Abstract
Solitary fibrous tumor (SFT) of the pancreas is rare, with 15 adult cases reported in the English literature. We described a 14-month-old boy who presented with obstructive jaundice. Dominantly elevated serum CA19-9 was detected. Imaging studies revealed a well-circumscribed, solid mass in the pancreatic head. A pancreaticoduodenectomy (child procedure) was performed using Shen’s anastomosis technique. After resection of the tumor, liver function and serum tumor markers normalized and clinical signs receded. The boy was disease free after a follow-up of 12 months. Histological examination showed the tumor consisted of “patternless pattern” arranged spindle tumor cells and keloid-like hyalinized collagen. Immunohistochemical staining was positive for CD34 and vimentin. Mutation analysis of CTNNB1 was negative. To the best of our knowledge, our patient was the first case of pancreatic SFT in a pediatric population. SFT should be considered in differential diagnosis when confronted with a pancreatic tumor in children. Complete resection should be meticulously pursued.
Introduction
Solitary fibrous tumor (SFT) arising in the pancreas is extremely rare, usually occurring in the pleura.Citation1 To date, all 15 reported pancreatic primary SFTs in the English literature are adult patients with a median age of 54 years.Citation2–Citation16 Here, we describe an SFT of the pancreas in a 14-month-old boy managed by pancreaticoduodenectomy (PD), with histopathological analysis and a review of the literature.
Case report
Written informed consent was obtained from the patient’s parents to have the case details and any accompanying images published. A 14-month-old boy presented with a 1-week history of painless jaundice, dark urine, and acholic stool. He had no fever, nausea, emesis, diarrhea, abdominal mass, or weight loss. Physical examination was unremarkable except icteric sclera. There was no history of hypoglycemia, and the patient’s random blood glucose level was within the normal range. Family history was negative for tumors or diabetes. His serum laboratory results were significant: slightly decreased hemoglobin (104 g/L, reference range: 110–160 g/L), hematocrit (32.1%, reference range: 34%–48%), elevated total bilirubin (64.63 µmol/L, reference range: 3.4–17.1 µmol/L), direct bilirubin (39.0 µmol/L, reference range: 0–6.8 µmol/L), alanine aminotransferase (431 U/L, reference range: 5–40 U/L), aspartate aminotransferase (467 U/L, reference range: 8–40 U/L), gamma-glutamyl transpeptidase (1,218 U/L, reference range: 11–50 U/L), and alkaline phosphatase (2,190 U/L, reference range: 0–500 U/L). Elevated serum CA19-9 was detected (268 U/mL, reference range: 0–35 U/mL), while other tumor markers including AFP, CEA, CA125, CA15-3, and NSE were within normal limits.
Abdominal ultrasonography revealed a well-demarcated hypoechoic solid mass with a diameter of 2.0 cm in the head of the pancreas. The mass showed slight hypoattenuation on unenhanced computed tomography (CT), with heterogeneous enhancement on the arterial phase after contrast injection (). There was also mass effect on the duodenum and obstruction of the common bile duct (dilated gallbladder, dilatation of both intrahepatic and extrahepatic bile ducts). The mass was hypointense on T1-weighted magnetic resonance imaging (MRI) and heterogeneous-intense on T2-weighted MRI. Magnetic resonance cholangiopancreatography (MRCP) demonstrated slight dilatation of the main pancreatic duct (with a diameter of 2.5 mm). Regional lymphadenopathy and distant metastasis (liver, brain, lung, bone, bone marrow) was not noted.
Figure 1 Abdominal computed tomography findings.
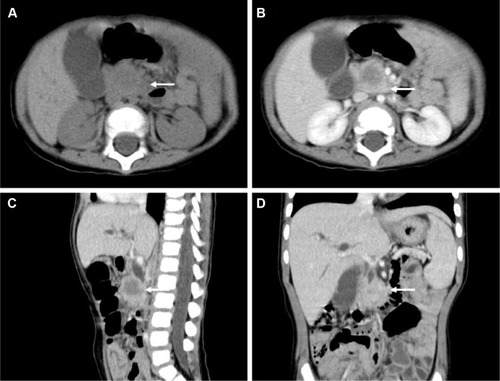
The patient was taken to surgery. Tumor biopsy was interpreted as mesenchymal origin on frozen analysis. A PD and cholecystectomy was performed using Shen’s anastomosis technique.Citation17 In brief, the pancreas was dissected according to the classical procedure. Then the pancreatic duct was identified by inspection and a probe (assisted by magnifying loupes), with a 3F internal stent for draining the pancreatic juice. Clear tissue planes between the tumor and the pancreatic parenchyma were established. After removal of the specimen, reconstruction of the digestive system was performed in the following sequence (child procedure): end-to-side duct-to-mucosal pancreaticojejunostomy, end-to-side choledochojejunostomy, and end-to-side gastrojejunostomy. A Roux jejunal loop was used for biliopancreatic anastomosis via the retrocolic route. The details of Shen’s whole-layer tightly appressed technique (pancreaticojejunostomy) have been recently reported by our group. No drainage was placed and there was no blood transfusion during the operation. Feeding was started on the 7th day after surgery. Postoperative recovery was uneventful, with no complications such as pancreatic fistula, biliary fistula, hemorrhage, intra-abdominal infection, and wound infection being observed. No adjuvant chemotherapy or radiotherapy was given. After surgery (1 month), the liver function and serum tumor markers normalized and clinical signs (jaundice) receded. Abdominal CT scan performed at 6 months after resection revealed no recurrence. The boy was well and disease free within a follow-up of 12 months.
Histopathological and molecular characteristics
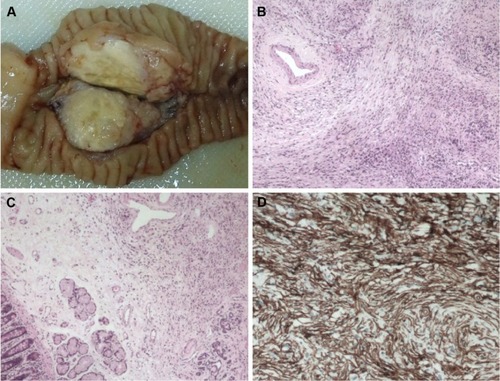
The mass appeared grossly well-circumscribed and non-capsulated with yellowish-gray cut surface (). The stomach, duodenum, proximal jejunum, and distal bile duct were macroscopically normal. The resected specimen was fixed in 10% buffered formalin, paraffin-embedded, micro-tome sectioned at 3 µm, and stained with hematoxylin and eosin. Genomic DNA was extracted from paraffin-embedded materials using a commercial kit (QIAamp DNA Mini Kit, Qiagen NV, Venlo, the Netherlands). Mutation analysis of CTNNB1 was performed by sequencing the polymerase chain reaction amplification products of exon 3. Immunohistochemistry (IHC) and mutation analysis (DNA isolation, amplification, purification, and sequencing) were performed as we previously reported.Citation18,Citation19 Sections were examined by an experienced pathologist (X Wang). Histological examination revealed the tumor consisted of “patternless pattern” arranged spindle tumor cells and keloid-like hyalinized collagen (). A mild to moderate degree of nuclear pleomorphism and a few atypical mitotic figures (2–5 per 10 high-power fields) were noted in the hypercellular area. The submucosa of the duodenum was microscopically infiltrated (). No tumor necrosis or vascular invasion was found. Ten regional lymph nodes were tumor free. IHC revealed that the tumor cells were strongly positive for CD34 () and vimentin, focally positive for SMA, and negative for CD117, CD99, CD56, NSE, ALK, EMA, DES, S-100, MYOG, CEA, SYP, CgA, BCL-2, and CK. The proliferation-associated Ki-67 index was <3%. The result of sequencing was wild type. Based on histopathology and mutation analysis, a diagnosis of SFT with low grade malignancy was suggested.
Figure 2 Histopathology of the pancreatic tumor.
Discussion
Primary mesenchymal tumor of the pancreas is a very rare entity both in adults and children, representing only 0.3% of surgically resected or biopsy-examined pancreatic tumor cases.Citation20,Citation21 Reported benign or borderline pancreatic mesenchymal tumors include schwannoma, hamartoma, inflammatory myofibroblastic tumor, aggressive fibromatosis (also known as desmoid tumor), and lymphangioma/hemangioma. Malignant pancreatic mesenchymal tumors include rhabdomyosarcoma, Ewing sarcoma/primitive neuroectodermal tumor, lymphosarcoma, malignant fibrous histiocytoma, and undifferentiated/unclassified sarcoma. Most of them are case reports or small series.Citation22–Citation25 SFT was formerly considered to be exclusively of pleural origin; however, many extra-thoracic sites of SFT have now been reported.Citation1,Citation26 Primary SFT arising in the pancreas is exceedingly rare. In the current study, we reported the first case of pancreatic SFT in a pediatric population.
A review of the English literature revealed 15 pancreatic SFT cases (, including the current case). The median age of the 16 patients was 53.5 years (range: 1–78 years). The ratio of female to male was 13:3. Almost half of patients presented with symptoms such as abdominal pain, abdominal distension, back pain, weight loss, and jaundice. The remaining tumors were found incidentally. The median size of tumor was 4.3 cm (range: 1.5–18.5 cm). At ultrasonog-raphy, SFT was typically well-defined and hypoechoic.Citation3,Citation27 On unenhanced CT scans, SFT was nearly iso-attenuated or slightly hypo-attenuated to pancreas, and there was progressive enhancement on arterial phase and portal venous phase. The attenuation likely depended on the content of collagen. At MRI, SFT was hypointense on T1-weighted images and hyperintense or variable on T2-weighted images. No or slight dilatation of main pancreatic duct was found on MRCP. There was no significant 18F-fluorodeoxyglucose uptake of the tumor observed on positron emission tomography/CT. Neuroendocrine tumor was always suggested based on imaging findings. No definitive cytologic diagnosis or only mesenchymal origin was established after fine needle aspiration due to cellular paucity. Estrella et alCitation14 and Vallat-Decouvelaere et alCitation28 have discussed histologic features of malignant SFTs in detail. Fibromatosis has considerable histologic overlap with SFT. High frequency of CTNNB1 heterozygous mutations (D32G, T41A, S45P, S45F, S45C, and in-frame deletion) has been reported by many groups.Citation29–Citation32 These mutations prevent phosphorylation and degradation of CTNNB1, resulting in increased nuclear CTNNB1 accumulation and transcriptional activation of target genes. No mutation was detected in our study.
Table 1 Clinicopathological characteristics of pancreatic solitary fibrous tumor in English literature
Radical surgical resection remains the gold standard of treatment, even in histopathology-approved malignant cases. Enucleation of the mass with clear margins has been achieved in three cases in the literature. PD is rarely performed in children, and this unfamiliarity is associated with a significant risk of postoperative mortality and morbidity.Citation33 However, d’Ambrosio et alCitation34 reported favorable outcomes after PD for malignancies in children. To reduce postoperative complications such as pancreatic fistula, a new anastomotic method (Shen’s whole-layer tightly appressed anastomosis technique) was introduced in the current case. The operation was tolerated well, and the postoperative course was uneventful. Impaired pancreatic endocrine function and atrophic changes of the pancreatic remnants after long-term follow-up have been observed by Sugito et al.Citation35 Surgeons should be aware of possible pancreatic endocrine insufficiency and morphological changes when performing pancreaticojejunal anastomosis in children.
Conclusion
To the best of our knowledge, our patient was the first case of pancreatic SFT in children. SFT should be borne in mind when confronted with a tumor in the pancreas. Complete excision, even at the risk of aggressive resection, is meticulously pursued. Although the boy in the present study seems to have been cured without postoperative morbidity, long-term follow-up is warranted.
Disclosure
The authors report no conflicts of interest in this work.
References
- GoldJSAntonescuCRHajduCClinicopathologic correlates of solitary fibrous tumorsCancer20029441057106811920476
- LüttgesJMentzelTHübnerGKlöppelGSolitary fibrous tumour of the pancreas: a new member of the small group of mesenchymal pancreatic tumoursVirchows Arch19994351374210431844
- MiyamotoHMolenaDASchoenigerLOXuHaodongSolitary fibrous tumor of the pancreas: a case reportInt J Surg Pathol200715331131417652547
- SrinivasanVDWayneJDRaoMSZyngerDLSolitary fibrous tumor of the pancreas: case report with cytologic and surgical pathology correlation and review of the literatureJOP20089452653018648147
- KwonHJByunJHKangJParkSHLeeMGSolitary fibrous tumor of the pancreas: imaging findingsKorean J Radiol20089SupplS48S5118607126
- ChettyRJainRSerraSSolitary fibrous tumor of the pancreasAnn Diagn Pathol200913533934319751911
- SugawaraYSakaiSAonoSSolitary fibrous tumor of the pancreasJpn J Radiol201028647948220661701
- SantosLASantosVMOliveiraOCDe MarcoMSolitary fibrous tumour of the pancreas: a case reportAn Sist Sanit Navar201235113313622552135
- van der VorstJRVahrmeijerALHuttemanMNear-infrared fluorescence imaging of a solitary fibrous tumor of the pancreas using methylene blueWorld J Gastrointest Surg20124718018422905287
- TasdemirASoyuerIYurciAKarahanliIAkyildizHA huge solitary fibrous tumor localized in the pancreas: a young womenJOP201213330430722572138
- ChenJWLüTLiuHBA solitary fibrous tumor in the pancreasChin Med J (Engl)201312671388138923557579
- HwangJKimJChangJImaging findings of a solitary fibrous tumor in pancreas: a case reportJ Korean Soc Radiol20147015357
- BaxterARNewmanEHajduCHSolitary fibrous tumor of the pancreasJ Surg Case Rep2015201512
- EstrellaJSWangHBhosalePREvansHLAbrahamSCmalignant solitary fibrous tumor of the pancreasPancreas201544698899426166470
- HanSBaekYHanSSolitary fibrous tumor of the pancreas: a case report and review of the literatureKorean J Med2015883293298
- ParamythiotisDKofinaKBangeasPTsiompanouFKarayannopoulouGBasdanisGSolitary fibrous tumor of the pancreas: Case report and review of the literatureWorld J Gastrointest Surg20168646146627358679
- ZhangTWangXHuoZShen’s whole-layer tightly appressed anastomosis technique for duct-to-mucosa pancreaticojejunostomy in pancreaticoduodenectomyMed Sci Monit20162254054826891466
- ShengQLvZXuWA case report of adrenocortical adenoma mimicking congenital adrenal hyperplasia in a young girlMedicine (Baltimore)20159425e104626107677
- ShengQLvZXuWLiuJWuYShiJXiZShort- term surgical outcomes of preterm infants with necrotizing enterocolitis: A single-center experienceMedicine (Baltimore)20169530e437927472729
- JohnsonPRSpitzLCysts and tumors of the pancreasSemin Pediatr Surg20009420921511112838
- LiuZDongCWangCLiuQSunDWangLMixed acinar-endocrine carcinoma of pancreas: A case report and brief review of the literatureOnco Targets Ther201581633164226170699
- GrosfeldJLVaneDWRescorlaFJMcGuireWWestKWPancreatic tumors in childhood: analysis of 13 casesJ Pediatr Surg19902510105710622262858
- ShorterNAGlickRDKlimstraDSBrennanMFLaquagliaMPMalignant pancreatic tumors in childhood and adolescence: The Memorial Sloan-Kettering experience, 1967 to presentJ Pediatr Surg200237688789212037756
- YuDCKozakewichHPPerez-AtaydeARShambergerRCWeldonCBChildhood pancreatic tumors: a single institution experienceJ Pediatr Surg200944122267227220006007
- NasherOHallNJSebireNJde CoppiPPierroAPancreatic tumours in children: diagnosis, treatment and outcomePediatr Surg Int201531983183526174862
- CranshawIMGikasPDFisherCThwayKThomasJMHayesAJClinical outcomes of extra-thoracic solitary fibrous tumoursEur J Surg Oncol200935999499819345055
- GinatDTBokhariABhattSDograVImaging features of solitary fibrous tumorsAJR Am J Roentgenol2011196348749521343490
- Vallat-DecouvelaereAVDrySMFletcherCDAtypical and malignant solitary fibrous tumors in extrathoracic locations: evidence of their comparability to intra-thoracic tumorsAm J Surg Pathol19982212150115119850176
- DômontJSalasSLacroixLHigh frequency of beta-catenin heterozygous mutations in extra-abdominal fibromatosis: a potential molecular tool for disease managementBr J Cancer201010261032103620197769
- TsukamotoYImakitaMNishitaniAItoTIzukuraMHirotaSPancreatic desmoid-type fibromatosis with beta-catenin gene mutation-Report of a case and review of the literaturePathol Res Pract2016212548448926907785
- TejparSNolletFLiCPredominance of beta-catenin mutations and beta-catenin dysregulation in sporadic aggressive fibromatosis (desmoid tumor)Oncogene199918476615662010597266
- LazarAJTuvinDHajibashiSSpecific mutations in the beta-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumorsAm J Pathol200817351518152718832571
- GoumaDJvan GeenenRCvan GulikTMRates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volumeAnn Surg2000232678679511088073
- d’AmbrosioGdel PreteLGrimaldiCPancreaticoduodenectomy for malignancies in childrenJ Pediatr Surg201449453453824726107
- SugitoKFuruyaTKanedaHLong-term follow-up of nutritional status, pancreatic function, and morphological changes of the pancreatic remnant after pancreatic tumor resection in childrenPancreas201241455455922158069
