Abstract
Published studies have investigated the prognostic role of cyclooxygenase-2 (COX-2) expression in patients with esophageal cancer (EC), but the result remains controversial. Thus, this meta-analysis was conducted to comprehensively evaluate the impact of COX-2 expression on the prognostic value in patients with EC. Relevant studies were identified from PubMed, EMBASE, and Web of Science databases. Studies that detected the COX-2 expression by immunohistochemistry and evaluated the relationship between COX-2 expression and overall survival (OS) or clinicopathological parameters were used in our analysis. The summary hazard ratios (HRs) or odds ratios were calculated to assess the risk or hazard association. A total of 25 studies, which included 2,465 patients, were included in our meta-analysis. Our analysis suggested that overexpression of COX-2 was associated with poor OS (HR =1.60, 95% CI =1.32–1.94, P<0.001). Subgroup analyses by race, percentage of high/positive COX-2 expression, histology type, treatment, and sample size all suggested significant association. Moreover, overexpression of COX-2 was significantly associated with depth of invasion, lymph node metastasis, distant metastasis, and TNM stage. This meta-analysis suggested that overexpression of COX-2 might serve as a prognostic biomarker for EC. Large well-designed prospective studies are needed to confirm our conclusion.
Introduction
Esophageal cancer (EC) ranks as the eighth most common cancer and the sixth most common cause of death from cancer worldwide.Citation1 EC can be classified as esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EADC).Citation1,Citation2 Over the past few decades, although significant advances have been made in surgical techniques, radiotherapy and chemotherapy, the prognosis of EC remains unsatisfactory. The overall 5-year survival rate of EC ranges from 15% to 25%. Poor outcomes in patients with EC are related to diagnosis at advanced (metastatic) stages and the propensity for metastases, even when tumors are superficial.Citation3
Cyclooxygenases (COXs) are rate-limiting enzymes that catalyze the conversion of arachidonic acid into prostaglandin. Two distinct forms of COX have been recognized: COX-1 and COX-2. COX-1 is constitutively expressed in a wide variety of cells and tissues and is responsible for the production of prostaglandins that are important for maintaining homeostasis. In contrast, COX-2 is normally absent, and its expression is induced by different mediators of inflammation, such as interleukin-1, tumor necrosis factor-1, and lipopolysaccharide. COX-2 is mostly expressed in pathological states, principally in inflammatory reactions and in oncogenesis.Citation4 Preclinical studies have shown that COX-2 is widely involved in carcinogenesis, including cell proliferation, apoptosis inhibition, angiogenesis, invasiveness, and immunosuppression.Citation5 On the basis of the evidence, a series of clinical studies have evaluated the prognostic role of COX-2 in patients with EC. Many studies suggested that the overexpression of COX-2 was associated with poor prognosis.Citation6–Citation14 However, other findings did not support this view;Citation15–Citation21 some studies even found the trend that the overexpression of COX-2 was related to favorable prognosis, although the association did not reach the significant level.Citation8,Citation22–Citation24 Considering the small sample size and the limited static power in individual study, a meta-analysis was necessary to comprehensively evaluate the prognostic significance of COX-2 expression in patients with EC.
Materials and methods
Literature search
A comprehensive literature search was conducted in the databases of PubMed, EMBASE, and Web of Science. The end search limit was December 31, 2016. The following search terms were used to identify the studies: (“cyclooxygenase-2” or “cyclooxygenase” or “COX-2”) and (“esophageal cancer” or “esophageal carcinoma” or “esophageal neoplasms” or “esophageal squamous cell carcinoma” or “ESCC” or “esophageal adenocarcinoma” or “EADC”). Furthermore, references of retrieved articles and reviews were manually screened for additional studies.
Inclusion and exclusion criteria
The inclusion criteria were used to identify the eligible studies: 1) human-based investigations; 2) pathologically confirmed EC; 3) articles with full texts published in English; 4) detecting COX-2 expression in the primary tumor tissues by immunohistochemistry (IHC) assay; 5) evaluating the correlation between COX-2 expression and OS, or clinicopathological parameters such as histological grade, depth of invasion, lymph-node metastasis, distant metastasis, and TNM stage; 6) providing sufficient information to estimate hazard ratio (HR) or odds ratio (OR) and their 95% confidence intervals (CIs). The exclusion criteria were as follows: 1) studies published in non-English; 2) cell line and animal studies, case reports, letters, reviews, or meta-analyses; 3) studies in which necessary data to extract the relationship between COX-2 expression and OS or clinicopathological parameters were not provided; 4) for overlapped studies, the studies with the small sample size and the insufficient data set were excluded.
Data extraction
Two investigators (YLY and ZXH) independently reviewed the eligible studies and extracted the following data: surname of the first author, publication year, country, ethnicity, sample size, disease stage, histology type, assay method, cutoff value, percentage of high/positive COX-2 expression, and the outcomes. All data were then examined by two investigators independently (YLY and ZXH). Disagreements were resolved by discussion among all authors.
Statistical analysis
The impact of COX-2 expression on overall survival (OS) was measured by the combined HRs and their 95% CIs extracted from each eligible study. The HR and its 95% CI in each eligible study were directly extracted from report or indirectly estimated by methods described by Tierney et al.Citation25 For the relationship between COX-2 expression and clinicopathological parameters, ORs and their 95% CIs were combined to estimate the effective value. The overall HR/OR and its 95% CI greater than 1 were considered statistically significant and indicated a worse effect for the group with high/positive COX-2 expression. Heterogeneity between studies was detected by the Q-test and the I2 metric (no heterogeneity: I2=0%–25%; moderate heterogeneity: 25%–50%; large heterogeneity: 50%–75%; and extreme heterogeneity: 75%–100%).Citation26 If P≥0.10 in the Q-test or I2<50%, the fixed-effects model (the Mantel–Haenszel method) was used.Citation27 Otherwise, the random-effects model (the DerSimonian–Laird method) was used.Citation28 Subgroup analyses by different analytical methods (race, percentage of high COX-2, histological type, treatment, and sample size) were performed in the analysis of OS. In addition, publication bias was assessed by the methods reported by Begg and MazumdarCitation29 and Egger et al.Citation30 Funnel plots were also applied for the assessment of possible publication biases. All P-values were two-tailed, and the P<0.05 was considered statistically significant. Most of the statistical analyses in this study were conducted by the STATA software (version 11.2; StataCorp., College Station, TX, USA).
Results
Eligible studies
A total of 399 studies were yielded by the systematic literature search. After screening of titles and abstracts, 345 irrelevant studies were excluded and the remaining 54 studies were further evaluated for potentially eligible studies. After carefully reading the full text, 29 studies were excluded for the following reasons: not an IHC method (n=3), cell-line or animal experiments (n=7), without outcome of interest (n=6), reviews and meta-analyses (n=5), and insufficient data (n=8). As a result, 25 studies, which included 2,465 EC patients, were included in our analysisCitation6–Citation24,Citation31–Citation36 ().
The general characteristics of 25 studies were sum-marized in . Four studies evaluated only clinico-pathological parameters,Citation8,Citation24,Citation31,Citation33 5 studies evaluated only OS,Citation8,Citation11,Citation18,Citation20,Citation21 and the remaining 16 studies investigated both clinicopathological parameters and OS.Citation6,Citation7,Citation9,Citation10,Citation12–Citation17,Citation19,Citation22,Citation23,Citation32–Citation36 Eighteen studies were conducted on Asian patients,Citation6,Citation9–Citation13,Citation16,Citation18,Citation19,Citation21–Citation24,Citation31,Citation33–Citation36 and 7 studies were conducted on Caucasian patients.Citation7,Citation8,Citation14,Citation15,Citation17,Citation20,Citation32 The percentage of high/positive COX-2 expression was >50% in 15 studies,Citation6,Citation8,Citation10,Citation11,Citation13–Citation19,Citation21,Citation22,Citation31,Citation34,Citation36 and the remaining 10 studies shared the percentage of high/positive COX-2 expression <50%.Citation7,Citation9,Citation12,Citation20,Citation21,Citation23,Citation24,Citation32,Citation33 Among these studies, ESCC was investigated in 17 studies,Citation6,Citation9–Citation13,Citation16,Citation18,Citation19,Citation21–Citation24, Citation31–Citation34,Citation36 EADC was investigated in 5 studies,Citation7,Citation8,Citation14,Citation17 and another 3 studies evaluated both histological types.Citation15,Citation20,Citation21 The patients in 17 studies were treated by surgery only;Citation7–Citation11,Citation13–Citation15,Citation17,Citation19,Citation21,Citation22,Citation24,Citation31,Citation33,Citation34 the surgery plus additional chemotherapy or radiotherapy was applied in 8 studies.Citation6,Citation12,Citation16,Citation18,Citation20,Citation23,Citation32,Citation36
Table 1 Main characteristics of all studies included in the meta-analysis
Impact of COX-2 expression on OS of EC
Twenty-four studies, which included 2,270 patients, reported the relationship between COX-2 expression and OS.Citation12–Citation15,Citation17–Citation22,Citation24,Citation26–Citation36 The pooled HR was 1.60 (95% CI: 1.32–1.94, P<0.001) with moderate heterogeneity (I2=49.1%, P=0.004), indicating that the EC patients with overexpression of COX-2 would have worse OS ( and ).
Figure 2 Funnel plot of the association of COX-2 expression with overall survival (OS) in patients with esophageal cancer.
Abbreviations: CI, confidence interval; COX-2, cyclooxygenase-2; HR, hazard ratio.
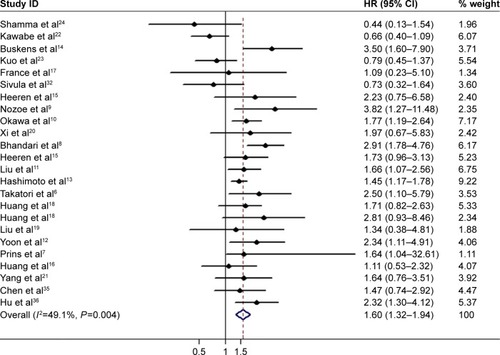
Table 2 Main meta-analysis results of COX-2 expression in patients with esophageal cancer
Further subgroup analysis by race was done; the analysis suggested that overexpression of COX-2 was related to worse OS in both Asian and Caucasian patients (Asian: HR =1.49, 95% CI =1.20–21.85, P<0.001, I2=50.0%, P=0.012; Caucasian: HR =2.04, 95% CI =1.54–2.71, P<0.001, I2=35.5%, P=0.145). When we conducted subgroup analysis by the percentage of high/positive COX-2 expression, we found that both studies with high/positive COX-2 expression ≥50% and <50% showed the significant association (high/positive COX-2 ≥50%: HR =1.55, 95% CI =1.35–1.78, P<0.001, I2=40.4%, P=0.064; low/negative COX-2 <50%: HR =1.53, 95% CI =1.02–2.29, P=0.038, I2=60.1%, P=0.005). When histology type was taken into consideration, high/positive COX-2 expression still had impact on OS in both ESCC and EADC patients (ESCC: HR =1.46, 95% CI =1.17–1.83, P=0.001, I2=52.0%, P=0.007; EADC: HR =2.13, 95% CI =1.62–2.79, P<0.001, I2=26.4%, P=0.246). When we focus on treatment strategy, for studies that received surgery only, the HR was 1.52 (95% CI =1.21–1.92, P<0.001, I2=52.6%, P=0.006); for studies that received surgery plus additional chemoradiotherapy, the pooled HR was 1.72 (95% CI =1.23–2.32, P=0.004, I2=53.7%, P=0.056). Subgroup analysis by sample size (≥100 and <100) also suggested the significant prognostic impact of high/positive COX-2 expression ().
COX-2 expression and clinicopathological parameters in patients with EC
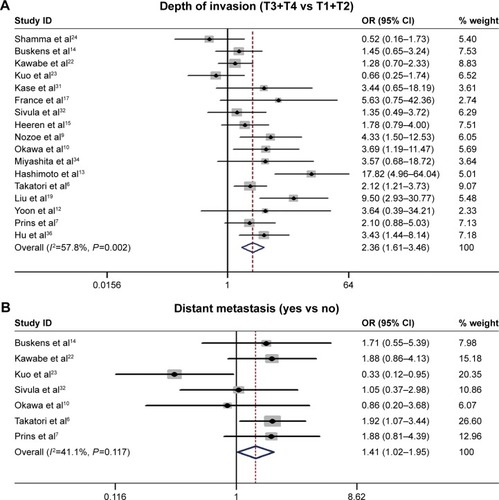
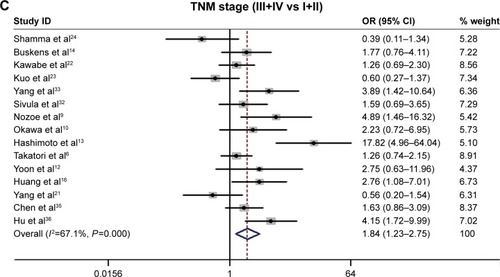
The relationship between the COX-2 expression and the following clinicopathological parameters was collected for analysis: histological grade,Citation6,Citation7,Citation9,Citation10,Citation13,Citation14,Citation19,Citation22–Citation24,Citation31–Citation36 depth of invasion,Citation6,Citation7,Citation9,Citation10,Citation12–Citation15,Citation17,Citation19,Citation22–Citation24,Citation31,Citation32,Citation34,Citation36 lymph-node metastasis,Citation6,Citation7,Citation9,Citation10,Citation12–Citation15,Citation17,Citation19,Citation22–Citation24,Citation31,Citation32,Citation34,Citation36 distant metastasis,Citation6,Citation7,Citation10,Citation14,Citation22,Citation23,Citation32 and TNM stage.Citation6,Citation7,Citation9,Citation10,Citation12–Citation14,Citation16,Citation22–Citation24,Citation32,Citation33,Citation35,Citation36 Our analysis suggested that high/positive COX-2 expression was significantly associated with the depth of invasion (T3/4 vs T1/2: OR =2.36, 95% CI =1.61–63.46, P<0.001, I2=57.8%, P=0.002; ), distant metastasis (yes vs no: OR =1.41, 95% CI =1.02–1.95, P=0.037, I2=41.1%, P=0.117; ), and TNM stage (III/IV vs I/II: OR =1.84, 95% CI =1.23–2.75, P=0.003, I2=67.1%, P<0.001; ). However, no significant association was found in the relationship between COX-2 expression and histological grade and lymph-node metastasis ().
Figure 3 Funnel plot of the association of COX-2 expression with the depth of invasion (T) (A), distant metastasis (M) (B), and TNM stage (C) in patients with esophageal cancer.
Abbreviations: CI, confidence interval; COX-2, cyclooxygenase-2; OR, odds ratio.
Publication bias
The assessment of publication bias showed that the Egger’s tests were not significant (P>0.05) for studies included in the analysis of OS and clinicopathological parameters. When Begg’s tests were applied to detect publication bias, except for distant metastasis (P=0.035), no additional publication bias was found in other comparisons ().
Discussion
In this meta-analysis, we explored the relationship between COX-2 expression and the prognosis in patients with EC. We analyzed 2,465 patients from 25 studies and demonstrated that overexpression of COX-2 might be associated with poor OS. In addition, we found that overexpression of COX-2 was related to the depth of invasion, distant metastasis, and TNM stage; however, no association was found in histological grade and lymph-node metastasis.
The relationship between COX-2 and other cancer was also investigated by other meta-analyses. Peng et alCitation37 found that COX-2 765G>C polymorphism was associated with colorectal cancer risk. Another study by Wang et alCitation38 found that COX-2 overexpression was associated with poor prognosis and cancer progression. COX-2 765G>C is a functional polymorphism located at 765 bp upstream (2,765 bp) from the transcription starting site. It changes a putative stimulatory protein (Sp1)-binding site in the promoter of COX-2 between 2,766 and 2,761 bp, but it creates an E2 promoter factor (E2F) binding site, leading to high transcription activity and increased COX-2 expressions, which might be involved in the development of cancers. All this evidence suggested that COX-2 plays an important role in cancer development and progression.
The results of our study also provided the evidence that the use of a COX-2 inhibitor could be an effective therapeutic strategy for patients with EC. COX-2 is considered to be a novel target for cancer prevention and therapy. As COX-2 may contribute to tumorigenesis, some nonsteroidal anti- inflammatory drugs such as aspirin and coxibs were introduced in clinical trials, initially for chemoprevention and later for cancer therapy.Citation5,Citation39 Clinical studies were designed to evaluate the efficacy of celecoxib in combination with adjuvant systemic chemotherapeutic or radiation therapy in various cancers, such as nonsmall cell lung cancer, prostate cancer, breast cancer, and colorectal cancer; however, the inconclusive results have generated.Citation5,Citation40–Citation43 Recently, a Phase 2 clinical trial (NCT00137852) evaluated the safety and efficacy of combining celecoxib with neoadjuvant irinotecan/cisplatin chemoradiation. They found that the addition of celecoxib to neoadjuvant cisplatin–irinotecan chemoradiation was tolerable; the OS appeared comparable to prior studies using neoadjuvant cisplatin–irinotecan chemoradiation alone.Citation44 The further mechanism of anticancer effects of COX-2 inhibitors should be elucidated, and results from large randomized clinical trials are needed to provide useful information in further establishing the efficacy of COX-2 inhibitors in adjuvant chemotherapy.
Our result was consistent with previous meta-analysis conducted by Li et al.Citation45 In previous meta-analysis, they only focus on ESCC and their literature search time was closed on December 2008. Our meta-analysis included more studies and larger sample size to comprehensively evaluate the prognostic and clinicopathological significance of COX-2 expression in patients with EC (including ESCC and EADC). Various subgroup analyses (such as race, percentage of high COX-2 expression, histology type, treatment strategy, and sample size) were done; all these subgroup analyses suggested the significant association. However, in the study by Li et al, they did not conduct subgroup analysis. To some extent, with the larger sample size, comprehensive analysis, the reliability of our analysis was largely enhanced.
The significant heterogeneity was a major concern in our analysis. The significant heterogeneity was detected in our analysis. Although various subgroup analyses were conducted, we still could not find the source of heterogeneity. This may come from the different characteristics of the subjects in different studies. Furthermore, the methodology for IHC could affect the heterogeneity due to the various detecting antibodies against COX-2 and the application of different cutoff values for determining high COX-2 levels. Moreover, the HRs and their 95% CIs we extracted from the OS data were not consistent. We have to estimate the HRs by reading the Kaplan–Meier curves because some studies did not report the HRs. For studies that reported the HRs, some provided the unadjusted HRs, whereas others provided the adjusted values. Even for adjusted HRs, the cofounders they adjusted in different studies were not the same. All of these factors more or less contributed to the heterogeneity.
At last, the potential publication bias may exist. Articles were not written in English and studies failed to get published because of negative or null results cannot be identified in our literature search, and thus were not included in this analysis. In addition, some reports that did not provide sufficient data were also excluded from our analysis.
Conclusion
Our study indicates that overexpression of COX-2 is correlated with tumor progression and prognosis of EC patients. COX-2 might be a predicative factor of progression and prognosis for patients with EC. With the limitations, het-erogeneities, and bias of meta-analysis, our conclusions in this study need to be interpreted with caution. Future large prospective studies with rigorously designed methodology are warranted to confirm our results.
Acknowledgments
This work was supported by the key project of the National Natural Science Foundation (No U1202224).
Disclosure
The authors report no conflicts of interest in this work.
References
- BrownLMDevesaSSChowWHIncidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and ageJ Natl Cancer Inst2008100161184118718695138
- LinYTotsukaYHeYEpidemiology of esophageal cancer in Japan and ChinaJ Epidemiol201323423324223629646
- PennathurAGibsonMKJobeBALuketichJDOesophageal carcinomaLancet2013381986440041223374478
- DempkeWRieCGrotheyASchmollHJCyclooxygenase-2: a novel target for cancer chemotherapy?J Cancer Res Clin Oncol2001127741141711469677
- RizzoMTCyclooxygenase-2 in oncogenesisClin Chim Acta20114129–1067168721187081
- TakatoriHNatsugoeSOkumuraHCyclooxygenase-2 expression is related to prognosis in patients with esophageal squamous cell carcinomaEur J Surg Oncol200834439740217553653
- PrinsMJVerhageRJten KateFJvan HillegersbergRCyclooxygenase isoenzyme-2 and vascular endothelial growth factor are associated with poor prognosis in esophageal adenocarcinomaJ Gastrointest Surg201216595696622258871
- BhandariPBatemanACMehtaRLPrognostic significance of cyclooxygenase-2 (COX-2) expression in patients with surgically resectable adenocarcinoma of the oesophagusBMC Cancer2006613416712734
- NozoeTEzakiTKabashimaABabaHMaeharaYSignificance of immunohistochemical expression of cyclooxygenase-2 in squamous cell carcinoma of the esophagusAm J Surg2005189111011515701502
- OkawaTNaomotoYNobuhisaTHeparanase is involved in angiogenesis in esophageal cancer through induction of cyclooxygenase-2Clin Cancer Res200511227995800516299228
- LiuJFJamiesonGWuTCZhangSWWangQZDrewPCyclooxygenase-2 expression in squamous cell carcinoma of the esophagusDis Esophagus200619535035416984531
- YoonMSNamTKLeeJSVEGF as a predictor for response to definitive chemoradiotherapy and COX-2 as a prognosticator for survival in esophageal squamous cell carcinomaJ Korean Med Sci201126451352021468258
- HashimotoNInayamaMFujishimaMShiozakiHClinicopathologic significance of expression of cyclooxygenase-2 in human esophageal squamous cell carcinomaHepatogastroenterology2007547575876017591056
- BuskensCJVan ReesBPSivulaAPrognostic significance of elevated cyclooxygenase 2 expression in patients with adenocarcinoma of the esophagusGastroenterology200212271800180712055587
- HeerenPPlukkerJvan DullemenHNapRHollemaHPrognostic role of cyclooxygenase-2 expression in esophageal carcinomaCancer Lett2005225228328915978332
- HuangJXChenWCLinMClinicopathological significance of cyclooxygenase-2 and cell cycle-regulatory proteins expression in patients with esophageal squamous cell carcinomaDis Esophagus201225212112921762277
- FranceMDrewPADoddTWatsonDICyclooxygenase-2 expression in esophageal adenocarcinoma as a determinant of clinical outcome following esophagectomyDis Esophagus200417213614015230726
- HuangWZFuJHWangDKOverexpression of cyclooxygenase-2 is associated with chemoradiotherapy resistance and prognosis in esophageal squamous cell carcinoma patientsDis Esophagus200821867968418459994
- LiuWKJiangXYZhangMPZhangZXThe relationship between HPV16 and expression of cyclooxygenase-2, P53 and their prognostic roles in esophageal squamous cell carcinomaEur J Gastroenterol Hepatol2010221677419730383
- XiHBaldusSEWarnecke-EberzUHigh cyclooxygenase-2 expression following neoadjuvant radiochemotherapy is associated with minor histopathologic response and poor prognosis in esophageal cancerClin Cancer Res200511238341834716322294
- YangZGuanBMenTFujimotoJXuXComparable molecular alterations in 4-nitroquinoline 1-oxide-induced oral and esophageal cancer in mice and in human esophageal cancer, associated with poor prognosis of patientsIn Vivo201327447348423812217
- KawabeAShimadaYUchidaSExpression of cyclooxygenase-2 is associated with carcinogenesis of the lower part of thoracic esophageal squamous cell carcinoma and p53 expressionOncology2002621465411810043
- KuoKTChowKCWuYCClinicopathologic significance of cyclooxygenase-2 overexpression in esophageal squamous cell carcinomaAnn Thorac Surg200376390991412963227
- ShammaAYamamotoHDokiYUp-regulation of cyclooxygenase-2 in squamous carcinogenesis of the esophagusClin Cancer Res2000641229123810778945
- TierneyJFStewartLAGhersiDBurdettSSydesMRPractical methods for incorporating summary time-to-event data into meta-analysisTrials200781617555582
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ2003327741455756012958120
- MantelNHaenszelWStatistical aspects of the analysis of data from retrospective studies of diseaseJ Natl Cancer Inst195922471974813655060
- DerSimonianRLairdNMeta-analysis in clinical trialsControl Clin Trials1986731771883802833
- BeggCBMazumdarMOperating characteristics of a rank correlation test for publication biasBiometrics1994504108811017786990
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ199731571096296349310563
- KaseSOsakiMHonjoSExpression of cyclooxygenase-1 and cyclooxygenase-2 in human esophageal mucosa, dysplasia and carcinomaPathobiology2004712849214707443
- SivulaABuskensCJvan ReesBPPrognostic role of cyclooxygenase-2 in neoadjuvant-treated patients with squamous cell carcinoma of the esophagusInt J Cancer2005116690390815856454
- YangGZLiLDingHYZhouJSCyclooxygenase-2 is over-expressed in Chinese esophageal squamous cell carcinoma, and correlated with NF-kappaB: an immunohistochemical studyExp Mol Pathol200579321421816202995
- MiyashitaMMakinoHKatsutaMCyclooxygenase-2 over-expression is associated with human esophageal squamous cell carcinomaJ Nippon Med Sch200673630831317220580
- ChenJWuFPeiHLAnalysis of the correlation between P53 and Cox-2 expression and prognosis in esophageal cancerOncol Lett20151042197220326622818
- HuDZhangMWangSWangZHigh expression of cyclooxygenase 2 is an indicator of prognosis for patients with esophageal squamous cell carcinoma after Ivor Lewis esophagectomyThorac Cancer20167331031527148416
- PengQYangSLaoXMeta-analysis of the association between COX-2 polymorphisms and risk of colorectal cancer based on case-control studiesPLoS One201494e9479024733273
- WangZHeMXiaoZWuHWuYQuantitative assessment of the association of COX-2 (Cyclooxygenase-2) immunoexpression with prognosis in human osteosarcoma: a meta-analysisPLoS One2013812e8290724358237
- GroschSMaierTJSchiffmannSGeisslingerGCyclooxygenase-2 (COX-2)-independent anticarcinogenic effects of selective COX-2 inhibitorsJ Natl Cancer Inst2006981173674716757698
- EdelmanMJWatsonDWangXEicosanoid modulation in advanced lung cancer: cyclooxygenase-2 expression is a positive predictive factor for celecoxib + chemotherapy – Cancer and Leukemia Group B Trial 30203J Clin Oncol200826684885518281656
- AntonarakisESHeathEIWalczakJRPhase II, randomized, placebo-controlled trial of neoadjuvant celecoxib in men with clinically localized prostate cancer: evaluation of drug-specific biomarkersJ Clin Oncol200927304986499319720908
- BundredNJCramerAMorrisJCyclooxygenase-2 inhibition does not improve the reduction in ductal carcinoma in situ proliferation with aromatase inhibitor therapy: results of the ERISAC randomized placebo-controlled trialClin Cancer Res20101651605161220179229
- BastiaannetESampieriKDekkersOMUse of aspirin postdiagnosis improves survival for colon cancer patientsBr J Cancer201210691564157022454078
- ClearyJMMamonHJSzymonifkaJNeoadjuvant irinotecan, cisplatin, and concurrent radiation therapy with celecoxib for patients with locally advanced esophageal cancerBMC Cancer20161646827412386
- LiLZhaoJWuZWangGChenGMeta-analysis: clinicopathological and prognostic significance of cyclooxygenase-2 expression on oesophageal squamous cell carcinomaAliment Pharmacol Ther200930658959619549265