Abstract
Purpose
The purpose of this study was to explore the frequencies of ALK and ROS1 fusion genes in EGFR-mutant lung adenocarcinoma patients and examine the therapeutic efficacies of EGFR-tyrosine kinase inhibitors (TKIs).
Materials and methods
A total of 421 EGFR-mutated patients taking EGFR-TKIs were examined for ALK and ROS1 fusion genes based on reverse transcription-polymerase chain reaction (RT-PCR). Progression-free survival (PFS) and overall survival (OS) were evaluated by the Kaplan–Meier method and compared by the log-rank test.
Results
The mutations of ALK rearrangement (n=10) and ROS1 rearrangement (n=3) were detected. All the patients received EGFR-TKIs, and eight took subsequent ALK/ROS1 inhibitor. PFS was longer in single EGFR mutants (n=408) than in EGFR/ALK or EGFR/ROS1 counterparts (n=13; 10.7 vs 6.6 months, P=0.004). No difference in OS existed between single EGFR and EGFR/ALK or EGFR/ROS1 mutants (21.0 vs 23.0 months, P=0.196). The median PFS of eight patients treated with ALK/ROS1 inhibitor was 6.0 months.
Conclusion
Concomitant ALK/ROS1 fusion genes occurred in 3.1% EGFR-mutated lung adenocarcinoma patients. Concomitant ALK/ROS1–EGFR mutations may influence the therapeutic efficacy of EGFR-TKIs.
Introduction
It is well known that the mutations of EGFR act as one of the most frequent driver genes in non-small cell lung cancer (NSCLC) patients, especially among East Asian female lung adenocarcinoma patients.Citation1,Citation2 Previous studies have shown that EGFR-tyrosine kinase inhibitors (EGFR-TKIs) could achieve a high response rate and yield a promising efficacy for EGFR mutants.Citation3–Citation6
Despite a response rate of 60%–70% in EGFR-mutated lung cancer patients treated with EGFR-TKIs, 10%–20% of individuals developed primary resistance.Citation4–Citation6 The mechanism is currently ill defined. Possible contributing factors included T790M mutation, MET amplification and PIK3CA mutation.Citation7–Citation9 Concomitant genetic alterations were found to be associated with primary resistance in EGFR-mutated NSCLC patients.Citation10,Citation11 However, the studies were somewhat limited, and only several case series were reported. Furthermore, the efficacies of EGFR-TKIs have remained elusive.
Here, ALK and ROS1 fusion gene panel was used for screening the patients har-boring EGFR-mutated samples and further detecting the therapeutic efficacies of EGFR-TKIs.
Materials and methods
Patient samples
Consecutive EGFR-mutated patients receiving EGFR-TKIs for lung adenocarcinoma were screened at Hangzhou Cancer Hospital between 2009 and 2015. The inclusion criteria were as follows: 1) uses of EGFR-TKIs during advanced stage; 2) sufficient residual tissues for detecting ALK and ROS1 fusion genes and 3) confirmed as having sensitive EGFR mutation. Resistant mutations such as T790M, S768I and exon 20 insertions were excluded. The study protocol was approved by the ethics committee of Hangzhou Cancer Hospital, and written informed consents were obtained from all patients.
Detection of EGFR/ALK/ROS1 fusion gene
EGFR gene was detected by amplification refractory mutation system (ARMS)-based kit (Amoy, Xiamen, China). It was capable of detecting the following 23 mutations: three in exon 18 (G719A, G719C and G719S), 13 deletions in exon 19, two mutations in exon 20 (T790M and S768I), three insertions in exon 20 and two mutations in exon 21 (L858R and L861Q).
The ALK and ROS1 fusion mRNAs were detected by polymerase chain reaction (PCR) using fusion gene detection kit (Amoy). Briefly, total RNA was extracted using Qiagen (Dusseldorf, Germany) RNeasy FFPE kit. mRNA was reverse transcribed into cDNA for 1 h at 42°C. β-actin was used as an internal control. The conditions of reverse transcription-polymerase chain reaction (RT-PCR) were as follows: initial denaturation at 95°C for 5 min, followed by 95°C for 25 s, 64°C for 20 s and 72°C for 20 s for ensuring specificity and 31 cycles at 93°C for 25 s, 60°C for 35 s and 72°C for 20 s. Data collection and sensitivity analysis were detailed previously.Citation12 The subtypes of EGFR/ALK/ROS1 genes are summarized in .
Evaluations of TKI treatment
Tumors were evaluated during the treatment with EGFR-TKIs or ALK/ROS1 inhibitor every 8 weeks. Objective tumor responses were evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1). Objective response rate (ORR) included complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD).
Statistical analyses
The Kaplan–Meier method was used for survival analysis. Progression-free survival (PFS) of TKIs was defined as the time from initiating TKI treatment to documented progression or mortality from any cause. Statistical analysis was performed using the SPSS 18 software (SPSS Inc., Chicago, IL, USA). The median follow-up period was 28.5 months (4.5–65 months). The last follow-up time was October 31, 2016.
Results
Patient characteristics
A total of 421 patients were enrolled, and their clinical characteristics are summarized in . Among the patients, 13 were confirmed as having concomitant ALK/ROS1 fusion gene. Their clinical characteristics are summarized in . There were six males and seven females with a median age of 55 years. One patient was a former smoker and 12 had never smoked. The comparisons of single EGFR versus concomitant gene mutations are listed in .
Table 1 Characteristics of the study population and comparison (n=421)
Table 2 Clinical characteristics of 13 patients with EGFR and ALK/ROS1 concurrent genes
Gene profiles
The genetic details of 421 EGFR mutations are as follows: exon 19 deletions (n=217), L858R mutation in exon 21 (n=169), G719X in exon 18 (n=21) and L861Q (n=14). Concomitant gene fusions were identified in 13 patients (3.1%), including ALK (n=10, 2.4%) and ROS1 (n=3, 0.7%). Their profiles are detailed in .
Efficacy of TKIs
All 421 patients received EGFR-TKIs. Among 13 patients with concomitant genes, eight switched to crizotinib after ineffective EGFR-TKIs. The agents of EGFR-TKIs included erlotinib (n=71), gefitinib (n=126) and icotinib (n=224). None of them received any second-generation EGFR-TKI since so far none have been approved in China.
The clinical efficacies of 408 single EGFR-mutated patients included CR (n=2, 0.5%), PR (n=249, 61.0%), SD (n=87, 21.3%) and PD (n=70, 17.2%). In concomitant ALK/ROS1 mutants, the outcomes of EGFR-TKIs were PR (n=6), SD (n=3) and PD (n=4). The efficacy comparisons between single EGFR and concomitant gene mutations are shown in .
Table 3 Clinical efficacy comparison of EGFR-TKI in single EGFR mutation and concurrent gene alterations
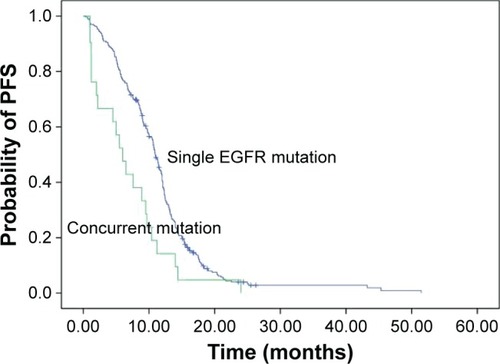
The overall value of PFS was 10.7 months (95% CI, 10.0–11.4). The median values of PFS were 10.7 and 6.6 months in mutants of single EGFR and concomitant ALK/ROS1 fusion gene, respectively (=0.004). For eight patients on crizotinib, the value of PFS was 6.0 months for concomitant ALK/ROS1 fusion gene mutations (95% CI, 3.2–8.8).
Figure 1 Comparison of PFS between single EGFR and concomitant ALK/ROS1 gene mutants on EGFR-TKI (10.7 vs 6.6 months, P=0.004).
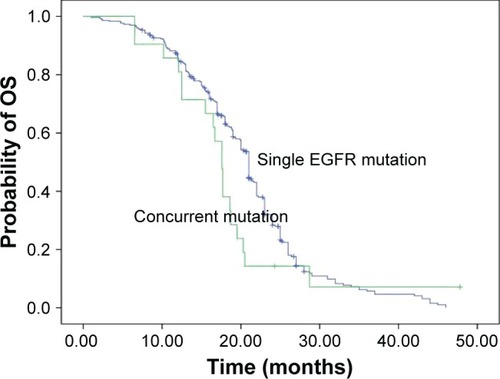
The overall median value of OS was 21.0 months (95% CI, 18.9–23.4). No survival difference existed between single EGFR mutants and those with concomitant ALK/ROS1 fusion gene mutations (21.0 vs 23.0 months, P=0.196; ).
Discussion
Our results showed that 3.1% of EGFR-mutated lung adeno-carcinoma Chinese patients harbored ALK/ROS1 fusion genes. Concurrent ALK/ROS1 gene decreased the therapeutic efficacy of EGFR-TKIs. However, it had no impact on the overall survival (OS).
Although gene alterations were presumably mutually exclusive in lung adenocarcinoma,Citation13 some reports revealed that genes might occur concomitantly.Citation14,Citation15 With the emerging of high-throughput sequencing, many concomitant genes have been detected. The frequency of concomitant EGFR/ALK or EGFR/ROS1 mutations was 3.1% in the present study, and this figure was consistent with previous reports.Citation16–Citation19
Owing to a low frequency of concomitant genes in EGFR-mutated lung cancer patients, the efficacy of EGFR-TKIs is largely unknown. The median PFS of first-generation EGFR-TKIs was 11.2 months in patients harboring concomitant EGFR/ALK genes in one report by Yang et al.Citation20 For concomitant ALK/ROS1 and EGFR mutants, there was a practical dilemma of therapeutic sequence of EGFR-TKIs and ALK/ROS1 inhibitors. Relative levels of phospho-EGFR or ALK could predict the efficacy of targeted treatment in EGFR/ALK mutants in the study of Yang et al.Citation20 In the present study, all patients took EGFR-TKIs initially and eight took subsequent ALK/ROS1 inhibitor. The median value of PFS was 6.6 months for 13 patients on EGFR-TKIs. However, among eight patients on crizotinib, six obtained disease control. Owing to efficacy difference for dosing order of targeted therapy, despite a low frequency, there is a future need of using a useful marker for selecting targeted treatment in patients with concomitant genes. Except at the level of phosphorylation, abundance of gene was identified previously as a predictor of targeted therapy;Citation21 the abundance level of different genes in one sample should be detected for patients with concomitant genes.
A small sample size was a major drawback of the present study. Second, not all patients received crizotinib after ineffective EGFR-TKIs. As a result, the clinical efficacy of further treatment could not be fully evaluated. Third, only RT-PCR was used for ALK/ROS1 genes so that false positives might ensue. Moreover, RT-PCR could not provide convincing evidence for the dominance of expression for one oncogene aberration over another in the same samples. In addition, it was impossible to check whether individual tumor cells had these two mutations simultaneously or singly.Citation22 However, our findings are meaningful for clinical practice.
Conclusion
A total of 3.1% of EGFR-mutated patients harbored ALK/ROS1 fusion genes. Concomitant genes of ALK/ROS1 might decrease the therapeutic efficacy of EGFR-TKIs.
Supplementary material
Table S1 The subtypes of EGFR/ALK/ROS1 genes
Disclosure
The authors report no conflicts of interest in this work.
References
- ShiYAuJSThongprasertSA prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER)J Thorac Oncol20149215416224419411
- ZhangYSunYPanYFrequency of driver mutations in lung adenocarcinoma from female never-smokers varies with histologic subtypes and age at diagnosisClin Cancer Res20121871947195322317764
- MokTSWuYLThongprasertSGefitinib or carboplatin-paclitaxel in pulmonary adenocarcinomaN Engl J Med20093611094795719692680
- ZhouCWuYLChenGErlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 studyLancet Oncol201112873574221783417
- MitsudomiTMoritaSYatabeYWest Japan Oncology GroupGefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trialLancet Oncol201011212112820022809
- HanJYParkKKimSWFirst-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lungJ Clin Oncol201230101122112822370314
- YuHAArcilaMERekhtmanNAnalysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancersClin Cancer Res20131982240224723470965
- JardimDLTangCGagliato DdeMAnalysis of 1,115 patients tested for MET amplification and therapy response in the MD Anderson Phase I ClinicClin Cancer Res201420246336634525326232
- LiSLiLZhuYCoexistence of EGFR with KRAS, or BRAF or PIK3CA somatic mutations in lung cancer: a comprehensive mutation profiling from 5125 Chinese cohortsBr J Cancer2014110112812282024743704
- KimGWSongJSChoiCMMultiple resistant factors in lung cancer with primary resistance to EGFR-TK inhibitors confer poor survivalLung Cancer201588213914625724261
- LeeJKShinJYKimSPrimary resistance to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in patients with non-small-cell lung cancer harboring TKI-sensitive EGFR mutations: an exploratory studyAnn Oncol20132482080208723559152
- WuCZhaoCYangYHigh discrepancy of driver mutations in patients with NSCLC and synchronous multiple lung ground-glass nodulesJ Thorac Oncol201510577878325629635
- LiHPanYLiYFrequency of well-identified oncogenic driver mutations in lung adenocarcinoma of smokers varies with histological subtypes and graduated smoking doseLung Cancer201379181323098378
- SongZYuXZhangYClinicopathologic characteristics, genetic variability and therapeutic options of RET rearrangements patients in lung adenocarcinomaLung Cancer2016101162127794403
- SongZYuXZhangYMutation and prognostic analyses of PIK3CA in patients with completely resected lung adenocarcinomaCancer Med20165102694270027554588
- SongZYuXZhangYClinicopathological characteristics and survival of ALK, ROS1 and RET rearrangements in non-adenocarcinoma non-small cell lung cancer patientsCancer Biol Ther Epub2016916
- WonJKKeamBKohJConcomitant ALK translocation and EGFR mutation in lung cancer: a comparison of direct sequencing and sensitive assays and the impact on responsiveness to tyrosine kinase inhibitorAnn Oncol201526234835425403583
- ZhangXZhangSYangXFusion of EML4 and ALK is associated with development of lung adenocarcinomas lacking EGFR and KRAS mutations and is correlated with ALK expressionMol Cancer2010918820624322
- ChaftJEArcilaMEPaikPKCoexistence of PIK3CA and other oncogene mutations in lung adenocarcinoma – rationale for comprehensive mutation profilingMol Cancer Ther201211248549122135231
- YangJJZhangXCSuJLung cancers with concomitant EGFR mutations and ALK rearrangements: diverse responses to EGFR-TKI and crizotinib in relation to diverse receptors phosphorylationClin Cancer Res20142051383139224443522
- ZhouQZhangXCChenZHRelative abundance of EGFR mutations predicts benefit from gefitinib treatment for advanced non-small-cell lung cancerJ Clin Oncol201129243316332121788562
- CaiWLinDWuCIntratumoral heterogeneity of ALK-rearranged and ALK/EGFR coaltered lung adenocarcinomaJ Clin Oncol201533323701370926416997