Abstract
Purpose
The purpose of this meta-analysis was to explore the influences of pretreatment de novo and posttreatment-acquired epidermal growth factor receptor (EGFR) T790M mutations in patients with advanced non-small cell lung cancer (NSCLC) who had received tyrosine kinase inhibitors (TKIs).
Methods
We searched PubMed, Embase, and the China National Knowledge Infrastructure database for eligible literature. Data were extracted to assess the hazard ratios (HRs) for progression-free survival (PFS), overall survival (OS), and post-progression survival (PPS) and the relative ratios (RRs) for objective response rate (ORR).
Results
This meta-analysis included 22 studies comprising 1,462 patients with NSCLC who harbored activating EGFR mutations and were treated with EGFR-TKIs. Compared to pretreatment T790M mutation-negative NSCLC, pretreatment T790M mutation-positive NSCLC was associated with decreased PFS (HR 2.23, P<0.001) and OS (HR 1.55, P=0.003). A trend toward significance of worsening ORR (RR 0.86, P=0.051) was evident. The acquired T790M mutation was correlated with improved PFS (HR 0.75, P=0.006) and PPS (HR 0.57, P<0.001), compared to patients without the T790M mutation who progressed after EGFR-TKI treatment. There were no significant differences in OS or ORR between patients with acquired T790M mutation-positive and T790M mutation-negative NSCLC. However, in the tumor tissue rebiopsy subgroup, patients with acquired T790M mutation had improved OS (HR 0.60, P<0.001) compared to T790M mutation-negative patients. In the plasma ctDNA subgroup, acquired T790M mutation decreased the OS (HR 1.87, P<0.001).
Conclusion
Pretreatment T790M mutation was associated with worse PFS and OS in patients with advanced NSCLC treated with EGFR-TKIs, while acquired T790M mutation was associated with longer PFS and PPS than T790M mutation-negative NSCLC. The effects on OS were different between acquired T790M mutation detected from rebiopsy of tumor tissue and that detected from plasma ctDNA.
Background
Non-small cell lung cancer (NSCLC) accounts for more than 85% of lung cancers; half of the cases of NSCLC are classified as adenocarcinoma. Approximately 30%–50% of Asian and 10%–17% of Caucasian patients with lung adenocarcinoma harbor activating epidermal growth factor receptor (EGFR) mutations.Citation1,Citation2 EGFR-tyrosine kinase inhibitors (TKIs) such as gefitinib and erlotinib are the preferred treatment for patients with activating EGFR mutations (a deletion in exon 19 [19del] and a point mutation in exon 21 leading to substitution of leucine for arginine at position 858 [L858R]). Treatment with EGFR-TKIs achieves a significantly improved objective response rate (ORR) of 60%–80% and a progression-free survival (PFS) of 9–13 months, which are significantly improved outcomes compared to those achieved with chemotherapy.Citation3,Citation4
Nevertheless, most patients who initially respond to EGFR-TKIs will eventually acquire resistance. Approximately 50%–60% of the cases of resistance are mediated by a secondary T790M mutation (ie, a threonine-to-methionine substitution at amino acid position 790 in exon 20 [T790M]). The T790M mutation can induce steric hindrance to EGFR-TKIs and increase the affinity of the receptor to adenosine triphosphate, relative to its affinity to EGFR-TKIs, which abolishes the effect of EGFR-TKIs.Citation5 AZD9291 (osimertinib [Tagrisso]) is the only third-generation EGFR-TKI approved by the US Food and Drug Administration for the treatment of acquired T790M mutation-positive advanced NSCLC after secondary resistance to first- or second-generation EGFR-TKIs. Significant improvements in PFS and ORR were observed in a phase I/II study of this drug.Citation6–Citation8 Some retrospective studies have observed that patients who experienced disease progression with or without acquired T790M mutation after EGFR-TKI therapy might have different prognoses.Citation9–Citation22 However, low rebiopsy rates and low sensitivities of detection methods after acquired resistance to EGFR-TKIs are challenging for clinical practice. Therefore, the development of noninvasive rebiopsy samples, such as plasma circulating tumor DNA (ctDNA), and high-sensitivity detection methods, such as digital polymerase chain reaction and next generation sequencing, is essential for monitoring dynamic changes in genes and selecting appropriate treatment strategies. Recently, the detection of EGFR mutations using plasma ctDNA and polymerase chain reaction-based or next generation sequencing methods has been confirmed as a feasible alternative strategy, if tumor tissue is not available. A moderate concordant rate of 65% in E20 T790M mutations between tumor and plasma ctDNA has been reported; this contrasts the high concordant rate of 90% in E19del and E21 L858R mutations.Citation23,Citation24
Recently, researchers have also explored the relationship between prognosis and pretreatment T790M mutation.Citation25–Citation32 Increasing evidence has indicated that T790M may exist at a low frequency within the tumor cells before EGFR-TKI treatment and may become the dominant clone only after drug selection pressure of EGFR-TKI treatment.Citation25 Although reliable and widely accepted methods for detecting EGFR T790M mutation status have not yet been established, some researchers have attempted to detect T790M mutation before EGFR-TKI treatment using different assays with sensitivities ranging from 0.001% to 0.4%.Citation25–Citation32 This meta-analysis explored the influences of acquired T790M mutation following EGFR-TKI treatment and de novo T790M mutation prior to EGFR-TKI treatment on survival and prognosis in patients with advanced NSCLC who had activating EGFR mutations.
Methods
Literature search
PubMed, Embase, China National Knowledge Infrastructure database, and abstracts from major scientific meetings were searched for relevant articles published up to July 5, 2016. The following search terms were used: 1) lung cancer OR non-small cell lung cancer OR NSCLC; 2) T790M; and 3) progression-free survival (PFS) OR overall survival (OS) OR progression. The computer searches were supplemented with a manual search of the references listed in all retrieved review articles, primary studies, and meeting abstracts.
Study selection
Eligible studies for the pretreatment T790M group met several criteria. First, patients were confirmed to have advanced or recurrent NSCLC with activating EGFR mutations (19del or L858R mutation), and the status of the T790M mutation was detected before treatment with single-agent EGFR-TKI, that is, erlotinib or gefitinib (there was no limitation to the detection method). In the studies, EGFR-TKIs must have been used for the first time. Also, the study must have contained PFS or OS outcome data based on T790M mutation status; the corresponding hazards ratios (HRs) and 95% confidence intervals (CIs) could be directly obtained or calculated. Finally, PFS was defined as the time from the start of EGFR-TKI treatment to the first disease progression or death from any reason without progression; OS was defined as the time from the start of EGFR-TKI treatment or first diagnosis to the date of death by any cause or the date patients were last known to be alive. In all of the studies, the prevalence of T790M mutation was higher than 10%.
Eligible studies for the posttreatment-acquired T790M group met several criteria. First, patients were confirmed to have advanced or recurrent NSCLC before treatment with single-agent EGFR-TKI (erlotinib or gefitinib), and acquired resistance to EGFR-TKI was established according to the Jackman criteriaCitation33 (ie, patients who were EGFR wild-type or EGFR status unknown had an objective response [according to RECIST criteria] to EGFR-TKIs or had a period of durable stable disease [≥6 months] and eventually developed acquired resistance to EGFR-TKIs). Also, the status of the T790M mutation was detected after resistance to EGFR-TKIs following treatment with single-agent EGFR-TKI, that is, erlotinib or gefitinib (there was no limitation to the detection method). The study must have contained PFS, OS, or post-progression survival (PPS) outcome data; HRs and the corresponding 95% CIs for PFS, OS, and PPS based on T790M mutation status could be acquired or calculated. There was no upper limit for the number of lines of chemotherapy. Finally, PFS was defined as the time from the start of EGFR-TKI treatment to the first disease progression or death from any reason without progression; PPS was defined as time from the date of the first progression according to RECIST criteria version 1.1 to the second progression or death; and OS was defined as the time from the start of EGFR-TKI treatment to the date of death by any cause or the date patients were last known to be alive. In all of the studies, the prevalence of T790M mutation was higher than 10%.
Studies were excluded if they mentioned the use of third-generation EGFR-TKIs, repeated published studies, or included patients with small cell lung cancer. Studies were also excluded if patients simultaneously received other therapies or multiple targeted drug combinations. Finally, studies in which the data were insufficient and unable to meet the inclusion criteria were excluded.
Data extraction and quality assessment
The primary outcomes were PFS and OS, and the secondary outcomes were PPS and ORR. Two reviewers independently extracted author name, published date, total number of patients, method of EGFR detection, T790M mutation status, study outcomes (OS, PFS, PPS, and ORR) and the corresponding HR or relative ratio (RR), and patient characteristics. Discrepancies were discussed with a third investigator to reach an agreement. The Joanna Briggs Institute Prevalence Critical Appraisal Tool was used to assess the quality of the enrolled studies.Citation34
Statistical analysis
All data were analyzed using the STATA 12.0 statistical software. The statistical heterogeneity of the enrolled studies was assessed using the inconsistency index (I2 statistic). If the I2 was >50% indicating significant heterogeneity, a random-effects model was used;Citation35 otherwise, a fixed-effects model was used.Citation36 HRs were extracted from the original studies or calculated from the reported number of events and the corresponding P-values of the log-rank statistics, as described by Tierney et al.Citation37 The PFS, OS, and PPS were pooled and the results were analyzed according to HR and the corresponding 95% CI; ORR was pooled and the results were analyzed according to RR and the corresponding 95% CI. A P-value <0.05 was considered statistically significant for all analyses. Publication bias was examined with Begg and Mazumdar’s rank correlation testCitation38 and Egger’s regression asymmetry test.Citation39
Results
Search results
In total, the meta-analysis included 22 studies comprising 1,462 patients according to the inclusion and exclusion criteria. illustrates the selection process.
Study characteristics
The pretreatment T790M mutation group included eight eligible studies involving 538 patients: 212 patients were T790M mutation positive and 326 patients were T790M mutation negative. The studies by Karachaliou et alCitation28 and Rosell et alCitation31 might share the same patients, despite the difference in survival-related data. Overall, seven studies including 447 patients reported PFStotal-related data, six studies including 420 patients reported PFSfirst-line-related data, four studies including 298 patients reported OStotal-related data, and five studies including 385 patients reported ORRtotal-related data. lists the details of the studies included in the meta-analysis.
Table 1 Studies of pretreatment T790M mutation
The posttreatment-acquired T790M mutation group included 14 eligible studies involving 924 patients: 445 patients were T790M mutation positive and 479 patients were T790M mutation negative. Three studiesCitation14,Citation17,Citation21 only provided initial data, so the P-values and corresponding HRs were calculated. Sorensen et alCitation17 only reported patients who received second-line treatment with EGFR-TKIs. The study by Uramoto et alCitation21 defined “TTP” as time-to-progression after gefitinib therapy, which was different from the definition in this meta-analysis, so TTP-related data were excluded. All the patients who had PPS-related data had rebiopsy tumor tissue specimens and almost received a rechallenge with TKIs (exclusive of third-generation TKIs) or standard chemotherapy regimens. Among the 14 studies included, nine studies including 405 patients reported PFS-related data, nine studies including 696 patients reported OS-related data, four studies including 282 patients reported PPS-related data, and five studies including 217 patients reported ORR-related data. lists the details of the studies included in the analysis.
Table 2 Studies of posttreatment-acquired T790M mutation
Quality assessment, heterogeneity analysis, and publication bias
The Joanna Briggs Institute Prevalence Critical Appraisal Tool was used to assess the quality of the enrolled studies ( and ). There was moderate heterogeneity in the pooled analysis of survival data (PFStotal, PFSfirst-line, OS, and ORR), and a random-effects model was used for final analysis (). Publication bias was assessed according to Egger’s and Begg’s regression methods and no significant publication bias was observed (P>0.05; ).
Table 3 Quality assessment of studies included in the pretreatment T790M mutation group
Table 4 Quality assessment of studies included in the posttreatment-acquired T790M mutation group
Table 5 Summary of heterogeneity and publication bias in all studies included in the meta-analysis
Meta-analysis of pretreatment de novo T790M mutation
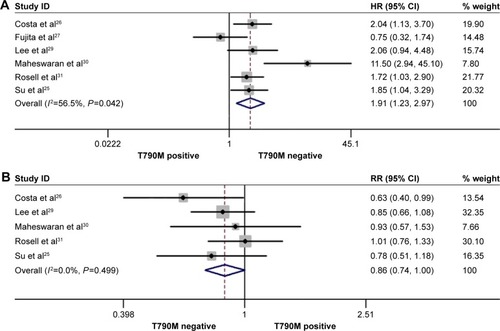
The rate of pretreatment T790M mutation-positive status ranged from 22.22% to 80% in the included studies. In the pretreatment T790M group, there was no significant heterogeneity between trials in the analysis of OStotal (I2=23.3%, P=0.271) or ORRtotal (I2=0.00%, P=0.499), so a fixed-effects model was used for analysis. A random-effects model was used when moderate heterogeneity existed between trials in the analysis of PFStotal (I2=60.6%, P=0.019) and PFSfirst-line (I2=56.5%, P=0.042). The heterogeneity did not decrease with the sensitivity analysis of the enrolled studies. Compared to T790M-negative patients, T790M-positive patients had significantly shorter PFStotal (HR 2.23, 95% CI 1.44–3.45, P<0.001; ) and OStotal (HR 1.55, 95% CI 1.16–2.07, P=0.003; ) in all treatment lines, as well as shorter PFSfirst-line (HR 1.91, 95% CI 1.23–2.97, P=0.004; ) in first-line treatments. Additionally, pretreatment T790M-positive patients had a decreased ORRtotal compared to T790M-negative patients; there was a trend toward significance (RR 0.86, 95% CI 0.74–1.00, P=0.051; ). Data showed that pretreatment T790M mutation may be predictive of worse survival in patients with NSCLC.
Figure 2 Forest plots of pooled HRs and 95% CIs for PFStotal (A) and OStotal (B) according to pretreatment de novo T790M mutation status.
Abbreviations: CI, confidence interval; HR, hazard ratio; OStotal, overall survival for all lines of epidermal growth factor-tyrosine kinase inhibitor treatment; PFStotal, progression-free survival for all lines of epidermal growth factor-tyrosine kinase inhibitor treatment.
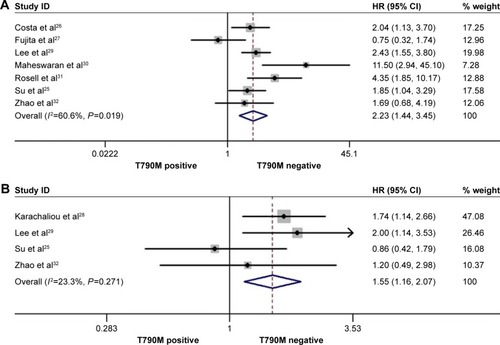
Figure 3 Forest plots of pooled HRs for all PFSfirst-line (A) and RRs for ORRtotal (B) according to pretreatment de novo T790M mutation status.
Abbreviations: CI, confidence interval; HRs, hazard ratios; PFSfirst-line, progression-free survival for first-line of epidermal growth factor-tyrosine kinase inhibitor treatment; ORRtotal, objective response rate for all lines of epidermal growth factor-tyrosine kinase inhibitor treatment; RR, relative ratio.
Meta-analysis of posttreatment-acquired T790M mutation
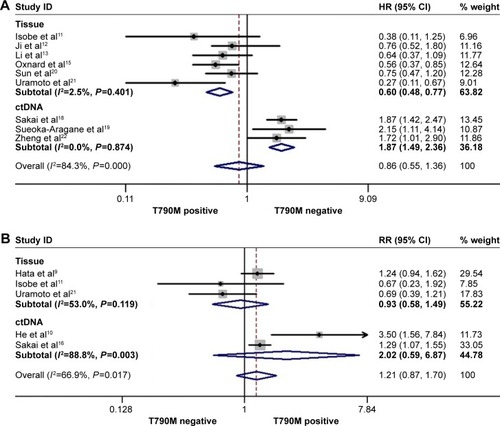
The rate of acquired T790M mutation-positive status after resistance to EGFR-TKI therapy ranged from 28% to 62.36% in the included studies. In the posttreatment-acquired T790M group, there was no significant heterogeneity between studies in the analysis of PFS (I2=30.2%, P=0.1.77) or PPS (I2=41.90%, P=0.16), so a fixed-effects model was used for analysis. The meta-analysis showed that T790M-positive patients had significantly longer PFS (HR 0.75, 95% CI 0.61–0.92, P=0.006; ) and PPS (HR 0.57, 95% CI 0.44–0.73, P<0.001; ) than T790M-negative patients. Heterogeneity was apparent in OS (I2=74.4%, P<0.001) and ORR (I2=66.9%, P=0.017) among the studies included, so a random-effects model was used. The heterogeneity of ORR did not change with the sensitivity analysis. The results showed that acquired T790M mutation-positive patients had similar OS (HR 0.86, 95% CI 0.55–1.36, P=0.526; ) and ORR (RR 1.21, 95% CI 0.89–1.70, P=0.256; ) compared to T790M-negative patients. PFS, OS, and ORR were further analyzed in two subgroups on the basis of type of rebiopsy specimen used for detection of EGFR mutation: tumor tissue or plasma ctDNA detection. In the tumor tissue rebiopsy subgroup, acquired T790M mutation significantly improved PFStissue (HR 0.76, 95% CI 0.59–0.98, P=0.037) and OStissue (HR 0.60, 95% CI 0.48–0.77, P<0.001) compared to T790M-mutation negative patients, but it did not increase ORRtissue (HR 0.93, 95% CI 0.85–1.49, P=0.759). In the plasma ctDNA subgroup, acquired T790M mutation significantly decreased OSctDNA (HR 1.87, 95% CI 1.49–2.36, P<0.001); no differences in PFSctDNA (HR 0.73, 95% CI 0.51–1.03, P=0.072) or ORRctDNA (HR 2.02, 95% CI 0.59–6.87, P=0.262) were observed between the two subgroups.
Figure 4 Forest plots of HRs and 95% CIs for PFS (A) and PPS (B) according to acquired T790M mutation status.
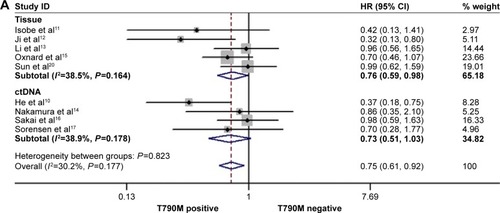
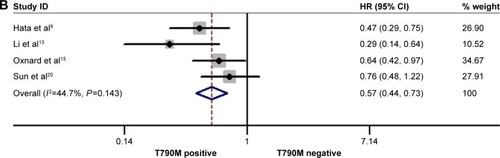
Figure 5 Forest plots of HRs for OS (A) and RR for ORR (B) according to acquired T790M mutation status.
Abbreviations: CI, confidence interval; ctDNA, circulating tumor DNA; HRs, hazard ratios; ORR, objective response rate; OS, overall survival; RR, relative ratio.
Discussion
The results of our meta-analysis indicate that pretreatment T790M mutation had a negative impact on PFS and OS in patients with NSCLC who harbored activating EGFR mutations and received EGFR-TKI treatment. In contrast, patients with acquired T790M mutation after resistance to EGFR-TKIs had significantly prolonged PFS and PPS, compared to patients without acquired T790M mutation. The subgroup analysis showed that OS benefit differed on the basis of the type of rebiopsy samples used for acquired T790M detection. In the tissue rebiopsy subgroup, OS was significantly improved, but, in the plasma ctDNA subgroup, OS was significantly inferior in patients with T790M mutation compared to those without T790M mutation.
Recently, highly sensitive genetic detection methods have been developed. Researchers are now largely able to identify pretreatment de novo T790M mutation existing at baseline before EGFR-TKI treatment. This achievement has attracted great interest related to drug sensitivity and survival prognosis. Pretreatment T790M mutation was reported to have no significant associations with the majority of the clinicopathologic characteristics such as age, stage, tumor size, or number of metastatic lymph nodes.Citation40 However, patients with pretreatment T790M mutation tended to present with higher proportions of never-smoker status and brain metastasis.Citation41 A previous meta-analysis by Ding et alCitation42 indicated that pretreatment T790M mutation predicted inferior PFS in patients with NSCLC who harbored activating EGFR mutations and received EGFR-TKI treatment. Nevertheless, the relationship between pretreatment T790M mutation and OS has not been evaluated. This analysis revealed that pretreatment T790M mutation had a negative impact on OS. In a randomized phase III trial, patients with pretreatment T790M mutation-positive NSCLC had decreased PFS compared to patients with T790M mutation-negative NSCLC (9.7 vs 15.8 months, P=0.0185) when given erlotinib treatment.Citation26 Among patients receiving chemotherapy, PFS was 6 months for T790M mutation-positive patients and 5.1 months for T790M mutation-negative patients (P<0.0001).Citation11 Despite the prediction of poor prognosis for pretreatment T790M mutation, patients harboring activating EGFR mutations with or without the T790M mutation had longer PFS and better ORR compared to wild-type EGFR mutations when given EGFR-TKI therapy.Citation25
It is not very clear why pretreatment T790M mutation predicts a negative effect on PFS and OS. A preclinical study showed that lung cancer cell lines with double-mutant T790M/L858R exhibited increased phosphorylated EGFR protein expression compared to cells with L858R mutation alone.Citation43 When the T790M mutation was present in cells at a low percentage, the sensitivity to EGFR-TKI was similar to the sensitivity in cells harboring an activating EGFR mutation. When the T790M mutation in cells reached a certain percentage, the sensitivity to EGFR-TKIs obviously decreased.Citation26,Citation44 Clinical studies have shown that, with an increased abundance of pretreatment T790M mutation, patients had worse clinical outcomes in response to EGFR-TKI therapy.Citation26,Citation28,Citation45 However, the European BELIEF study showed that patients with pretreatment T790M mutation benefitted more from erlotinib combined with bevacizumab than patients with T790M mutation-negative NSCLC (PFS 16.0 vs 10.5 months).Citation46 Similarly, it is unclear whether patients harboring pretreatment T790M mutation will benefit more from third-generation EGFR-TKIs than patients without pretreatment T790M mutation. The ongoing FLAURA study might help to explore this important question.
Until now, research about the predictive role of acquired T790M mutation after EGFR-TKI therapy has been inconsistent. Rebiopsy of tumors after acquired resistance is vital to identify the mechanisms of resistance and choose subsequent therapy strategies. However, this is often not easily accomplished. In this meta-analysis, acquired T790M mutation after resistance to EGFR-TKI treatment predicted longer PFS, which was contrary to outcomes associated with the pretreatment T790M mutation. This could be explained by the fact that cells with acquired T790M mutation are characterized by indolent biologic behaviors;Citation15,Citation44 other complicated mechanisms of resistance to EGFR-TKIs might lead to patients being more refractory to subsequent treatment. In fact, the low abundance of pretreatment T790M mutation in tumor cells might gradually increase after EGFR-TKI therapy under selective pressure from drugs.Citation25,Citation27,Citation47 One study involving 83 patients with activating EGFR mutations showed that patients with an increasing trend for T790M quantity from pretreatment to posttreatment of EGFR-TKIs had superior PFS and OS, compared to patients with a decreasing trend of T790M quantity.Citation44,Citation48 Thanks to recent advances in the era of third-generation EGFR-TKIs such as osimertinib, it is better understood that patients with acquired T790M mutation will benefit more from osimertinib treatment than patients without T790M mutation. Similar to resistance to first-generation EGFR-TKIs, acquired resistance to osimertinib is almost inevitable after a progression-free period of approximately 10 months. EGFR C797S, L718Q mutation, and amplification of HER-2, MET, and ERBB2 were found to be responsible for this resistance. Importantly, another drug, EAI045, has been developed to partially overcome the acquired resistance to AZD9291.Citation49 In this analysis, all the included studies neither described the third generation of EGFR-TKIs nor mentioned that patients had ever received third-generation EGFR-TKI treatment. Therefore, our pooled results were not influenced by third-generation EGFR-TKI treatment. We believe acquired T790M mutation, relative to other resistance mechanisms after secondary resistance to EGFR-TKIs, suggests a better prognosis.
Several studies support these results. One study by Kuiper et alCitation48 reported that acquired T790M mutation had a positive effect on PFS compared to T790M mutation-negative status after EGFR-TKI resistance (14.2 vs 11.1 months, P=0.034); no difference in OS was observed between the two arms (45.9 vs 29.8 months, P=0.213). Another study by Yu et alCitation50 indicated that patients with acquired T790M mutation had improved PPS compared to T790M mutation-negative patients (1.9 vs 1.6 years, P=0.015). However, another study by Otsuka et alCitation51 showed that acquired T790M mutation had a negative effect on PFS compared to T790M mutation-negative status after EGFR-TKI resistance (3.3 vs 4.1 months, P=0.048); no difference in OS was observed between the groups (15.1 vs 13.5 months, P=0.996). Interestingly, a randomized, controlled phase III trial (the IMPRESS study) indicated that patients who developed resistance to first-line gefitinib treatment failed to achieve benefits in PFS and OS from continuous gefitinib combined with chemotherapy compared to patients who received chemotherapy alone, regardless of T790M status.Citation18 Other studies showed patients who progressed without the T790M mutation did benefit from the combination of continuous EGFR-TKIs and chemotherapy.Citation9,Citation13,Citation15
This meta-analysis showed that acquired T790M mutation was not predictive of improved OS. Nevertheless, the subgroup analysis showed that OS was significantly superior in the tissue rebiopsy subgroup, but significantly inferior in the plasma ctDNA subgroup in patients with the T790M mutation compared to those without the T790M mutation. These findings suggest that high plasma levels of T790M mutation might be associated with an increased tumor burden, as well as tendencies for tumor progression and metastases.Citation19,Citation22 Thus, in order to individualize treatment, assessment of T790M status with both qualitative and quantitative analyses may be required. Combined detection of T790M in both tumor tissue and plasma ctDNA is a promising method for screening patients who might be appropriate candidates for osimertinib treatment.Citation52,Citation53
Limitations
There are some limitations to this meta-analysis. Significant heterogeneities were observed among the included studies. First, the enrolled studies used different EGFR detection methods with different sensitivities and specificities; these methods likely yielded false-negative and false-positive results.Citation54,Citation55 Second, the mutation tended to be heterogeneously distributed within the tumor tissue or plasma and some mutations (especially the T790M mutation) are only present in low proportions.Citation56 In addition, the asymmetrical distribution of patient characteristics also influenced the results.
Conclusion
The clinical data included in this meta-analysis indicated that pretreatment T790M mutation was associated with worse PFS and OS in patients with advanced NSCLC who harbored activating EGFR mutations and received EGFR-TKI treatment, compared to patients without pretreatment T790M mutation. In contrast, acquired T790M mutation after resistance to EGFR-TKIs was associated with longer PFS and PPS, compared to T790M mutation-negative patients. Despite the fact that no significant difference was observed in OS in the total group, acquired T790M mutation detected from rebiopsy of tumor tissue had a positive effect on OS and mutation detected from plasma ctDNA had a negative effect on OS.
Disclosure
The authors report no conflicts of interest in this work.
References
- EttingerDSAkerleyWBorghaeiHNon-small cell lung cancerJ Natl Compr Canc Netw201210101236127123054877
- ReguartNRemonJCommon EGFR-mutated subgroups (Del19/L858R) in advanced non-small-cell lung cancer: chasing better outcomes with tyrosine kinase inhibitorsFuture Oncol20151181245125725629371
- SunLMaJTZhangSLZouHWHanCBEfficacy and safety of chemotherapy or tyrosine kinase inhibitors combined with bevacizumab vs chemotherapy or tyrosine kinase inhibitors alone in the treatment of non-small cell lung cancer: a systematic review and meta-analysisMed Oncol201532247325603953
- SavasPHughesBSolomonBTargeted therapy in lung cancer: IPASS and beyond, keeping abreast of the explosion of targeted therapies for lung cancerJ Thorac Dis20135Suppl 5S579S59224163750
- KobayashiSBoggonTJDayaramTEGFR mutation and resistance of non-small-cell lung cancer to gefitinibN Engl J Med2005352878679215728811
- JännePAYangJCKimDWAZD9291 in EGFR inhibitor-resistant non-small-cell lung cancerN Engl J Med2015372181689169925923549
- YangJRamalingamSSJännePACantariniMMitsudomiTLBA2_PR: osimertinib (AZD9291) in pre-treated pts with T790M-positive advanced NSCLC: updated Phase 1 (P1) and pooled Phase 2 (P2) resultsJ Thorac Oncol2016114 SupplS152S153
- WangSCangSLiuDThird-generation inhibitors targeting EGFR T790M mutation in advanced non-small cell lung cancerJ Hematol Oncol2016913427071706
- HataAKatakamiNYoshiokaHRebiopsy of non-small cell lung cancer patients with acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitor: Comparison between T790M mutation-positive and mutation-negative populationsCancer2013119244325433224105277
- HeCZhengLXuYLiuMLiYXuJHighly sensitive and noninvasive detection of epidermal growth factor receptor T790M mutation in non-small cell lung cancerClin Chim Acta201342511912423886554
- IsobeKHataYTochigiNUsefulness of nanofluidic digital PCR arrays to quantify T790M mutation in EGFR-mutant lung adenocarcinomaCancer Genomics Proteomics2015121313725560642
- JiWChoiCMRhoJKMechanisms of acquired resistance to EGFR-tyrosine kinase inhibitor in Korean patients with lung cancerBMC Cancer20131360624369725
- LiWRenSLiJT790M mutation is associated with better efficacy of treatment beyond progression with EGFR-TKI in advanced NSCLC patientsLung Cancer201484329530024685306
- NakamuraTSueoka-AraganeNIwanagaKA noninvasive system for monitoring resistance to epidermal growth factor receptor tyrosine kinase inhibitors with plasma DNAJ Thorac Oncol20116101639164821921847
- OxnardGRArcilaMESimaCSAcquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutationClin Cancer Res20111761616162221135146
- SakaiKHoriikeAIrwinDLDetection of epidermal growth factor receptor T790M mutation in plasma DNA from patients refractory to epidermal growth factor receptor tyrosine kinase inhibitorCancer Sci201310491198120423721103
- SorensenBSWuLWeiWMonitoring of epidermal growth factor receptor tyrosine kinase inhibitor-sensitizing and resistance mutations in the plasma DNA of patients with advanced non-small cell lung cancer during treatment with erlotinibCancer2014120243896390125103305
- SoriaJCWuYLNakagawaKGefitinib/chemotherapy vs chemotherapy in EGFR mutation-positive NSCLC after progression on 1st line gefitinib (IMPRESS study): Final overall survival (OS) analysisAnn Oncol201627suppl_6416454
- Sueoka-AraganeNKatakamiNSatouchiMMonitoring EGFR T790M with plasma DNA from lung cancer patients in a prospective observational studyCancer Sci2016107216216726577492
- SunJMAhnMJChoiYLAhnJSParkKClinical implications of T790M mutation in patients with acquired resistance to EGFR tyrosine kinase inhibitorsLung Cancer201382229429824035188
- UramotoHYamadaTYanoSKondoNHasegawaSTanakaFPrognostic value of acquired resistance-related molecules in Japanese patients with NSCLC treated with an EGFR-TKIAnticancer Res20123293785379022993320
- ZhengDYeXZhangMZPlasma EGFR T790M ctDNA status is associated with clinical outcome in advanced NSCLC patients with acquired EGFR-TKI resistanceSci Rep201662091326867973
- OxnardGRThressKSAldenRS135O_PR: Plasma genotyping for predicting benefit from osimertinib in patients (pts) with advanced NSCLCJ Thorac Oncol2016114 SupplS154
- ZhouQYangJJChenZHSerial cfDNA assessment of response and resistance to EGFR-TKI for patients with EGFR-L858R mutant lung cancer from a prospective clinical trialJ Hematol Oncol2016918627619632
- SuKYChenHYLiKCPretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancerJ Clin Oncol201230443344022215752
- CostaCMolinaMADrozdowskyjAThe impact of EGFR T790M mutations and BIM mRNA expression on outcome in patients with EGFR-mutant NSCLC treated with erlotinib or chemotherapy in the randomized phase III EURTAC trialClin Cancer Res20142072001201024493829
- FujitaYSudaKKimuraHHighly sensitive detection of EGFR T790M mutation using colony hybridization predicts favorable prognosis of patients with lung cancer harboring activating EGFR mutationJ Thorac Oncol20127111640164422899358
- KarachaliouNCostaCGimenez-CapitaBRCA1, LMO4, and CtIP mRNA expression in erlotinib-treated non-small-cell lung cancer patients with EGFR mutationsJ Thorac Oncol20138329530023407556
- LeeYLeeGKLeeYSClinical outcome according to the level of preexisting epidermal growth factor receptor T790M mutation in patients with lung cancer harboring sensitive epidermal growth factor receptor mutationsCancer2014120142090209824737599
- MaheswaranSSequistLVNagrathSDetection of mutations in EGFR in circulating lung-cancer cellsN Engl J Med2008359436637718596266
- RosellRMolinaMACostaCPretreatment EGFR T790M mutation and BRCA1 mRNA expression in erlotinib-treated advanced non-small-cell lung cancer patients with EGFR mutationsClin Cancer Res20111751160116821233402
- ZhaoJFengHHZhaoJYA sensitive and practical method to detect the T790M mutation in the epidermal growth factor receptorOncol Lett20161142573257927073519
- JackmanDPaoWRielyGJClinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancerJ Clin Oncol201028235736019949011
- MunnZMoolaSRiitanoDLisyKThe development of a critical appraisal tool for use in systematic reviews addressing questions ofInt J Health Policy Manage201433123128
- DerSimonianRLairdNMeta-analysis in clinical trialsControl Clin Trials198671771883802833
- MantelNHaenszelWStatistical aspects of the analysis of data from retrospective studies of diseaseJ Natl Cancer Inst19592271974813655060
- TierneyJFStewartLAGhersiDBurdettSSydesMRPractical methods for incorporating summary time-to-event data into meta-analysisTrials200781617555582
- BeggCBMazumdarMOperating characteristics of a rank correlation test for publication biasBiometrics1994504108811017786990
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ199731571096296349310563
- OhJEAnCHYooNJLeeSHDetection of low-level EGFR T790M mutation in lung cancer tissuesAPMIS2011119740341121635547
- LeeYLeeGKHwangJAYunTKimHTLeeJSClinical likelihood of sporadic primary EGFR T790M mutation in EGFR-mutant lung cancerClin Lung Cancer2015161465025450875
- DingDYuYLiZNiuXLuSThe predictive role of pretreatment epidermal growth factor receptor T790M mutation on the progression-free survival of tyrosine-kinase inhibitor-treated non-small cell lung cancer patients: a meta-analysisOnco Targets Ther2014738739324623981
- MulloyRFerrandAKimYEpidermal growth factor receptor mutants from human lung cancers exhibit enhanced catalytic activity and increased sensitivity to gefitinibCancer Res20076752325233017332364
- ChmieleckiJFooJOxnardGROptimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modelingSci Transl Med201139090ra59
- WangZChenRWangSQuantification and dynamic monitoring of EGFR T790M in plasma cell-free DNA by digital PCR for prognosis of EGFR-TKI treatment in advanced NSCLCPLoS One2014911e11078025405807
- StahelABDafniUGautschiOA phase II trial of erlotinib (E) and bevacizumab (B) in parents with advanced non-small-cell lung cancer (NSCLC) with activating epidermal growth factor receptor (EGFR) mutations with and without T790M mutation. The Spanish Lung Cancer Group (SLCG) and the European Thoracic Oncology Platform (ETOP) BELIEF trialEur J Cancer201551Suppl 3S711S712
- NguyenKSKobayashiSCostaDBAcquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathwayClin Lung Cancer200910428128919632948
- KuiperJLHeidemanDAThunnissenEIncidence of T790M mutation in (sequential) rebiopsies in EGFR-mutated NSCLC-patientsLung Cancer2014851192424768581
- WangSSongYYanFLiuDMechanisms of resistance to third-generation EGFR tyrosine kinase inhibitorsFront Med201610438338827770386
- YuHArcilaMERekhtmanNAnalysis of mechanisms of acquired resistance to EGFR TKI therapy in 155 patients with EGFR-mutant lung cancersClin Cancer Res20131982240224723470965
- OtsukaKHataATakeshitaJEGFR-TKI rechallenge with bevacizumab in EGFR-mutant non-small cell lung cancerCancer Chemother Pharmacol201576483584126349474
- OxnardGRThressKSAldenRSAssociation between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancerJ Clin Oncol201634283375338227354477
- YangJCAhnMJKimDWOsimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA Study Phase II Extension ComponentJ Clin Oncol2017 JCO2016703223
- YeXZhuZZZhongLHigh T790M detection rate in TKI-naive NSCLC with EGFR sensitive mutation: truth or artifact?J Thorac Oncol2013891118112023945382
- YuHAArcilaMEHellmannMDKrisMGLadanyiMRielyGJPoor response to erlotinib in patients with tumors containing baseline EGFR T790M mutations found by routine clinical molecular testingAnn Oncol201425242342824478319
- NagaiYMiyazawaHHuqunGenetic heterogeneity of the epidermal growth factor receptor in non-small cell lung cancer cell lines revealed by a rapid and sensitive detection system, the peptide nucleic acid-locked nucleic acid PCR clampCancer Res200565167276728216105816