Abstract
Aim
The aim of this study was to compare the efficacy and adverse effects of radioiodine (131I) therapy between two groups of patients with low-risk differentiated thyroid cancer (DTC) who received 30 mCi or 100 mCi radioiodine for ablation of the thyroid remnant after total thyroidectomy.
Methods
The study cohort was 173 patients, 85 of whom were given 30 mCi of radioiodine and the others were given 100 mCi of radioiodine. Follow-up involved neck ultrasonography, measurement of serum levels of thyroglobulin and whole-body scans to evaluate the response of radioiodine treatment. All patients were assessed for adverse effects.
Results
Of the 173 patients, 170 (98.3%) patients finally achieved successful ablation. The prevalence of successful ablation was 77.6% in the low-dose group versus 71.5% in the high-dose group after the first dose administration (P=0.36), 79% in the low-dose group versus 88% in the high-dose group after the second dose administration (P=0.416), and 97.6% in the low-dose group versus 98.9% in the high-dose group after the final ablation (P=0.54). We found no significant differences between the two groups. No patient had an adverse effect with a severity grade ⩾2 and the prevalence of adverse effects in the high-dose group was higher than that in the low-dose group, especially for nausea, neck pain, and sore throat.
Conclusion
These data suggest that a low dose of radioiodine is as effective as a high dose of radioiodine for ablation of the thyroid remnant after total thyroidectomy for low-risk DTC. Moreover, low-dose radioiodine therapy is associated with a lower prevalence of adverse events.
Introduction
During recent few decades, thyroid cancer (TC) has been the most prevalent malignant endocrine tumor. The rise in TC incidence seems to be attributable to better detection, such as the increased use of high-resolution ultrasonography and fine-needle aspiration biopsy, which has led to enhanced detection and diagnosis of subclinical TCs.Citation1 Approximately 90% of TCs are differentiated thyroid cancers (DTCs). That is, they arise from thyroid follicular cells and can, in general, take up iodine. This is the biologic foundation of postoperative complementary radioiodine (131I) therapy.Citation2 According to guidelines set by the Chinese Association of Nuclear Medicine, most TC patients should be treated with total or near-total thyroidectomy and then undergo adjuvant radioiodine treatment.
Postoperative complementary radioiodine therapy is effective and low-risk treatment for DTC because the long-term risk of recurrence and death is low. This beneficial effect results from the destruction of potentially malignant cells or occult multifocal disease that may occur in ≤30% of patients with papillary tumors.Citation3,Citation4 Furthermore, the specificity of thyroglobulin as a tumor marker is increased and the sensitivity of subsequent whole-body scans (WBSs) seems to be improved because residual thyroid tissue may compete with recurrent/metastatic TC cells for radioiodine uptake.Citation5–Citation10
Nevertheless, the postoperative dose of radioiodine is controversial. There is a trend toward low-dose radioiodine for patients who have a good prognosis. A higher dose means higher costs – hospital admission and inconvenience to the patient, and is associated with nausea, xerophthalmia, neck pain, altered taste, and dry mouth. Therefore, recent guidelines set by the American Thyroid Association (ATA) (2015) have emphasized using the minimum effective dose of radioiodine. They state that postoperative radioiodine treatment should not be given routinely to any patient who is considered to be low-risk according to ATA guidelines.Citation11 However, the European Association of Nuclear Medicine and the Chinese Association of Nuclear Medicine have refused to endorse the 2015 ATA management guidelines for adult patients with thyroid nodules and DTC and believe that the current strategy may not be based on robust evidence.Citation12
We undertook a retrospective study to compare the efficacy and adverse effects of adjuvant radioiodine therapy between two groups of patients with low-risk DTC treated by total thyroidectomy and lymph-node dissection and who were then given, respectively, 30 mCi or 100 mCi of radioiodine for ablation of thyroid remnants.
Patients and methods
This retrospective analysis involved 173 patients treated in our department from January 2007 to January 2010. All patients met the following criteria: age ≥18 years; low-risk DTC (papillary or follicular, excluding aggressive histologic subtypes) in the range of 1–4 cm; stage pT1–T2/N0-Nx/M0 disease according to the Cancer Staging Manual (seventh edition) of the American Joint Committee on Cancer and ATA guidelines (2015); all patients underwent total thyroidectomy and lymph-node dissection if there was evidence of lymph-node involvement. We excluded patients: positive for anti-thyroglobulin antibody (TgAb); who were pregnant; with major coexisting conditions (including other cancer types) within the previous 5 years. The study was approved by the ethics committee of Taian City Central Hospital.
All participants were allocated randomly to receive low-or high-dose radioiodine. Twenty-four patients with low-risk DTC were given 100 mCi of radioiodine in 2007, whereas 28 patients were given this dose in 2008, and 36 in 2009. Since 2008, this low-dose protocol was phased into our practice gradually, and 30 mCi of radioiodine was administered to 32 patients in 2008 and to 53 patients in 2009.
Thyroid-stimulating hormone (TSH) stimulation was achieved by abstaining from levothyroxine administration for ≥28 days before radioiodine administration, with radioiodine administered when the serum concentration of thyrotropin reached ⩾30 μIU/mL. A diet containing a low content of iodine was recommended before ablation. All patients underwent physical examination and biochemical evaluation before ablation of thyroid remnants. Before radio-iodine administration, patients underwent an overnight fast for ≥9 h and then, after 2 h, food was consumed. The WBS was done 3–6 days after radioiodine administration using single-photon emission computed tomography equipped with parallel-hole high-energy collimators.
Diagnostic WBS was undertaken 6–8 months after radioiodine (5 mCi) administration. Simultaneously, we determined concentrations of TSH, stimulated thyroglobulin (STg), and TgAb in serum, and neck ultrasonography was carried out. Levels of TSH, STg, and TgAb were measured by an immunometric assay. Complete radioiodine ablation of thyroid remnants was defined by a combination of: i) normal results of neck ultrasonography; ii) undetectable (≤1 ng/mL) serum levels of thyroglobulin; iii) absence of abnormal uptake in a diagnostic WBS. Radioiodine ablation was repeated if serum levels of thyroglobulin were detectable, if there was abnormal uptake in the thyroid bed or at extra-thyroid sites in a WBS, or if there were abnormal results on neck ultrasonography. The radioactivity at repeat administration was identical to that at the first administration. Patients who underwent successful ablation were followed-up. In the first 2 years, serum levels of thyroglobulin were measured and neck ultrasonography was done every 6–8 months and WBS once a year. If there was no evidence of recurrence in the first 2 years, the follow-up cycle was prolonged to 1 year.
Adverse events related to radioiodine treatment at 1 week as well as 1 and 3 months after administration were classified according to the Common Terminology Criteria for Adverse Events v3.0 and recorded on structured forms by participants.Citation4
Continuous variables are presented as the mean ± SD and were compared between groups by the Student’s t-test. Categorical variables are expressed as proportions and were compared between groups with the chi-squared test or Fisher’s exact test. Statistical analyses were done using SPSS v19.0 (IBM Corporation, Armonk, NY, USA). Differences were considered significant at P⩽0.05.
Ethics
The study was approved by the ethics committee of Taian City Central Hospital. This was a retrospective study, involving no additional procedures. All procedures described herein were in accordance with the ethical standards of the institutional research committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required. However, before the radioiodine treatment described herein, written informed consent was obtained from all individual study participants. This consent included allowing use of their data in analyses such as the present study.
Results
Patients
Between January 2007 and January 2010, 173 patients with low-risk DTC were included in the present study. They were divided into two groups: low-dose and high-dose. Eighty-five patients received 30 mCi of radioiodine, and the others were given 100 mCi of radioiodine. Baseline patient characteristics are reported in . The study population comprised 136 women and 37 men (ratio: 3.68) and the mean age was 46.63±14.77 (range, 19–75) years. Histology showed a papillary thyroid carcinoma in 89.6% of patients, follicular thyroid carcinoma in 8.7% of patients, and a follicular variant in the rest. Apart from proportion of primary tumor diameter (P=0.034), we found no significant differences in the characteristics between the two groups.
Table 1 Patient and tumor characteristics
Treatments, efficacy, recurrence, and adverse effects
In total, 170 patients underwent ablation successfully (). The prevalence of successful ablation with low-dose radioiodine was non-inferior to that with high-dose radioiodine (). The prevalence of successful ablation after first-dose administration was 74.6% overall and 77.6% in the low-dose group versus 71.5% in the high-dose group (P=0.36). Then, patients in whom ablation was unsuccessful were given a second dose; the prevalence of successful ablation was 84.1% overall and 79% in the low-dose group versus 88% in the high-dose group (P=0.416). There was no significant difference between the two groups with regard to the final successful ablation (P=0.54). In addition, ablation was not successful in one patient in the high-dose group after three attempts, and so this case underwent surgery because the thyroid remnant had become too large. Ablation was not successful in two patients in the low-dose group; one of them was given high-dose radioiodine after three attempts at ablation, and ablation was finally successful; the other patient was considered to be refractory to iodine.
Figure 1 The images of WBS and thyroid static imaging of one successful ablation patient.
Abbreviations: WBS, whole-body scan; 99mTcO4− pertechnetate.
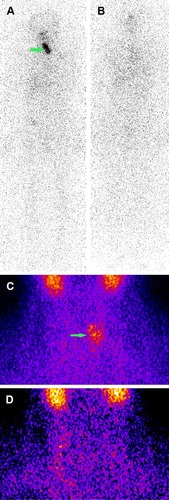
Table 2 Success and recurrence of ablation of the thyroid remnant using radioiodine
Radioiodine ablation was, in general, low-risk and well-tolerated, and the frequency and grade of adverse effects decreased with time. During ablation and ≤3 months after the procedure, no patient had an adverse effect of severity grade ⩾2. Among the adverse effects related to radioiodine ablation, the prevalence of nausea, neck pain, and sore throat was significantly different between the two groups. The proportion of patients with nausea was 11.76% among the low-dose group versus 28.41% among the high-dose group (P=0.008). The proportion of patients with neck pain was 16.47% among the low-dose group versus 34.09% among the high-dose group (P=0.006). The proportion of patients with a sore throat was 5.88% among the low-dose group versus 15.91% among the high-dose group (P=0.035). However, the prevalence of most of the other adverse effects was lower in the low-dose group, but was not significantly different between the two groups (). During follow-up, 14 patients had married and produced 18 healthy offspring, and no patient was found to have bone-marrow damage. Only one patient in the high-dose group was diagnosed with hyponatremia after radioiodine ablation on account of drinking a considerable amount of water because of fear of radiation damage. However, this hyponatremia had no direct relationship with radioiodine ablation.
Table 3 Common adverse effects related to radioiodine ablation
We did not observe local or distant recurrence in any of the patients throughout follow-up (). No patient died from TC during follow-up, but two patients died of other reasons. One died of heart disease, and the other died in a traffic accident, neither of which were related to radio-iodine ablation.
Discussion
The prognosis of DTC is good and 10-year survival can reach >90%.Citation2,Citation13 Radioiodine treatment remains the cornerstone of DTC therapy combined with surgery and thyroid hormone replacement/inhibition. Radioiodine treatment can elicit many benefits: i) reduce the prevalence of recurrence and death due to DTC; ii) facilitate follow-up; iii) enable early detection of metastases.Citation14 Nevertheless, radioiodine treatment also carries potential risks because it can cause i) short-term discomfort in the head and neck; ii) digestive-system disorders; iii) transient dysfunction in the salivary glands and lacrimal gland; iv) secondary tumors or fertility abnormalities. Thus, radioiodine treatment must be considered carefully before use for DTC patients, especially for patients with low-risk DTC.
Since the introduction of radioiodine in the 1940s, radioiodine treatment has been employed in doses ranging from 100 mCi to 150 mCi to ablate residual thyroid tissue. However, the optimal dose of radioiodine for ablation of thyroid remnants is not known. High radioactivity is most effective for the ablation of large normal-thyroid remnants and the eradication of micro-metastases; low radioactivity may be just as effective to ablate small normal-thyroid remnants after total thyroidectomy.Citation15,Citation16 Nevertheless, in 1976, McCowen et al reported the advantages of using a smaller dose of radioiodine (30 mCi). They concluded that low-dose therapy (≤30 mCi) was as effective as high-dose therapy (80–100 mCi) in their patient cohort.Citation17 After that provocative report, in the late 1970s, low-dose radioiodine ablation of thyroid remnants was introduced and, subsequently, many centers confirmed the effectiveness of such therapy. However, in some studies, the success rate was greater with higher doses. Siddiqui et al reported that a high dose (100–150 mCi) was a more conventional dose and a low dose (30 mCi) could not be used to ablate thyroid remnants using the clinical protocols stated by McCowen et al.Citation18 Doi and Woodhouse supported those findings and reported that doses ⩽75 mCi were less effective than 100 mCi.Citation19 Interestingly, Kukulska et al showed that 30 mCi of radioiodine was less effective than 60 mCi of radioiodine and that 60 mCi of radioiodine was as effective as 100 mCi of radioiodine.Citation20
Recently, in a multicenter, randomized non-inferiority study conducted by Mallick et al, 438 patients with DTC were randomized to receive low-dose (30 mCi) radioiodine and high-dose (100 mCi) radioiodine. The success of ablation was 85.0% in the low-dose group versus 88.9% in the high-dose group. In a randomized Phase III study conducted by Schlumberger et al, 752 patients with low-risk DTC were randomized to receive low-dose (30 mCi) radioiodine and high-dose (100 mCi) radioiodine, and the success of ablation between the two groups was found to be equivalent.Citation3,Citation4 Those two studies had important methodological advantages not only in sample size, but also in prospective randomized design. Also, they reported clearly better success rates (85%–94%) and they compared differences in preparation by means of recombinant human thyrotropin or withholding of thyroid hormone, and equivalent results were achieved. Four randomized studies comparing therapeutic doses of 30 mCi and 100 mCi showed that 30 mCi was equivalent to that of 100 mCi.Citation21–Citation24
We retrospectively analyzed the outcomes of 173 Han Chinese patients with low-risk DTC treated by radioiodine after total thyroidectomy. We wanted to know if different ethnicities, lifestyles, and dietary habits could affect study results. We found no conclusive evidence that the high-dose (100 mCi) radioiodine was more effective than low-dose (30 mCi) radioiodine. The success rate in our study was 77.6% in the low-dose group versus 71.5% in the high-dose group after the first course of treatment and, finally, was 97.6% in the low-dose group versus 98.9% in the high-dose group. Previously reported success rates for radioiodine ablation after thyroidectomy using low or high radioiodine doses have been 10%–98%. In our opinion, the different success rates for radioiodine ablation are due mainly to variations in the extent of surgery and inconsistent definitions of “successful ablation”. In our study, successful ablation was confirmed by normal findings of neck ultrasonography, undetectable serum levels of thyroglobulin, and an absence of abnormal uptake in a diagnostic WBS.
Notably, in our study, the severity of all adverse effects associated with radioiodine treatment was mild (grade 1 or 2), and most of the adverse effects between the two groups were not different except for nausea, neck pain, and sore throat 1 week after the procedure. All patients could tolerate symptomatic treatment and there were no unexpected serious adverse events. Mild adverse effects associated with radioiodine treatment have also been noted by other researchers.Citation3,Citation4,Citation22 They reported that radioiodine treatment was, in general, well-tolerated and that lower radioactivity was associated with less nausea, taste disturbances, and pain in the salivary glands. After follow-up for ⩾5 years, we did not find that radioiodine treatment (even if carried out more than once) resulted in secondary tumors, fertility abnormalities, or other serious adverse effects. This finding is despite the fact that, according to recent ATA guidelines, radioiodine treatment is used only for more advanced DTC stages in the USA.Citation11 In our opinion, radioiodine treatment should be used for low-risk DTC because it allows elimination of postoperative remnants and better follow-up through improvement in the measurement specificity of serum levels of thyroglobulin and sensitivity of the WBS; moreover, it is safe for patients.
During follow-up, we did not find recurrence in either group, so current treatments for low-risk DTC were effective. However, one patient in the low-dose group was considered to be refractory to iodine after three treatments. Our study cohort was small, so we could not verify the theory advanced by Wartofsky et al: during radioiodine treatment, if the first dose does not kill TC cells but just damages them, then cells will no longer absorb radioiodine, and the patient will become refractory to iodine.Citation25 A larger study cohort, multicenter setting, and long-term follow-up studies are needed to confirm our findings.
A potential limitation of our study was that short-term follow-up was not sufficient to investigate more important endpoints (survival, the prevalence of local relapse, distant metastases, and recurrence) or to evaluate the long-term adverse events of the radioiodine treatment. Other limitations included the retrospective design of our study and the small number of patients.
Conclusion
The present study showed a similar prevalence of thyroid-remnant ablation among Han Chinese participants with low-risk DTC after total thyroidectomy when 30 mCi or 100 mCi radioiodine was used. Moreover, low-dose radioiodine therapy was associated with a lower prevalence of adverse effects. Thus, use of a low dose of radioiodine for postoperative radioiodine ablation could be an effective and attractive option for the treatment of low-risk DTC because it reduces the prevalence of adverse effects and maintains a good quality of life.
Acknowledgments
The authors thank the nursing staff of the Department of Nuclear Medicine of Taian City Central Hospital, for skillful assistance. We thank Su Jun, PhD, for help in the statistical analysis of the data. The first author had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This was an investigator-initiated study funded from the regular budgets of the Department of Nuclear Medicine of Taian City Central Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
- KitaharaCMSosaJAThe changing incidence of thyroid cancerNat Rev Endocrinol2016121164665327418023
- SiposJAMazzaferriELThyroid cancer epidemiology and prognostic variablesClin Oncol (R Coll Radiol)201022639540420627675
- SchlumbergerMCatargiBBorgetIStrategies of radioiodine ablation in patients with low-risk thyroid cancerN Engl J Med2012366181663167322551127
- MallickUHarmerCYapBAblation with low-dose radioiodine and thyrotropin alfa in thyroid cancerN Engl J Med2012366181674168522551128
- HanJMKimWGKimTYEffects of low-dose and high-dose postoperative radioiodine therapy on the clinical outcome in patients with small differentiated thyroid cancer having microscopic extrathyroidal extensionThyroid201452482082524328997
- SongXMengZJiaQDifferent radioiodine dose for remnant thyroid ablation in patients with differentiated thyroid cancer: a meta-analysisClin Nucl Med2015401077477926204220
- CeccarelliCBencivelliWMorcianoDPincheraAPaciniF131I therapy for differentiated thyroid cancer leads to an earlier onset of menopause: results of a retrospective studyJ Clin Endocrinol Metab20018683512351511502772
- TsangRWBrierleyJDSimpsonWJPanzarellaTGospodarowiczMKSutcliffeSBThe effects of surgery, radioiodine, and external radiation therapy on the clinical outcome of patients with differentiated thyroid carcinomaCancer19988223753889445196
- MazzaferriELKloosRTClinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancerJ Clin Endocrinol Metab20018641447146311297567
- SawkaAMBrierleyJDTsangRWAn updated systematic review and commentary examining the effectiveness of radioactive iodine remnant ablation in well-differentiated thyroid cancerEndocrinol Metab Clin North Am200837245748018502337
- HaugenBRAlexanderEKBibleKC2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancerThyroid2016261113326462967
- VerburgFAAktolunCChitiAWhy the European Association of Nuclear Medicine has declined to endorse the 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancerEur J Nucl Med Mol Imaging20164361001100526883666
- QuYHuangRLiLLow- and high-dose radioiodine therapy for low-/intermediate-risk differentiated thyroid cancer: a preliminary clinical trialAnn Nucl Med2016311718327757803
- PaciniFSchlumbergerMDralleHEliseiRSmitJWWiersingaWEuropean Thyroid Cancer TaskforceEuropean consensus for the management of patients with differentiated thyroid carcinoma of the follicular epitheliumEur J Endocrinol2006154678780316728537
- SawkaAMThephamongkholKBrouwersMThabaneLBrowmanGGersteinHCClinical review 170: a systematic review and meta analysis of the effectiveness of radioactive iodine remnant ablation for well-differentiated thyroid cancerJ Clin Endocrinol Metab20048983668367615292285
- HackshawAHarmerCMallickUHaqMFranklynJA131I activity for remnant ablation in patients with differentiated thyroid cancer: a systematic reviewJ Clin Endocrinol Metab2007921283817032718
- McCowenKDAdlerRAGhaedNVerdonTHofeldtFDLow dose radioiodide thyroid ablation in postsurgical patients with thyroid cancerAm J Med19766115258937370
- SiddiquiAREdmondsonJWellmanHNFeasibility of low doses of 131I for thyroid ablation in postsurgical patients with thyroid carcinomaClin Nucl Med1981641581617214770
- DoiSAWoodhouseNJAblation of the thyroid remnant and 131I dose in differentiated thyroid cancerClin Endocrinol (Oxf)2000526765773
- KukulskaAKrajewskaJGawkowska-SuwinskaMRadioiodine thyroid remnant ablation in patients with differentiated thyroid carcinoma (DTC): prospective comparison of long-term outcomes of treatment with 30, 60 and 100 mCiThyroid Res201031921040579
- MaenpaaHOHeikkonenJVaalavirtaLTenhunenMJoensuuHLow vs. high radioiodine activity to ablate the thyroid after thyroidectomy for cancer: a randomized studyPLoS One200834e188518382668
- JohansenKWoodhouseNJOdugbesanOComparison of 1073 and 3700MBq iodine-131 in postoperative ablation of residual thyroid tissue in patients with differentiated thyroid cancerJ Nucl Med19913222522541992028
- TaubeALundellGProspective randomized clinical trial to evaluate the optimal dose of 131I for remnant ablation in patients with differentiated thyroid carcinomaCancer19977911901918988747
- FallahiBBeikiDTakavarALow versus high radioiodine dose in postoperative ablation of residual thyroid tissue in patients with differentiated thyroid carcinoma: a large randomized clinical trialNucl Med Commun201233327528222124360
- WartofskyLVan NostrandDRadioiodine treatment of well-differentiated thyroid cancerEndocrine201242350651322733393
