrovics et als’ studyCitation19 (PBegg =0.174072, PEgger =0.079).
Abbreviations: OR, odds ratio; PBegg, P-value for Begg’s test; PEgger, P-value for Egger’s test; SE, standard error.
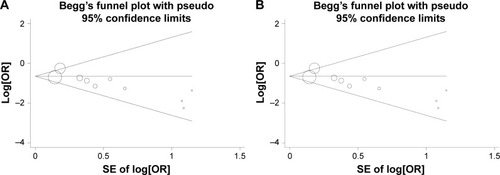
Further analysis suggested that after deleting the Mithal’s study and the African American cohort of Petrovics’ study, the publication bias was reduced (; PBegg=0.174, PEgger=0.079). Considering that the patients in Mithal’s study consisted of a high proportion of African Americans (42/77), we speculated that the publication bias might be resulted from the African American population.
Sensitivity analysis
We conducted sensitivity analyses through sequentially excluding individual studies. When a sensitivity analysis was carried out from ten pooled analysis, the recalculated ORs using a fixed-effects model remained stable (the ORs were <1), as shown in . The Kim’s study had the greatest impact on OR among ten studies, but after deleting it, the OR was still <1. In other words, the results demonstrated that the pooled ORs were not affected by removing every single study each time.
Figure 4 Sensitivity analyses of studies from (A) all ten eligible studies and (B) the eight studies after excluding Mithal et als’ studyCitation13 and the African American cohort of Petrovics et als’ study.Citation19
Abbreviations: AA, African American; CA, Caucasian American.
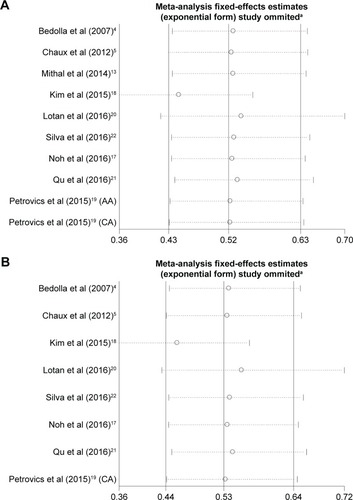
Due to the Mithal’s study and the African American cohort of Petrovics’ study contributing to the publication bias, the sensitivity analysis was also performed after deleting Mithal’s study and the African American cohort of Petrovics’ study. The results suggested that the pooled ORs were <1 and still significant ().
Discussion
PCa is one of the most common malignancies around the world. The mainly established prognostic factors for PCa are GS, pT stage, and serum level of PSA. Recently, a series of novel prognostic biomarkers have also been found for PCa management. For example, transmembrane protease, serine 2 (TMPRSS2)–ETS-related gene (ERG), cysteine-rich secretory protein 3 (CRISP3), fatty acid synthase (FASN), and PTEN were found to be altered in PCa, including fused genes and chromosomal deletion, and have been considered as important prognostic factors for PCa.Citation23 Furthermore, measurement of circulating or urinary microRNAs (miR-NAs) is emerging as a noninvasive tool for PC diagnosis and prognosis.Citation24
The prostate-specific gene TMPRSS2 is fused with ERG in a large proportion of PCa cases and is considered as a prognostic predictor. TMPRSS2 is a prostate-specific, androgen-regulated gene, and its rearrangement leads to high expression of the ETS members.Citation25 ERG, one of the EST members, plays an important role in a variety of biological processes, including cell proliferation, differentiation, angiogenesis, and tumor invasiveness. It is reported that ERG expression was a significantly strong and independent predictor of all stages of PCa progression. The association between ERG fusion and poor prognostic indicators, including higher Gleason grade, pathologic stage, BCR, metastases, and cancer-specific mortality, has been proved.Citation26
CRISP3, belonging to a large family of cysteine-rich secretory proteins, is highly expressed in several exocrine glands, including salivary, pancreas, and prostate glands.Citation6 CRISP3 has been reported to be a direct target of the transcription factor ERG. ERG and CRISP3 mRNA levels were strongly correlated. Both were associated with pT3 disease staging. A higher expression of CRISP3 has been linked to poor prognosis of PCa. It is reported that CRISP3 mRNA was significantly associated with higher GS and BCR.Citation17
FASN is an enzyme that synthesizes long-chain fatty acids. FASN upregulation can lead to Warburg effect in multiple cancer types, including kidney, pancreas, lung, and colorectal cancers. FASN overexpression has also been described in PCa as a significant predictor of cancer progression and pathologic stage.Citation26
miRNAs, the small sequences of noncoding RNAs, regulate specific genes involved in the development of PCa.Citation24 Stable miRNAs have already been found in serum and plasma and played a critical role in tumor initiation, development, and progression. Some miRNAs, including miR-106a, miR-223, and miR-1207, correlated with the Cancer of the Prostate Risk Assessment Post-Surgical (CAPRA-S) score (a PCa risk assessment based on patient age), PSA serum levels, clinical tumor stage, and the GS.Citation26,Citation27 Thus, the measurement of PCa-associated miRNAs is emerging as a promising tool for PCa monitoring.
Other emerging immune-histochemical biomarkers of PCa, membrane-associated guanylate kinase, WW and PDZ domain-containing 2 (MAGI-2), and serine peptidase inhibitor Kazal-type 1 (SPINK1), were also associated with an increased risk of disease progression and BCR.Citation26
PTEN, mapped to chromosome 10q23.3, regulates cell growth, survival, and genome stability by targeting proteins in signaling pathways.Citation10,Citation28 Its genomic deletion has been observed in several human malignancies including PCa and is the major hallmark of tumor invasion and progression.Citation12,Citation29–Citation32 In PCa, PTEN acts as a tumor suppressor by negatively regulating the PIK3/Akt pathway.Citation7
Previous studies have revealed that PTEN deletion is associated with some clinical features in PCa such as higher GS, higher clinical stage, and larger tumor size and is also a prognostic factor.Citation15,Citation20,Citation22,Citation33,Citation34 Some studies have focused on the relationship between PTEN deletion and BCR rate, a principal cause for treatment failure and death in patients with PCa.Citation4,Citation6,Citation21 However, the results are inconclusive and insufficient, thus we performed a systematic meta-analysis to clarify the relationship.
In our analysis, nine articles containing ten independent cohort studies with a total of 3,160 PCa cases were included, and the pooling analyses suggested that positive expression of PTEN was associated with lower BCR rate (in other words, PTEN deletion was an indicator of higher BCR rate in PCa). Subgroup analyses stratified by race or sample size suggested that the associations were significant in subgroups of Multiple races, Caucasian, sample size <100, and sample size >100 but not in Asian, indicating the different genetic contribution to PCa BCR rate among different races. In addition, the previous studies have shown that African Americans have a higher rate of PCa incidence and mortality compared with Caucasian American men, which may be due to the different genetic background. Petrovics et al have found that genomic deletions of LSAMP were more prevalent, while the rate of PTEN and ERG loss was significantly lower in African American cases than that in Caucasian American cases. In our meta-analysis, subgroup analysis of African American could not be performed because only one study reported the association between PTEN and PCa rate in African American population. Publication bias analysis revealed that the Mithal’s study (with a high proportion of African American) and the African American cohort of Petrovics’ study might be responsible for the publication bias. This also demonstrated the different genetic contribution to PCa BCR rate among different races.
Our study is the first meta-analysis to evaluate the effect of PTEN expression on the PCa BCR rate. However, some limitations of this meta-analysis should be noted. First, because the number of studies included in our analysis was small, there is a disproportionate effect on the final analysis, which could not be avoided. Second, the data were only from studies meeting our inclusion criteria, and many other published studies that did not meet these criteria may be missed out. Third, this meta-analysis was based on positive expression of PTEN, so we were only able to evaluate the relationship between positive expression of PTEN and BCR rate, not the individual positive PTEN values.
Conclusion
Our meta-analysis provides evidence of an association between positive expression of PTEN and PCa BCR rate, suggesting that positive expression of PTEN could increase PCa BCR rate. It indicated that PTEN deletion is a potentially novel clinical event to identify individuals at an increased risk for the PCa progression.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2017CA Cancer J Clin201767173028055103
- FerlayJSoerjomataramIDikshitRCancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012Int J Cancer20151365E359E38625220842
- BurdelskiCReiswichVHube-MaggCCytoplasmic accumulation of sequestosome 1 (p62) is a predictor of biochemical recurrence, rapid tumor cell proliferation, and genomic instability in prostate cancerClin Cancer Res201521153471347925925890
- BedollaRPrihodaTJKreisbergJIDetermining risk of biochemical recurrence in prostate cancer by immunohistochemical detection of PTEN expression and Akt activationClin Cancer Res200713133860386717606718
- ChauxAPeskoeSBGonzalez-RoibonNLoss of PTEN expression is associated with increased risk of recurrence after prostatectomy for clinically localized prostate cancerMod Pathol201225111543154922684219
- GruppKKohlSSirmaHCysteine-rich secretory protein 3 overexpression is linked to a subset of PTEN-deleted ERG fusion-positive prostate cancers with early biochemical recurrenceMod Pathol201326573374223196798
- ZhangSYuDPI(3)king apart PTEN’s role in cancerClin Cancer Res201016174325433020622047
- CourtneyKDCorcoranRBEngelmanJAThe PI3K pathway as drug target in human cancerJ Clin Oncol20102861075108320085938
- De VelascoMAUemuraHPreclinical remodeling of human prostate cancer through the PTEN/AKT pathwayAdv Urol20122012112
- WangXJiangXPTEN: a default gate-keeping tumor suppressor with a versatile tailCell Res200818880781618626510
- LinP-CLinJ-KLinH-HA comprehensive analysis of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) loss in colorectal cancerWorld J Surg Oncol201513118625986931
- FerraldeschiRNava RodriguesDRiisnaesRPTEN protein loss and clinical outcome from castration-resistant prostate cancer treated with abiraterone acetateEur Urol201567479580225454616
- MithalPAllottEGerberLPTEN loss in biopsy tissue predicts poor clinical outcomes in prostate cancerInt J Urol201421121209121425099119
- MulhollandDJTranLMLiYCell autonomous role of PTEN in regulating castration-resistant prostate cancer growthCancer Cell201119679280421620777
- KluthMRunteFBarowPConcurrent deletion of 16q23 and PTEN is an independent prognostic feature in prostate cancerInt J Cancer2015137102354236326009879
- EpsteinJIFengZTrockBJPierorazioPMUpgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: incidence and predictive factors using the modified Gleason grading system and factoring in tertiary gradesEur Urol20126151019102422336380
- NohB-JSungJYKimYChangSGParkYKPrognostic value of ERG, PTEN, CRISP3 and SPINK1 in predicting biochemical recurrence in prostate cancerOncol Lett20161163621363027284364
- KimSHKimSHJoungJYOverexpression of ERG and wild-type PTEN are associated with favorable clinical prognosis and low biochemical recurrence in prostate cancerPLoS One2015104e012249825897494
- PetrovicsGLiHStumpelTA novel genomic alteration of LSAMP associates with aggressive prostate cancer in African American menEBioMedicine20152121957196426844274
- LotanTLWeiWMoraisCLPTEN loss as determined by clinical-grade immunohistochemistry assay is associated with worse recurrence-free survival in prostate cancerEur Urol Focus20162218018827617307
- QuXJeldresCGlaskovaLIdentification of combinatorial genomic abnormalities associated with prostate cancer early recurrenceJ Mol Diagn201618221522426752304
- SilvaMPBarros-SilvaJDErsvaerECancer prognosis defined by the combined analysis of 8q, PTEN and ERGTransl Oncol20169657558227916292
- McGrathSChristidisDPereraMProstate cancer biomarkers: are we hitting the mark?Prostate Int20164413013527995111
- BertoliGCavaCCastiglioniIMicroRNAs as biomarkers for diagnosis, prognosis and theranostics in prostate cancerInt J Mol Sci201617342144127011184
- LuLIZhangHPangJHouGLLuMHGaoXERG rearrangement as a novel marker for predicting the extra-prostatic extension of clinically localised prostate cancerOncol Lett20161142532253827073512
- GiannicoGAArnoldSAGellertLLHameedONew and emerging diagnostic and prognostic immunohistochemical biomarkers in prostate pathologyAdv Anat Pathol20171243544
- FilellaXFojLProstate cancer detection and prognosis: from prostate specific antigen (PSA) to exosomal biomarkersInt J Mol Sci2016171117841805
- YoshimotoMCunhaIWCoudryRAFISH analysis of 107 prostate cancers shows that PTEN genomic deletion is associated with poor clinical outcomeBr J Cancer200797567868517700571
- AtreyaCESangaleZXuNPTEN expression is consistent in colorectal cancer primaries and metastases and associates with patient survivalCancer Med20132449650624156022
- ZhuXQinXFeiMLoss and reduced expression of PTEN correlate with advanced-stage gastric carcinomaExp Ther Med201351576423251242
- TanMHMesterJLNgeowJRybickiLAOrloffMSEngCLifetime cancer risks in individuals with germline PTEN mutationsClin Cancer Res201218240040722252256
- PriceTJHardinghamJELeeCKPrognostic impact and the relevance of PTEN copy number alterations in patients with advanced colorectal cancer (CRC) receiving bevacizumabCancer Med20132327728523930204
- TsourlakisMCWeigandPGruppKβIII-tubulin overex-pression is an independent predictor of prostate cancer progression tightly linked to ERG fusion status and PTEN deletionAm J Pathol2014184360961724378408
- BurdelskiCMenanDTsourlakisMCThe prognostic value of SUMO1/Sentrin specific peptidase 1 (SENP1) in prostate cancer is limited to ERG-fusion positive tumors lacking PTEN deletionBMC Cancer20151553826202067
