Abstract
The aim of this work was to investigate the role of chemokines in proliferation and migration of tongue squamous cell carcinoma (TSCC). Out of the 80 cytokines surveyed by a human cytokine antibody array, three chemokines, macrophage inflammatory protein-3α (MIP-3α), macrophage inflammatory protein-1β (MIP-1β), and interferon gamma-induced protein 10 (IP-10), showed elevated expression in TSCC cells (CAL-27 and UM-1), compared to the oral mucosal epithelial cells. Immunohistochemistry confirmed the high level of expression of MIP-3α in the TSCC tissues, especially in the high clinical stages. Furthermore, Western blot and immunofluorescence staining indicated that C-C chemokine receptor type 5, C-C chemokine receptor type 6, and C-X-C motif chemokine receptor 3, which are the receptors for MIP-3α, MIP-1β, and IP-10, respectively, were expressed in the TSCC cells. Viability assay showed MIP-3α, MIP-1β, and IP-10 led to the proliferation of the CAL-27 cells. Interestingly, MIP-1β and IP-10 also induced apoptosis in the TSCC cells. Transwell invasion assay showed MIP-3α and IP-10 could increase the invasive capability of TSCC cells; consistently, the enzymatic activities of matrix metalloproteinase-2 and matrix metalloproteinase-9 increased in the MIP-3α- and IP-10-treated cells. In summary, our results indicate the expression of MIP-3α, MIP-1β, and IP-10 increased in the TSCC cells. The elevated expression of MIP-3α and IP-10 promoted proliferation and migration of TSCC. These chemokines, along with their receptors, could be potential biomarkers and therapeutic targets for TSCC, especially for those in the high clinical stages.
Introduction
Squamous cell carcinoma (SCC) is a common skin cancer, and more than 50% cases are cancers of the oral tongue.Citation1 Tongue squamous cell carcinoma (TSCC) develops through a series of histopathologic stages from mild dysplasia to invasive disease. Despite recent advances in primary prevention and therapeutic intervention, the morbidity and mortality remain high with a 5-year survival of ~50%.Citation2 As such, the community has shown a rising interest in developing new and effective biomarkers and therapeutic targets for the prediction and treatment of TSCC.Citation3
Inflammatory responses play decisive roles at different stages of tumor development.Citation4 Immune/inflammatory cell infiltration into tumor leads to an active and extensive cross talk with cancer cells.Citation4,Citation5 Chemokines are a group of cytokine superfamilies that are capable of inducing and controlling the migration of chemotactic cells, such as monocytes, natural killer cells and dendritic cells.Citation6
Chemokines are divided into four subfamilies – CC, CXC, C and CX3C – based on the signature sequence at the amino terminus region, with CC and CXC being the major classes. Chemokines and their G-protein-coupled receptors are known to play a crucial role in tissue homeostasis, inflammatory responses and the pathogenesis of a variety of diseases.Citation7–Citation10 Chemokines can be induced by other factors, such as inflammatory cytokines, growth factors and pathogenic stimuli, in a variety of cells including tumor cells and tumor-infiltrating immune cells. Blocking the activities of chemokines leads to preclinical antitumor activity in various malignancies.Citation11–Citation13 Chemotherapy and radiotherapy, which are routinely used to kill cancer cells, have shown effects on the expression of chemokines and their receptors, leading to weakened and ineffective therapy.Citation14 Tremendous efforts are under way in searching for antagonists to neutralize chemokine receptors, in combination with conventional chemotherapeutic treatments. These strategies are some of the promising new directions in the therapy of metastatic disease.Citation15 However, the role of many chemokines is not fully understood in the pathogenesis of TSCC. An in-depth study of the chemokine response in patients with TSCC is beneficial for the diagnosis, prognosis and treatment of this type of cancer.
The major objective of this study was to investigate the expression of chemokines in TSCC and their function in proliferation and migration of the cancer cells. We evaluated the expression of 80 cytokines in TSCC cells by a human cytokine antibody array. This assay showed that the expression of three chemokines, macrophage inflammatory protein-3α (MIP-3α), macrophage inflammatory protein-1β (MIP-1β) and interferon gamma-induced protein 10 (IP-10), was significantly elevated in SCC. The functions of these chemokines were further studied by immunofluorescence staining, Western blot analysis, viability assay, apoptosis assay and Transwell invasion assay. Our study provides important insights into the role of MIP-3α, MIP-1β and IP-10 in TSCC, which may serve as biomarkers and potential therapeutic targets for therapy of this type of cancer.
Materials and methods
Ethics statement
Samples of patients were collected during a routine checkup by medical professionals. The study was approved by the Ethics Committee of Stomatological Hospital affiliated with Southern Medical University, and carried out in accordance with the approved guidelines. The study was conducted according to the guidelines of the Declaration of Helsinki.
Cell culture
CAL-27 cells were donated by Shanghai Ninth People’s Hospital affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China. UM-1 cells were donated by the First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China. TCA-8113 cells were purchased from the China Center for Type Culture Collection, Wuhan, China. Human oral mucosa epithelial cells were the keratinized squamous cells from the masticatory mucosa distributed in hard palate. CAL-27, UM-1, TCA-8113 and human oral mucosa epithelial cells were cultured in Dulbecco’s modified eagle’s medium (DMEM), Dulbecco’s modified eagle medium: nutrient mixture F-12 (DMEM/F12), Roswell Park Memorial Institute (RPMI) 1640 and minimum essential medium (MEM) (Thermo Fisher Scientific, Waltham, MA, USA), respectively, all of which were supplied with 10% fetal bovine serum (Hyclone, Logan, UT, USA).
Human cytokine antibody assay
To investigate the expression of cytokines, cells in serum-free media (24 h) were collected and subjected to analysis by Human Cytokine Antibody Array 5 (RayBiotech, Norcross, GA, USA) according to the manufacturer’s instructions. The slide was analyzed using a GenPix 4000B scanner (Axon Instrument, Burlingame, CA, USA). The values were normalized and the background was reduced. The relative expression level of cytokines was determined by comparing signal intensities.
Enzyme-linked immunosorbent assay
Out of the 80 cytokines, the expression of MIP-3α, MIP-1β and IP-10 was further validated by enzyme-linked immunosorbent assay (ELISA) kits (Sigma-Aldrich Co., St Louis, MO, USA) following the manufacturer’s instructions. The supernatant was taken, and the absorbance at 450 nm was determined using the ELISA kits for MIP-1I3, MIP-3α and IP-10. The concentrations of MIP-3α, MIP-1β and IP-10 were calculated based on the standard curve of the OD values against known concentrations of MIP-3α, MIP-1β and IP-10 standard solutions.
Immunohistochemistry
A total of 46 paraffin-embedded oral TSCC sections from patients diagnosed between January 2006 and December 2012 were obtained from the Department of Pathology, Guangdong Provincial Stomatological Hospital, Guangzhou, China, with the consent of the patients. None of the patients had received any form of tumor-specific therapy before the surgery, and no distant metastases were observed. A total of 20 normal tongue mucosal tissues obtained from the same hospital were used as the control group. The volunteers in the control group have provided written informed consent. According to the International Union against Cancer TNM classification, the cases can be classified into stages I (14), II (10), III (14) and IV (8). According to the criteria set by the World Health Organization in 1997, the cases can be classified into grades I (20) and II (26). Among these, 17 cases had lymph node metastasis.
Tissue sections of 4 μm thickness from the paraffin- embedded TSCC blocks were used for immunohistochemical (IHC) staining. These sections were deparaffinized in xylene and rehydrated through an alcohol gradient. They were subjected to antigen retrieval by conventional microwave heating (750 W) in 0.01 M sodium citrate retrieval buffer (pH 6.0) for 5 min. Endogenous peroxidase activities were blocked with 3% hydrogen peroxide. The sections were then incubated with a rabbit CCL20/MIP-3α monoclonal antibody (1:100; Shanghai Tianyuan Biotech Inc., Shanghai, China) overnight at 4°C and subsequently incubated with goat anti-rabbit immunoglobulin (1:5,000; Shanghai Shifeng Biotech Inc., Shanghai, China) at 20°C for 20 min. The sections were then washed three times with PBS (pH 7.2) for 2 min. The reaction product was developed with 3,3′-diaminobenzidine and counterstained with hematoxylin. Immunoreactivity in the tissue was judged independently by two pathologists who were blinded to the clinical data and other IHC results. TSCC tissues were used as positive control. Negative controls were included in each slide run with omission of primary and secondary antibodies.
Immunoreactivity was semiquantitatively evaluated based on the immunoreactive score (IRS) method.Citation16 IRS is the product of the intensity of staining (SI) and the percentage of positive cells (PP). The SI was defined as: 0, negative; 1, weak, light yellow; 2, moderate, tan and 3, strong, dark brown; the PP was scored as: 0, negative; 1, <10%; 2, 10%–50%; 3, >50%–80% and 4, >80% positive cells. IRS ranged from 0 to 12. IHC results with an IRS of 0–1 were considered negative (−), 2–3 as weak positive (+), 4–5 as moderate positive (++) and ≥6 as strong positive (+++).
Western blotting
CAL-27, UM-1 and TCA-8113 cell lysates were electrophoresed on a 10% acrylamide gel and immunoblotted onto nitrocellulose membranes. The nitrocellulose membranes were washed, blocked with 5% nonfat dry milk in tris-buffered saline (TBS) for 90 min at 25°C and then incubated overnight with rabbit polyclonal antibodies for C-C chemokine receptor type 5 (CCR5; 1:1,000), C-C chemokine receptor type 6 (CCR6; 1:1,000) and C-X-C motif chemokine receptor 3 (CXCR3; 1:1,000), following the manufacturer’s instructions (Bioss Inc., Beijing, China). Membranes were washed and then incubated with goat anti-rabbit IgG for 1 h, before they were visualized by enhanced chemiluminescence (ECL; Amersham, Piscataway, NJ, USA) on an X-ray film.
Immunofluorescence staining
CAL-27, UM-1 and TCA-8113 cells were subjected to trypsin digestion before being placed in six-well plates filled with a small amount of culture medium. Cells were seeded on coverslips for incubation overnight at 37°C in a saturated air humidity/5% CO2 incubator. The cells were fixed in 4% (v/v) paraformaldehyde in PBS for 30 min at room temperature, before incubating them with rabbit polyclonal antibodies for CCR5 (1:1,000), CCR6 (1:1,000) and CXCR3 (1:1,000) at 4°C. Negative controls were samples with the omission of primary antibodies. The cells were incubated in Cy3 conjugated goat anti-rabbit IgG secondary antibody at 37°C for 2 h. The nuclei were counterstained with 4′,6′-diaminino-2-phenlindole. The cells were mounted over fluorescence mounting medium (Dako Denmark A/S, Glostrup, Denmark) and observed using a confocal laser scanning microscope imaging system (Olympus Corporation, Tokyo, Japan).
Cell proliferation assay
CAL-27 cells were digested by 0.25% trypsin and resuspended in DMEM containing 10% fetal bovine serum. The cell density was adjusted to 3.5×104 cells per milliliter before the cells were planted into 96-well plates. The cells were divided into three groups (l0, 20 and 40 ng/mL of the chemokines) and one control group (PBS), according to the concentrations of MIP-3α, MIP-1β and IP-10. After being stimulated for 12, 24 and 48 h, cell viability was analyzed with a Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) according to the manufacturer’s instructions. For each well, 10 μL Cell Counting Kit-8 solution was added, and the cells were incubated at 37°C for 3 h. The absorbance was measured at 450 nm.
Cell invasion assay
The invasion of CAL-27 cells was evaluated by using a 24-Transwell insert with 8-μm pores coated with Matrigel (Corning Inc., Corning, NY, USA). CAL-27 cells (1×105) were suspended in fetal bovine serum (FBS)-free medium and cocultured with 80 ng/mL MIP-3α, MIP-1β and IP-10 cells for 24 h. A total of 200 mL cells were placed in the Transwell chambers, and the lower chambers were then filled with 500 mL RPMI 1640 media containing 10% FBS. The plates were incubated at 37°C in a 5% CO2 atmosphere for 24 h. After incubation, the CAL-27 cells remaining in the upper side of the insert were removed with a swab, and the cells that had infiltrated the lower side of the insert were fixed with 100% methanol and stained with 0.1% crystal violet for 10 min. The stained nuclei were counted. All experiments were performed in triplicate.
Statistical analysis
Statistical analysis was performed with the SPSS 1.3.0 software. The clinical specimens were analyzed by chi-square test. The ELISA and apoptosis data were analyzed using one-way analysis of variance (ANOVA). The differences in cell proliferation were tested by a factorial analysis, and intragroup differences were evaluated using the least significant difference test. Independent samples t-test was used for the evaluation of statistical significance for gelatin zymography and invasion experiments. The results were expressed as mean ± SD of at least three experiments. P<0.05 was considered to be statistically significant.
Results
Screening of the cytokines with elevated expression in the TSCC cell lines
Among the 80 cytokines tested by the human cytokine antibody array, 12 showed significant expression changes in the TSCC, including interleukin (IL)-lβ, monocyte chemotactic protein 4, regulated on activation normal T-cell expressed and secreted, insulin-like growth cytokine binding protein 4, transforming growth cytokine-β3, IL-6, IL-8, tumor necrosis factor-α (TNF-α), IP-10, MIP-1β, MIP-3α and osteoprotegerin (OPG).
Among these cytokines, IL-1β, IL-6, IL-8, TNF-α and OPG had increased the expression in TSCC cell lines compared with the squamous cells, whereas monocyte chemotactic protein 4 had decreased expression (). The expression levels of IP-l0, MIP-1β and MIP-3α were increased in UM-1 and CAL-27, though their expression showed a slight decrease in TCA-8113 (). The expression of IP-l0, MIP-1β and MIP-3α was further validated by ELISA, which was in agreement with the antibody assay (), indicating these cytokines may participate in different mechanisms in these cell lines. In this study, we mainly focused on the investigation of the functions of IP-l0, MIP-1β and MIP-3α in TSCC. Other cytokines, such as IL-1β, IL-6 and TNF-α, will be further investigated in the future.
Table 1 Relative intensity of fluorescence signals between UM-1, CAL-27, TCA-8113 and squamous cells
Table 2 Expression of IP-l0, MIP-1β, and MIP-3α detected by ELISA
Expression of MIP-3α increased in the TSCC
MIP-3α was expressed in both normal tongue tissues and tongue cancer tissues as shown by the immunohistochemistry assay. The positive rates of MIP-3α in normal tongue tissues and TSCC tissues were 30% (6/20) and 78% (36/46), respectively. The expression of MIP-3α in tongue cancer carcinoma was significantly higher than that in the squamous cell group (χ2=14.030, P<0.05; ). MIP-3α was mainly located in the cytoplasm, with a small amount in the nucleus. The high expression level of MIP-3α in the cytoplasm and nucleus of the TSCC was indicated by a dark yellow staining, whereas in the tongue mucosa epithelial cells, the staining was lighter (). The expression of MIP-3α in the TSCC was correlated with the lymph node metastasis and the clinical stages (P<0.05), but not with the pathologic grade of the tongue cancer (P>0.05; ). Our results also showed that MIP-3α had high expression levels in TSCC in the high clinical stages.
Figure 1 High expression of MIP-3α in the cytoplasm and nucleus of TSCC.
Abbreviations: MIP, macrophage inflammatory protein; TSCC, tongue squamous cell carcinoma.
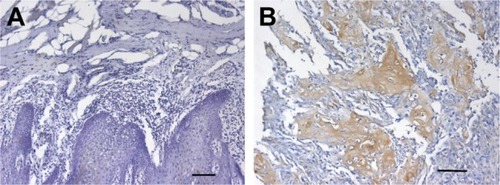
Table 3 Expression of MIP-3α in the TSCC and squamous cells
Table 4 Expression of MIP-3α in the TSCC cells was correlated with the lymph node metastasis and the clinical stages (P<0.05), but not with the pathologic grade of the tongue cancer
Expression of the corresponding receptors of MIP-3α, MIP-1β and IP-10 in the TSCC
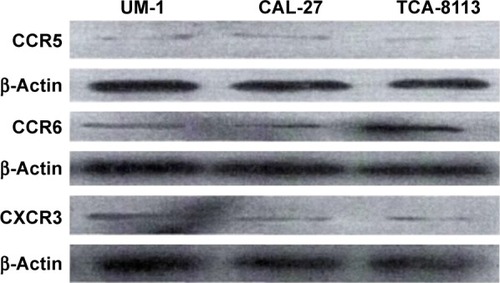
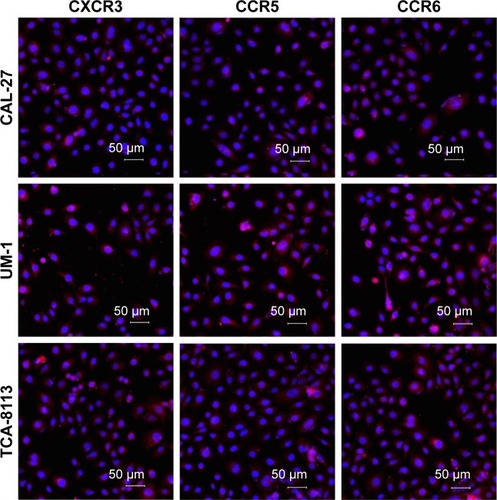
The expression levels of the corresponding receptors of MIP-3α, MIP-1β and IP-10 were investigated by Western blot analysis and immunofluorescence staining. Bands at 40.5, 42.5 and 40.7 kDa were observed for CCR5, CCR6 and CXCR3, respectively, indicating CCR5, CCR6 and CXCR3 were expressed in all three human TSCC cell lines (CAL-27, UM-1 and TCA-8113; ). Expression of CCR5, CCR6 and CXCR3 was further investigated by immunofluorescence staining, which showed that these receptors were expressed in both cell membrane and cytoplasm in CAL-27, UM-1 and TCA-8113 cells ().
MIP-3α, MIP-1β and IP-10 promoted proliferation in CAL-27 cells
The results showed that the proliferation of CAL-27 cells was MIP-1β concentration dependent (F=23.476, P=0.000), but not time dependent (F=1.761, P=0.150), as shown in . ANOVA showed that MIP-1β in different concentrations could promote the proliferation of CAL-27 cells at 12 and 24 h (P<0.05). At 48 h, 10 and 20 ng/mL MIP-1β could promote proliferation of CAL-27 cells (P<0.05), but 40 ng/mL MIP-1β could not (P>0.05), which means when set at 40 ng/mL, the effect of MIP-1β on proliferation declined with time and no effect was observed at 48 h.
Figure 4 Viability assays show the proliferation of CAL-27 cells by IP-10 (A), MIP-3α (B), and MIP-1β (C).
Abbreviations: IP-10, interferon gamma-induced protein 10; MIP-1β, macrophage inflammatory protein-1β; MIP-3α, macrophage inflammatory protein-3α.
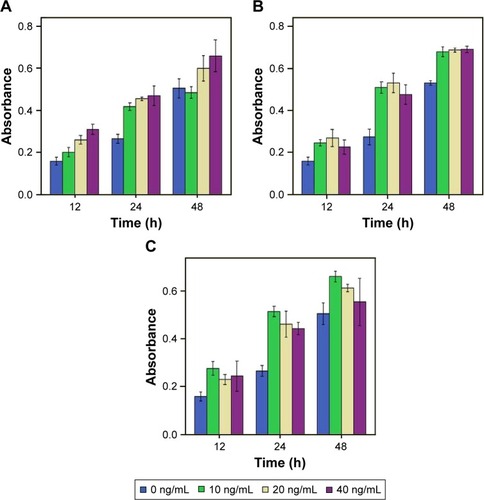
For MIP-3α and IP-10, both the concentration and the time of treatment had a significant effect on the proliferation of CAL-27 cells. ANOVA showed that MIP-3α could promote the proliferation of CAL-27 cells at 12, 24 and 48 h (P<0.05). However, the effect was not concentration dependent for MIP-3α (P>0.05). At 12 h, IP-10 could promote the proliferation of CAL-27 (P<0.05) in a concentration-dependent manner, as shown in . At 24 h, the effect was concentration dependent. At 48 h, only IP-10 at 40 ng/mL could promote CAL-27 proliferation (P<0.05).
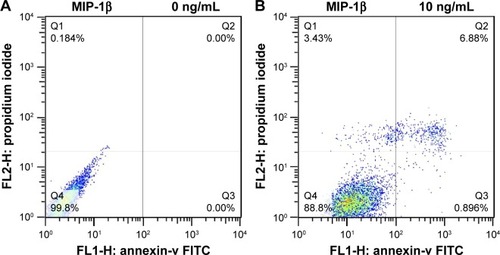
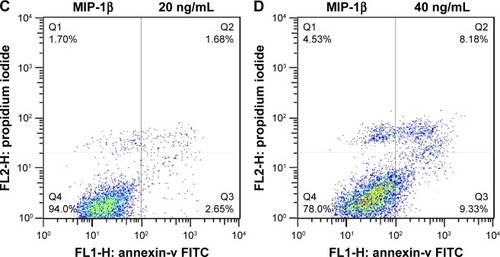
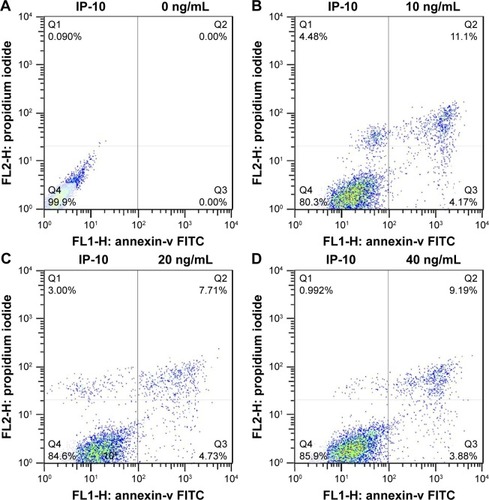
MIP-1β and IP-10 induced apoptosis in CAL-27 cells
MIP-1β and IP-10 had significant effects on CAL-27 cell apoptosis at a higher concentration (40 ng/mL; and ), while at lower concentrations, there was no obvious effect. MIP-3α did not show any significant effect on CAL-27 cell apoptosis at the concentrations tested (10, 20 and 40 ng/mL).
MIP-3α and IP-10 increased invasive capability of the TSCC cells
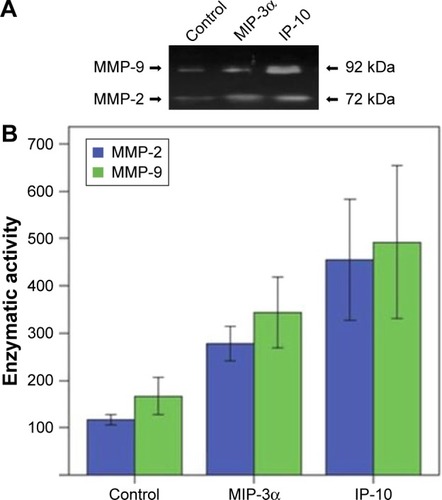
Transwell invasion assay showed MIP-3α and IP-10 could increase the invasive capability of TSCC cells (P<0.05), while MIP-1β could not (P>0.05; ). The enzymatic activities of matrix metalloproteinase (MMP)-2 and MMP-9 were compared in the TSCC cells (). The results showed that the MMP-2 and MMP-9 activities were significantly increased in the MIP-3α- and IP-10-treated cells (P<0.05), which is consistent with the results of the Transwell invasion assay.
Figure 7 Invasive capability of TSCC cells by Transwell invasion assay.
Abbreviations: IP-10, interferon gamma-induced protein 10; MIP, macrophage inflammatory protein; TSCC, tongue squamous cell carcinoma.
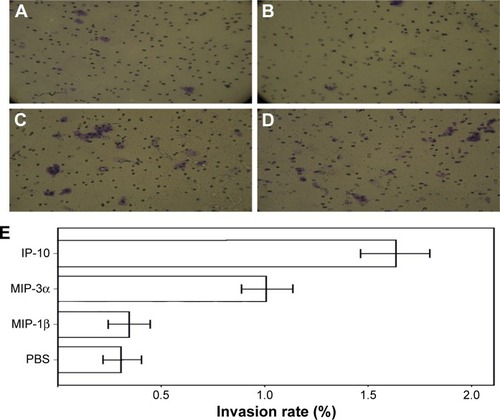
Figure 8 MMP-2 and MMP-9 activities were significantly increased in the MIP-3α-and IP-10-treated cells.
Abbreviations: IP-10, interferon gamma-induced protein 10; MIP-1β, macrophage inflammatory protein-1β; MIP-3α, macrophage inflammatory protein-3α; MMP, matrix metalloproteinase; TSCC, tongue squamous cell carcinoma.
Discussion
Previous studies have noted the importance of chemokines and chemokine receptors in SCC.Citation6,Citation17,Citation18 In this study, we surveyed the expression of 80 cytokines in TSCC by a human cytokine antibody array, which is a rapid and accurate screening method to find the cytokines with different expression in different human tongue cancer cell lines and squamous cells. High expression levels of three chemokines, MIP-3α, MIP-1β and IP-10, were observed in the TSCC cells, especially CAL-27 and UM-1, indicating the chemokines play different roles in these cell lines.
IP-10, expressed from the CXCL10 gene, was first identified in a lymphoma cell line treated with recombinant interferon-γ.Citation19 IP-10 induces chemotaxis, apoptosis, cell growth inhibition and angiostasis, and has been linked to infectious diseases, immune dysfunction and tumor development.Citation20 The elevated expression of IP-10, as well as its receptor CXCR3,Citation21–Citation23 has been found to be associated with advanced-stage tumors, such as malignant melanoma, ovarian carcinoma and B-cell lymphoma.Citation24–Citation26 Rentoft et al showed the expression of CXCL10 was upregulated by 16-fold response to radiotherapy, which is correlated with the low overall survival in SCC of the tongue.Citation18 As such, it can be potentially a biomarker for a variety of diseases.
MIP-1β, also know as chemokine (C-C motif) ligand 4 (CCL4), was also observed with a higher expression. MIP-1β is a C-C chemokine executing its function through CCR5 receptors. It provokes chemotaxis for natural killer cells, monocytes and a variety of other immune cells.Citation27 MIP-1β is involved in the macrophage-modulated migration and invasion of human TSCC by recruiting M1 macrophages to tumor sites.Citation28 In addition to lymph node metastasis, CCL4 expression can also be used as an independent predictor for esophageal SCC patients’ survival.Citation20 A significant decrease in serum MIP-1β level was observed in patients with head and neck squamous cell cancer, irrespective of the primary tumor site.Citation29 However, in our study, we observed a significant increase of MIP-1β.
MIP-3α, also known as chemokine (C-C motif) ligand 20 (CCL20), is located on 2q35–36, including four exons and three introns. MIP-3α is a chemokine of C-C subfamily, and its specific receptor is CCR6. The interaction between MIP-3α and dendritic cells and T cells plays an important role in tumor cell immunity and autoimmune diseases.Citation30 MIP-3α is involved in oral immune response to bacterial infection and may cause the growth of oral SCC (OSCC).Citation31 MIP-3α was expressed in OSCC samples and in six different OSCC lines.Citation31 One study showed that overexpression of MIP-3α in oral cavity SCC is associated with nodal metastasis.Citation17 When MIP-3α was knocked down by interfering RNA, the suppression of CCL20 in the SCC cell lines reduced migration and invasion.Citation17
Elevated expression was observed for other proteins in the assay. For example, OPG had an elevated expression in the UM-1 and TCA-8113 cells, but not as significant as in the CAL-27 cells. A previous study has observed a similar phenomenon, which shows that notch signaling regulated OPG expression in HSC-4 cells, but not in another human OSCC cell line (HSC-5) or a human head and neck SCC cell line (HN22).Citation32 OPG and other chemokines that were not investigated in this study deserve a further pursue.
Immunohistochemistry confirmed the elevated expression of MIP-3α in the TSCC tissues, especially during the high clinical stages. The chemokine receptors, CCR5, CCR6 and CXCR3, were also expressed in the TSCC cells. Our results showed that MIP-3α, MIP-1β and IP-10 promoted the proliferation of TSCC cells. However, MIP-1β and IP-10 could also induce apoptosis of TSCC cells at a high concentration (40 ng/mL). Some chemokines, such as TNF-α, can induce apoptosis in many cell types by recruiting death-effector domain containing protein caspase-8 to the receptor complex.Citation33 For IP-10, one study showed that it induces cell apoptosis and inhibits viral replication in Tet-On HeLa cells;Citation34 another study showed IP-10, in combination with IL-2 and/or interferon-α, induces apoptosis in T lymphocytes.Citation35 MIP-1β and IP-10 could play different roles under different microenvironments.
Transwell invasion assay showed MIP-3α and IP-10 could promote TSCC cell invasion, while MIP-1β could not. MMP-2 and MMP-9 activity increased in MIP-3α- and IP-10-treated cells, which is consistent with a previous study. Campbell et al demonstrated MIP-3α can promote tumor cell migration by upregulation of MMPs and pancreatic cancer invasion through upregulation of MMP-9 by binding to the transmembrane receptor CCR6.Citation36 The effect of IP-10 on cancer cell invasion was also investigated. Zipin-Roitman et al showed that IP-10 can significantly upregulate invasion by promoting the expression of MMP-9 in colorectal carcinoma cells.Citation37
Our study suggests that although the expression levels of these chemokines were elevated, their functions and impact on TSCC cell proliferation and migration are different. Further experiments, such as siRNA knockdown, need to be undertaken to fully understand the function of these chemokines in TSCC.
In summary, our results showed that MIP-3α, MIP-1β and IP-10 had high expression in CAL-27 and UM-1. Immunohistochemistry confirmed the high level of expression of MIP-3α in TSCC tissues, especially in the high clinical stages. According to cell proliferation assay, the proliferation of TSCC cells was promoted by MIP-3α, MIP-1β and IP-10. Invasive cell assay demonstrated that the invasive capability of the TSCC cells was increased by MIP-3α and IP-10. Taken together, our results showed these chemokines played important roles in TSCC cells. MIP-3α, MIP-1β and IP-10 and their receptors could be potentially used as biomarkers and therapeutic targets for TSCC, especially for those in high clinical stages.
Disclosure
The authors report no conflicts of interest in this work.
References
- GanlyIPatelSShahJEarly stage squamous cell cancer of the oral tongue–clinicopathologic features affecting outcomeCancer2012118110111121717431
- RothenbergSMEllisenLWThe molecular pathogenesis of head and neck squamous cell carcinomaJ Clin Invest201212261951195722833868
- SahuNGrandisJRNew advances in molecular approaches to head and neck squamous cell carcinomaAnticancer Drugs201122765666421178766
- GrivennikovSIGretenFRKarinMImmunity, inflammation, and cancerCell2010140688389920303878
- MantovaniAAllavenaPSicaABalkwillFCancer-related inflammationNature2008454720343644418650914
- da SilvaJMSoaveDFMoreira Dos SantosTPSignificance of chemokine and chemokine receptors in head and neck squamous cell carcinoma: a critical reviewOral Oncol20165681627086481
- BalkwillFCancer and the chemokine networkNat Rev Cancer20044754055015229479
- CharoIFRansohoffRMThe many roles of chemokines and chemokine receptors in inflammationN Engl J Med2006354661062116467548
- ChowMTLusterADChemokines in cancerCancer Immunol Res20142121125113125480554
- OoYHShettySAdamsDHThe role of chemokines in the recruitment of lymphocytes to the liverDig Dis Basel Switz20102813144
- BranaICallesALoRussoPMCarlumab, an anti-C-C chemokine ligand 2 monoclonal antibody, in combination with four chemotherapy regimens for the treatment of patients with solid tumors: an open-label, multicenter phase 1b studyTarget Oncol201510111112324928772
- PientaKJMachielsJPSchrijversDPhase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancerInvest New Drugs201231376076822907596
- SandhuSKPapadopoulosKFongPCA first-in-human, first-in-class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumorsCancer Chemother Pharmacol20137141041105023385782
- MatsumuraSWangBKawashimaNRadiation-induced CXCL16 release by breast cancer cells attracts effector T cellsJ Immunol Baltim Md 19502008181530993107
- MullerASonkolyEEulertCChemokine receptors in head and neck cancer: association with metastatic spread and regulation during chemotherapyInt J Cancer200611892147215716331601
- RemmeleWStegnerHERecommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissuePathol198783138140 German
- ChangKPKaoHKYenTCOverexpression of macrophage inflammatory protein-3α in oral cavity squamous cell carcinoma is associated with nodal metastasisOral Oncol201147210811321163685
- RentoftMCoatesPJLoljungLWilmsTLaurellGNylanderKExpression of CXCL10 is associated with response to radiotherapy and overall survival in squamous cell carcinoma of the tongueTumor Biol J Int Soc Oncodevelopmental Biol Med201435541914198
- LusterADUnkelessJCRavetchJVGamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteinsNature198531560216726763925348
- LiuJYLiFWangLPCTL- vs Treg lymphocyte-attracting chemokines, CCL4 and CCL20, are strong reciprocal predictive markers for survival of patients with oesophageal squamous cell carcinomaBr J Cancer2015113574775526284335
- LoBKKYuMZlotyDCowanBShapiroJMcElweeKJCXCR3/ligands are significantly involved in the tumorigenesis of basal cell carcinomasAm J Pathol201017652435244620228225
- LoetscherMGerberBLoetscherPChemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytesJ Exp Med199618439639699064356
- SallustoFLenigDMackayCRLanzavecchiaAFlexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytesJ Exp Med199818768758839500790
- FuruyaMSuyamaTUsuiHUp-regulation of CXC chemokines and their receptors: implications for proinflammatory microenvironments of ovarian carcinomas and endometriosisHum Pathol200738111676168717707463
- JonesDBenjaminRJShahsafaeiADorfmanDMThe chemokine receptor CXCR3 is expressed in a subset of B-cell lymphomas and is a marker of B-cell chronic lymphocytic leukemiaBlood200095262763210627472
- MonteagudoCMartinJMJordaELlombart-BoschACXCR3 chemokine receptor immunoreactivity in primary cutaneous malignant melanoma: correlation with clinicopathological prognostic factorsJ Clin Pathol200760659659916522748
- BystryRSAluvihareVWelchKAKallikourdisMBetzAGB cells and professional APCs recruit regulatory T cells via CCL4Nat Immunol20012121126113211702067
- PiriläEVäyrynenOSundquistEMacrophages modulate migration and invasion of human tongue squamous cell carcinomaPLoS One2015103e012089525811194
- KaskasNMMoore-MedlinTMcClureGBEkshyyanOVanchiereJANathanCAOSerum biomarkers in head and neck squamous cell cancerJAMA Otolaryngol – Head Neck Surg2014140151124232368
- OppenheimJJYangDBiragynAHowardOZPlotzPChemokine receptors on dendritic cells promote autoimmune reactionsArthritis Res Ther20024Suppl 3S183S188
- AbikoYNishimuraMKusanoKExpression of MIP-3alpha/CCL20, a macrophage inflammatory protein in oral squamous cell carcinomaArch Oral Biol200348217117512642237
- ManokawinchokeJOsathanonTPavasantPRegulation of osteoprotegerin expression by Notch signaling in human oral squamous cell carcinoma cell lineAsian Pac J Trop Biomed201668692697
- SpragueAHKhalilRAInflammatory cytokines in vascular dysfunction and vascular diseaseBiochem Pharmacol200978653955219413999
- ZhangHMYuanJCheungPGamma interferon-inducible protein 10 induces HeLa cell apoptosis through a p53-dependent pathway initiated by suppression of human papillomavirus type 18 E6 and E7 expressionMol Cell Biol200525146247625815988033
- SidahmedAMELeónAJBosingerSECXCL10 contributes to p38-mediated apoptosis in primary T lymphocytes in vitroCytokine201259243344122652417
- CampbellASAlboDKimseyTFWhiteSLWangTNMacrophage inflammatory protein-3alpha (mip-3alpha) promotes pancreatic cancer cell invasion by upregulation of matrix metalloproteinase-9 (MMP-9) productionJ Surg Res20031142250251
- Zipin-RoitmanAMeshelTSagi-AssifOCXCL10 Promotes invasion-related properties in human colorectal carcinoma cellsCancer Res20076773396340517409450