Abstract
Current evidence indicates that long noncoding RNAs (lncRNAs) play pivotal roles in human cancers. The present study aims to assess differentially expressed lncRNAs related to diffuse-type gastric carcinoma (DGC). Next-generation RNA sequencing was carried out to detect aberrantly expressed lncRNAs in DGC. Real-time polymerase chain reaction (RT-PCR) was performed to evaluate RP11–357H14.17 gene expression levels in DGC cell lines/tissues comparatively with normal gastric epithelial cell lines and adjacent normal tissues. The associations of RP11–357H14.17 expression levels with the clinicopathological features were also analyzed. The regulatory effects of RP11–357H14.17 on the biological behaviors of DGC cells were evaluated by MTT, colony formation assays, flow cytometry for apoptosis, wound healing assay, and transwell migration and invasion assays. RP11–357H14.17 expression was remarkably increased in DGC tissues and cell lines compared with normal gastric epithelial cells and adjacent normal tissues. High levels of RP11–357H14.17 were associated with increased tumor size, deeper depth of invasion, lymphatic metastasis, and advanced pathological stage. Further experiments demonstrated that the DGC cells MGC-803 transfected with si-RP11–357H14.17 showed reduced cell proliferation, migration, invasion, enhanced G1 phase arrest and cell apoptosis. These findings suggest that the novel lncRNA RP11–357H14.17 is associated with poor prognosis, and may serve as a potential prognostic biomarker and target for new antineoplastic therapies in human DGC.
Introduction
Gastric cancer (GC) is the fourth most common malignancy and second leading cause of cancer death worldwide, especially in East Asia.Citation1 Diffuse-type gastric carcinoma (DGC) is one of the major histological types (diffuse and intestinal types) according to the classification scale originally described by Lauren in 1965.Citation2,Citation3 Diffuse-type carcinomas are poorly differentiated and composed of solitary or poorly cohesive tumor cells in the absence of gland formation. Despite recent advances in surgical techniques and the medical treatment of DGC, the prognosis of patients with DGC remains relatively poor, in the absence of reliable early diagnostic markers and therapeutic targets for the suppression of tumor metastasis.Citation4–Citation6 The 5-year survival rate remains low.Citation7–Citation9 DGC has poorer prognosis, and occurs more frequently in younger individuals with GC.Citation10,Citation11 Previous studies have reported many oncogenes and tumor suppressor genes closely associated with DGC, but the highly complex molecular mechanisms underlying invasion and metastasis remain obscure.Citation12 Therefore, identifying reliable biomarkers associated with early DGC diagnosis, effective therapy, and prognosis evaluation is of great clinical relevance.
Different from conventional coding genes, long noncoding RNAs (lncRNAs) are a class of RNA transcripts longer than 200 nucleotides, with no or limited protein-coding potential.Citation13 Emerging evidence demonstrates that lncRNAs are closely involved in multiple biological processes, including stem cell pluripotency, cell differentiation, cell growth, cell apoptosis, and cancer metastasis.Citation14–Citation16 Dysregulation of lncRNAs has been reported in different cancers, including DGC.Citation17,Citation18 However, the overall pathophysiological contributions of lncRNAs to DGC remain largely unknown. To explore the function and mechanism of lncRNAs in DGC, we performed next-generation sequencing of 6 clinical DGC samples, and found that RP11–357H14.17 was upregulated in DGC tissues compared with adjacent normal tissues. RP11–357H14.17 was mapped to 17q21.32 with a transcript length of 11,101 bp. However, the role of RP11–357H14.17 in DGC has not been reported, and its expression and function in DGC are poorly understood.
In this research, RP11–357H14.17 expression levels in DGC issues and cell lines were evaluated by real-time polymerase chain reaction (RT-PCR). We also evaluated the associations of RP11–357H14.17 levels with clinicopathological characteristics. Moreover, its role in DGC cell proliferation and metastasis was also researched. Our findings may help further understand the role of RP11–357H14.17 as a regulator of DGC pathogenesis, which may serve as a candidate target for new diagnostics and therapies.
Material and methods
Patients and clinical specimens
Fresh primary DGC tumor tissues and matched normal adjacent tissues were collected from 48 pathologically confirmed DGC patients in Changhai Hospital (Shanghai, People’s Republic of China) between May 2015 and May 2016. A total of 6 cases were used for sequencing; RP11– 357H14.17 expression was evaluated in 42 cases. Patients with two or more different malignancies were excluded. No patients had received preoperative radiotherapy or chemotherapy. The patient characteristics are shown in . Samples were frozen immediately in liquid nitrogen and stored at −80°C until analysis. The ethics committee of ChangHai Hospital affiliated to the Second Military Medical University approved this study; written informed consent was obtained from all patients (Registration number: ChiCTR-CDC-15007379) for this study.
Table 1 Correlation between RP11–357H14.17 expression and different clinicopathological features in patients with diffuse-type gastric cancer
Cell lines and culture
Human GC cell lines (AGS, SGC-7901, MGC-803, and MKN-45) and the human normal gastric epithelial cell line GES-1 were obtained from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, People’s Republic of China). They were maintained in Roswell Park Memorial Institute 1640 medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL of penicillin, and 100 μg/mL streptomycin sulfate. Cultures were incubated in a humidified atmosphere containing 5% CO2 at 37°C.
Whole transcriptome library preparation and sequencing
Total RNA from 6 paired DGC and adjacent normal tissue samples was isolated and quality controlled. The preparation of whole transcriptome libraries and deep sequencing were performed by Novogene Bioinformatics Technology Cooperation (Shanghai, People’s Republic of China). Ribosomal RNA was removed and strand-specific sequencing libraries were generated following the manufacturer’s instructions. RNA-Seq was performed on an Illumina; HiSeq 2000 platform (San Diego, CA, USA), and 100 bp paired-end reads were generated according to the Illumina’s protocol.
Lentivirus infection and construction of stable cell lines with RP11–357H14.17 knockdown lncRNA RP11–357H14.17 short hairpin RNA (LV-lncRNA-RNAi (52661–1), si-RP11–357H14.17, 5′-GGCCAGGAA-GTCCCAGAAATA-3′) and non-targeting shRNA (si-NC, 5′-TTCTCCGAACGTGTCACGT-3′) were purchased from GenePharma (Shanghai, People’s Republic of China). The DGC MGC-803 cells were cultured in 6-well plates at optimal density until transfection, using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. The cells were harvested for further assays after 48 h for quantitative RT-PCR (qRT-PCR) or Western blot analyses.
RNA preparation and quantitative RT-PCR
Total RNA was extracted from tissues or cultured cells using TRIzol reagent (Thermo Fisher Scientific). For qRT-PCR, 1 μg RNA was reverse transcribed into cDNA with Reverse Transcription Kit (Takara, Dalian, People’s Republic of China). Real-time PCR was performed with SYBR Premix ExTaq II Kit (Takara). Data were normalized to glyceraldehyde 3-phosphate dehydroge-nase levels (5′-TGACTTCAACAGCGACACCCA-3′ (forward) and 5′-CACCCTGTTGCTGTAGCCAAA-3′ (reverse)). The PCR primers for RP11–357H14.17 were 5′-GAACTCGGCTTCTGGCACCTC-3′ (forward) and 5′-CCCCACTCCCTTTCTTCCTTG-3′ (reverse). The qRT-PCR assays and data collection were performed on ABI 7500, and relative RP11–357H14.17 expression was assessed by the 2−ΔCt method, and converted to fold changes using the 2−ΔΔCt method.
MTT assay
Cell proliferation was analyzed by MTT assay. Briefly, ~1×103 cells were seeded into 96-well plates, and incubated for 1, 2, 3, 4, and 5 days. Then, 20 μL of MTT (5 mg/mL) (Sigma-Aldrich, St Louis, MO, USA) was added into each well, and incubated for another 4 h. Then, the supernatants were removed and 150 μL of dimethyl sulfoxide (Sigma-Aldrich) was added to solubilize the formazan crystals. Absorbance (optical density) was measured at 490 nm on a microplate reader (Molecular Devices, Sunnyvale, CA, USA). Triplicate experiments were independently repeated three times.
Colony formation assay
For the colony formation assay, cells were placed in 6-well plates and maintained in media containing 10% FBS. The medium was replaced every 4 days; after 14 days, the cells were fixed with methanol and stained with 0.1% crystal violet (Sigma-Aldrich). Visible colonies were then counted.
Wound healing assay
A total of 3×105 cells were seeded in 6-well plates. The next day, once cultures reached 85% confluence, the cell monolayer was scratched with a sterile plastic tip and washed with culture medium. Cells were then cultured for 48 h with medium containing 1% FBS. At different time points, images of the plates were acquired under a microscope (IX71; Olympus, Tokyo, Japan). The distance between the two edges of the scratch was measured using the Digimizer software system (MedCalc Software, Ostend, Belgium). The assay was independently repeated three times.
Cell migration and invasion assays
For the migration assay, 5×104 cells in serum-free media were placed into the upper chamber of an insert (8-μm pore size; Millipore). In invasion assays, 1×105 cells in serum-free medium were placed into the upper chamber of an insert coated with Matrigel (Sigma-Aldrich). Culture medium containing 10% FBS was added to the lower chamber. After incubation for 24 h, the cells remaining on the upper membrane were removed with cotton wool. Those that had migrated or passed through the membrane were stained with 0.1% crystal violet in methanol, imaged, and counted using an IX71 inverted microscope (Olympus). Experiments were independently repeated three times.
Flow cytometric analysis of the cell cycle and apoptosis
Cells were cultured in 6-well plates for 48 h, washed with ice-cold phosphate-buffered saline (PBS), and fixed in cold 70% ethanol overnight at −20°C. The cells were then washed and stained with propidium iodide (PI; Beyotime, Shanghai, People’s Republic of China) containing 0.25 mg/mL of RNase A. After incubation in the dark at 4°C for 15 min, the cells were analyzed by flow cytometry (FACScan; BD Biosciences, San Jose, CA, USA) using the MODFIT software (Verity Software House, Topsham, ME, USA). The percentages of cells in various cycle phases (G1, S, and G2/M phases) were recorded and compared.
Apoptosis was examined using an Annexin-V fluorescein isothiocyanate (FITC)/PI dual staining kit (KeyGen, Nanjing, People’s Republic of China). Briefly, MGC-803 cells were treated with lncRNA-RP11–357H14.17 or control siRNA for 48 h and washed three times in cold PBS. The cells were then stained with Annexin V-FITC and PI in 500 μL of binding buffer, in the dark at room temperature for 15 min, and assessed by flow cytometry.
Statistical analysis
All experiments were independently performed at least three times. Statistical analyses were performed with the SPSS 20.0 software (IBM Corp., Armonk, NY, USA). Enumeration data were presented as percentage (%), and compared by Chi-squared (χ2) test. Values were mean ± standard deviation; two groups were compared by paired samples t-test, and multiple groups by one-way analysis of variance. Non-normally distributed data were presented as median (interquartile range), and compared with the Wilcoxon test. Statistical differences were set at *P<0.05, **P<0.01. P<0.05 was considered statistically significant.
Results
LncRNA expression profiles in diffuse-type GC and adjacent normal tissues
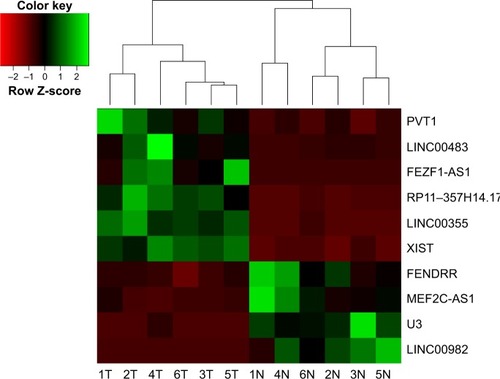
First, next-generation sequencing was used to analyze differentially expressed lncRNAs in 6 paired DGC and adjacent normal tissues. The differentially expressed lncRNAs were filtered by the criteria of fold change >2 and P<0.05 between cancer and adjacent normal tissues. There were 73 up-regulated and 39 down-regulated lncRNAs in DGC samples compared with adjacent normal specimens, including 30 antisense lncRNAs, 11 intronic lncRNAs, and 71 intergenic lncRNAs. Hierarchical clustering analysis on most significantly dysregulated cancer-related lncRNAs is summarized in . The lncRNAs showing the highest absolute expression levels in DGC tissues (fold change >8) were further assessed. This filtering process yielded 3 lncR-NAs (XIST, PVT1 and RP11–357H14.17). Notably, the lncRNAs XIST and PVT1 were shown to be overexpressed in GC.Citation19,Citation20 Our filtering approach was validated by the identification of XIST and PVT1. Therefore, we selected the intergenic lncRNA RP11–357H14.17 for further studies.
RP11–357H14.17 expression and clinicopathological factors in DGC
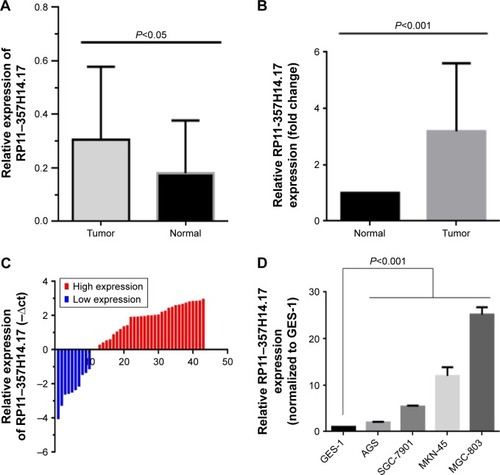
To verify the involvement of RP11–357H14.17 in DGC progression, we detected RP11–357H14.17 expression levels in 42 paired DGC and adjacent normal tissues by qRT-PCR. As shown in , RP11–357H14.17 amounts were remarkably up-regulated in DGC tissues compared with adjacent normal tissues (P<0.05). Compared with the normal gastric epithelial cell line, GES-1, RP11–357H14.17 expression was increased in the GC cells MGC-803 (P<0.0001), MKN-45 (P=0.0094), AGS (P=0.0079), and SGC-7901 (P=0.0009), especially MGC-803 cells, a DGC cell line ().
Figure 2 Relative RP11–357H14.17 expression levels in diffuse-type gastric cancer tissues and cell lines.
Abbreviations: DGC, diffuse-type gastric cancer; GC, gastric cancer; qRT-PCR, quantitative real-time polymerase chain reaction.
We further analyzed the associations of RP11–357H14.17 expression with clinicopathological features in patients with DGC. According to median RP11–357H14.17 levels, we divided the samples into high (above median value, n=21) and low (below median value, n=21) RP11–357H14.17 expression groups. Chi-square test was then performed to evaluate the clinicopathological features between the two groups. As shown in , increased RP11–357H14.17 expression was significantly associated with larger tumor size (P=0.029), deeper depth of invasion (P=0.040), more advanced pathological stage (P=0.027) and lymphatic metastasis (P=0.006). However, RP11–357H14.17 levels were not associated with other clinicopathological features, such as age (P=0.203) and sex (P=0.476) in DGC.
RP11–357H14.17 depletion inhibits proliferation and cell cycle progression, and promotes apoptosis in diffuse-type GC cells
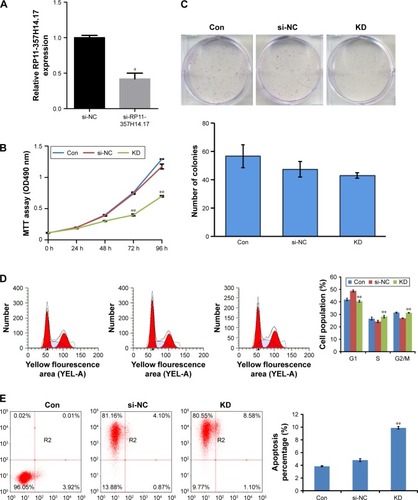
To assess the biological functions of RP11–357H14.17 in DGC, we designed si-RP11–357H14.17 and transfected into the MGC-803 cell line. RP11–357H14.17 expression was overtly downregulated (P<0.005) in si-RP11–357H14.17 transfected cells (). MTT assay indicated that RP11–357H14.17 knockdown significantly inhibited cell proliferation compared with control cells after 72 h (). However, in colony formation assays, clono-genic survival was not significantly decreased following inhibition of RP11–357H14.17 in the MGC-803 cell line (), probably due to the short cycle. Furthermore, flow cytometry revealed that RP11–357H14.17 silencing promoted G1 phase arrest in MGC-803 cells (). Moreover, RP11–357H14.17 downregulation obviously promoted apoptosis compared with the si-NC group ().
Figure 3 The effects of RP11–357H14.17 on cell viability.
Abbreviations: CON, MGC-803 cell line; si-NC, si-negative control/non-targeting shRNA; KD, si-RP11–357H14.17.
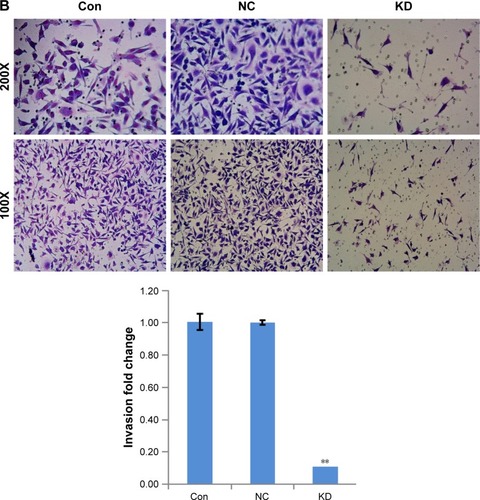
RP11–357H14.17 downregulation inhibits cell migration and invasion in DGC
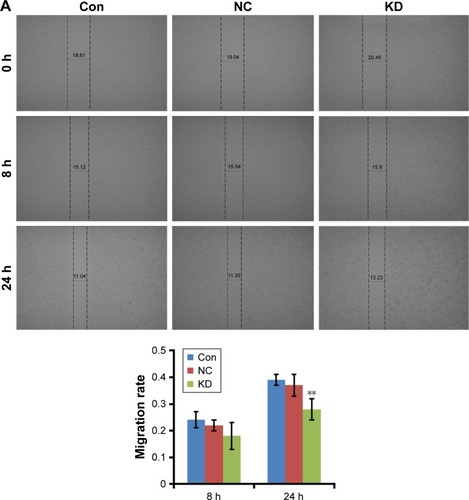
To assess whether RP11–357H14.17 is involved in DGC cell migration and invasion, we evaluated cell migration and invasion by wound healing and transwell assays. As shown in , the migration ability of RP11–357H14.17 knockdown MGC-803 cells was significantly reduced compared with the negative control (NC) cells (P<0.05). Furthermore, migration and invasion were also markedly repressed, as shown in transwell invasion assays (P<0.05, ).
Figure 4 Knock-down RP11–357H14.17 suppresses migration and invasion of DGC cells. Cell migration and invasion were determined by wound healing assay and transwell assay.
Abbreviations: DGC, diffuse-type gastric carcinoma; CON, MGC-803 cell line; NC, negative control; KD, si-RP11–357H14.17.
Discussion
Multiple studies have revealed the emerging significance of lncRNAs in various human diseases, including cancer.Citation21–Citation24 LncRNAs play critical roles in tumor invasion and metastasis, by regulating tumor suppressor and oncogenetic pathways.Citation25,Citation26 The relationship between lncRNAs and tumors attracts increasing attention from oncologists. Recently, more evidence has emerged that abnormal lncRNAs might play critical roles in GC invasion and metastasis.Citation27,Citation28 For example, our previous study found that high lncRNA H19 levels contribute to the proliferation of GC cells.Citation29 The lncRNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331–3p in GC.Citation30 Accumulating evidence suggests that further lncRNA assessment would provide new insights that may help find more efficient biomarkers of GC for early diagnosis, effective therapy, and prognosis evaluation.
To the best of our knowledge, this is the first report to illustrate RP11–357H14.17 function in DGC. In the present study, we first selected the lncRNA RP11–357H14.17 from a total of 112 lncRNAs associated with DGC by high-throughput sequencing, and confirmed RP11–357H14.17 was highly expressed in DGC cells and tissues by RT-PCR. These findings may be the first evidence that high RP11–357H14.17 expression is closely associated with the development of DGC. Moreover, we evaluated the associations of RP11–357H14.17 expression levels with clinicopathological characteristics of DGC. Interestingly, high RP11–357H14.17 expression was more frequently correlated with aggressive tumor characteristics (greater invasion depth, higher tumor stage, lymphatic metastasis, and advanced tumor, node, metastasis (TNM) stage). This suggested that RP11–357H14.17 might be useful in the development of novel prognostic and progression markers in DGC.
Due to the abnormally high expression of RP11– 357H14.17 in DGC cells and tissues, we hypothesized that RP11–357H14.17 plays a significant role in tumor biology. To examine this possibility, RP11–357H14.17 expression levels in four representative GC cell lines were evaluated comparatively with the normal gastric epithelial cell lines GES-1. As shown above, RP11–357H14.17 expression was significantly increased in the DGC cell line (MGC-803) compared with the normal gastric epithelial cell line GES-1, which corroborated our previous findings. We next used siRNA to knockdown RP11–357H14.17 in DGC cells; this inhibited cell proliferation, cell cycle progression, cell migration and cell invasion, and promoted apoptosis. These results revealed that RP11–357H14.17 might be involved in the progression of DGC by altering cell migration and invasion, and could constitute a potential prognostic biomarker and target for new antineoplastic therapies.
The present study had some limitations. First, due to the short cycle of clinical specimens, survival analyses were not performed. Second, the effects of RP11–357H14.17 were evaluated in vitro, and in vivo effects of RP11–357H14.17 on DGC and the related mechanism were not analyzed in this study.
Conclusion
In summary, this study first confirmed that RP11–357H14.17 is significantly increased in DGC tissues and cell lines, with high RP11–357H14.17 expression correlated with invasion depth, larger tumor size, lymphatic metastasis, and TNM stage in DGC patients. Regulation of RP11–357H14.17 expression affected proliferation, migration and invasion of DGC. These findings suggested that RP11–357H14.17 is a potential therapeutic target for highly aggressive and malignant DGCs in clinical practice.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No 81172156 and 81372048).
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2016CA Cancer J Clin201666173026742998
- LaurenPThe two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classificationActa Pathol Microbiol Scand196564314914320675
- Van CutsemESagaertXTopalBHaustermansKPrenenHGastric cancerLancet2016388100602654266427156933
- JinawathNFurukawaYHasegawaSComparison of gene-expression profiles between diffuse- and intestinal-type gastric cancers using a genome-wide cDNA microarrayOncogene200423406830684415273739
- MingSCCellular and molecular pathology of gastric carcinoma and precursor lesions: a critical reviewGastric Cancer199811315011957042
- SipponenPGastric cancer: pathogenesis, risks, and preventionJ Gastroenterol200237Suppl 13394412109664
- HiroseSHonjouHNakagawaHScirrhous carcinoma of the stomach: a clinical and pathological study of 106 surgical casesGastroenterol Jpn19892454814872553523
- OtsujiEKuriuYOkamotoKOutcome of surgical treatment for patients with scirrhous carcinoma of the stomachAm J Surg2004188332733215450843
- Marques-LespierJMGonzalez-PonsMCruz-CorreaMCurrent perspectives on gastric cancerGastroenterol Clin North Am201645341342827546840
- CorreaPHoughtonJCarcinogenesis of Helicobacter pyloriGastroenterology2007133265967217681184
- AdachiYYasudaKInomataMSatoKShiraishiNKitanoSPathology and prognosis of gastric carcinoma: well versus poorly differentiated typeCancer20008971418142411013353
- TaharaEMolecular biology of gastric cancerWorld J Surg1995194484488 discussion 489–4907676688
- NaganoTFraserPNo-nonsense functions for long noncoding RNAsCell2011145217818121496640
- MuersMRNA: Genome-wide views of long non-coding RNAsNat Rev Genet20111211742
- PontingCPOliverPLReikWEvolution and functions of long non-coding RNAsCell2009136462964119239885
- LoewerSCabiliMNGuttmanMLarge intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cellsNat Genet201042121113111721057500
- LiHYuBLiJOverexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancerOncotarget2014582318232924810858
- ZhangEBKongRYinDDLong noncoding RNA ANRIL indicates a poor prognosis of gastric cancer and promotes tumor growth by epigenetically silencing of miR-99a/miR-449aOncotarget2014582276229224810364
- MaLZhouYLuoXGaoHDengXJiangYLong non-coding RNA XIST promotes cell growth and invasion through regulating miR-497/MACC1 axis in gastric cancerOncotarget2017834125413527911852
- YuanCLLiHZhuLLiuZZhouJShuYAberrant expression of long noncoding RNA PVT1 and its diagnostic and prognostic significance in patients with gastric cancerNeoplasma201663344244926925791
- GibbEABrownCJLamWLThe functional role of long non-coding RNA in human carcinomasMol Cancer2011103821489289
- UlitskyIBartelDPlincRNAs: genomics, evolution, and mechanismsCell20131541264623827673
- GeislerSCollerJRNA in unexpected places: long non-coding RNA functions in diverse cellular contextsNat Rev Mol Cell Bio2013141169971224105322
- WapinskiOChangHYLong noncoding RNAs and human diseaseTrends Cell Biol201121635436121550244
- GuttmanMAmitIGarberMChromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammalsNature2009458723522322719182780
- HuarteMGuttmanMFeldserDA Large intergenicnoncoding RNA induced by p53 mediates global gene repression in the p53 responseCell2010142340941920673990
- FangXYPanHFLengRXYeDQLong noncoding RNAs: novel insights into gastric cancerCancer Lett2015356235736625444905
- ZhouXYinCDangYYeFZhangGIdentification of the long non-coding RNA H19 in plasma as a novel biomarker for diagnosis of gastric cancerSci Rep201551151626096073
- YangFBiJXueXUp-regulated long non-coding RNA H19 contributes to proliferation of gastric cancer cellsFEBS J2012279173159316522776265
- LiuXHSunMNieFQLnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR31–3p in gastric cancerMol Cancer2014139224775712