Abstract
This study was conducted to identify gene expression profile changes associated with β-estradiol (E2) treatment in U2OS osteosarcoma cells by high-throughput RNA sequencing (RNA-seq). Two U2OS cell samples treated with E2 (15 μmol/L) and two untreated control U2OS cell samples were subjected to RNA-seq. Differentially expressed genes (DEGs) between the groups were identified, and main biological process enrichment was performed using gene ontology (GO) analysis. A protein–protein interaction (PPI) network was constructed using Cytoscape based on the Human Protein Reference Database. Finally, NFKB1 expression was confirmed by quantitative real-time polymerase chain reaction (qRT-PCR). The map ratios of the four sequenced samples were >65%. In total, 128 upregulated and 92 downregulated DEGs were identified in E2 samples. After GO enrichment, the downregulated DEGs, such as AKT1, were found to be mainly enriched in cell cycle processes, whereas the upregulated DEGs, such as NFKB1, were involved in the regulation of gene expression. Moreover, AKT1 (degree =117) and NFKB1 (degree =72) were key nodes with the highest degrees in the PPI network. Similarly, the results of qRT-PCR confirmed that E2 upregulated NFKB1 expression. The results suggest that E2 upregulates the expression of NFKB1, ATF7IP, and HDAC5, all of which are involved in the regulation of gene expression and transcription, but downregulates that of TCF7L2, ALCAM, and AKT, which are involved in Wnt receptor signaling through β-catenin and morphogenesis in U2OS osteosarcoma cells.
Introduction
17β-Estradiol (E2) is a primary sex hormone in human beings that is essential for the development and maintenance of female reproductive organs.Citation1 However, it also has important effects on many other tissues, such as bone,Citation2 liver,Citation3 and brain.Citation4 E2 is mainly produced by the granulosa cells of ovaries in women,Citation5 but it can also be produced by the testes in men.Citation6,Citation7 E2 has been used for treating menopausal syndromeCitation8 and preventing osteoporosis in postmenopausal women.Citation9 In addition, it has been reported that older men with total E2 deficiency are more likely to be osteoporotic.Citation10 E2 has also been implicated in cancer progression.Citation11,Citation12 Recently, Tchafa et alCitation13 have found that E2 promotes the cellular invasion and proliferation of breast cancer cells. Gunter et alCitation12 have reported that endogenous E2 levels are positively associated with the risk of colorectal cancer.
Osteosarcoma is the most common primary malignancy of bone and exhibits a high risk of metastasis and poor prognosis.Citation14,Citation15 Although E2 is known to play a critical role in osteosarcoma, its effects in this disease are controversial. E2 can inhibit purine metabolic and biosynthetic pathways in human osteosarcoma cells to achieve an antagonistic effect on cell proliferation.Citation16 Previous studies have also shown that E2 protects against cell death in estrogen receptor-α and -β-expressing human U2OS osteosarcoma cells.Citation17,Citation18 Furthermore, 2-methoxyestradiol, a mammalian metabolite of E2, has been reported to induce cell cycle arrest and osteosarcoma cell apoptosis.Citation19 Therefore, defining the molecular mechanism(s) of E2 actions in osteosarcoma cells is necessary. In the present study, high-throughput RNA sequencing (RNA-seq) and bioinformatics methods were used to identify changes in the gene expression profile that are associated with E2 treatment of U2OS osteosarcoma cells.
Materials and methods
Cell lines and culture conditions
Human U2OS osteosarcoma cells were purchased from Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, People’s Republic of China). Cells were maintained in phenol red-free Roswell Park Memorial Institute (RPMI)-1640 medium supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin (Thermo Fisher Scientific, Waltham, MA, USA) in a humidified atmosphere of 95% air and 5% CO2 at 37°C. Before beginning treatments, cells were washed twice with phosphate-buffered saline (PBS) to remove residual serum and were grown in serum-free RPMI-1640 medium for 24 h. Subsequently, E2 (15 μmol/L, dissolved in dimethyl sulfoxide [DMSO]) was added to the medium, and an equal volume of DMSO was added to the control U2OS cell medium. Two U2OS cell samples (14710C-3 and 14710C-4) treated with E2 and two untreated control U2OS samples (14710C-1 and 14710C-2) were subjected to RNA-seq.
RNA extraction and sequencing
After an incubation period of 48 h, cells were washed twice with PBS and harvested. Total RNA was isolated from cultured cells using TRIzol® Reagent (Thermo Fisher Scientific) according to the manufacturer’s instruction. RNA quality and quantity were assessed by an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). cDNA libraries were constructed using an NEBNext® Ultra™ RNA Library Prep Kit (Illumina, Shanghai, People’s Republic of China) following the manufacturer’s instruction. Subsequently, libraries were sequenced on Illumina HiSeq 2000 at Beijing Berry Genomics Co., Ltd. Sequenced reads were generated by base calling using the Illumina standard pipeline. Paired-end RNA-seq data were generated with a read length of 100 bp. The raw sequencing data have been uploaded to the National Center for Biotechnology Information database under the BioProject accession no SRP101761.
Alignment of sequenced reads
Raw reads were first filtered to obtain clean reads using FASTX-Toolkit (http://hannonlab.cshl.edu/fastx_toolkit/).Citation20 High-quality reads were mapped to the human reference genome hg19 using TopHat (version 2.0.12) softwareCitation21 with default parameters. Alignment was independently performed for reads from each sample, and reads mapping to more than three genomic sites were discarded.
Differentially expressed genes (DEGs) screening
Based on the value of reads per kilobase per million mapped reads, gene expression levels were determined using Cufflinks software (version 2.21).Citation22 Subsequently, the Cuffdiff programCitation23 of Cufflinks was used to identify DEGs. Only the genes with |log(fold change)| >1 and P<0.01 were considered as DEGs.
Gene ontology (GO) enrichment analysis
Database for Annotation, Visualization and Integrated Discovery (DAVID)Citation24 is an online tool used for functional annotation of genes. A GO functional enrichment analysis of DEGs was performed using the DAVID. P<0.05 was chosen as the cutoff criterion.
Construction of protein–protein interaction (PPI) network
The Human Protein Reference Database (http://www.hprd.org/)Citation25 is a database for experimentally derived information about the human proteome, including that on PPIs, post-translational modifications, and tissue expression. DEG-encoding proteins were mapped to the Human Protein Reference Database to search for interaction relationships. The PPI network was visualized using Cytoscape.Citation26 Finally, the hub nodes with a high degree of connectivityCitation27 in the PPI network were also identified.
NFKB1 expression levels using quantitative real-time polymerase chain reaction (qRT-PCR)
To confirm RNA-seq results, NFKB1 and AKT1 expression levels were detected using qRT-PCR. Total RNA was extracted using TRIzol Reagent following the manufacturer’s instructions (TaKaRa, Dalian, People’s Republic of China). Subsequently, the first-strand cDNA was prepared from total lens RNA using a TaKaRa PrimeScript II First Strand cDNA Synthesis Kit (RR036A-1; TaKaRa) according to the manufacturer’s instructions. Glyceraldehyde-3-phosphate dehydrogenase was used as a control. The primers used for NFKB1, AKT1, and glyceraldehyde-3-phosphate dehydrogenase were based on the rat sequences: 5′-AACAGCAGATGGCCCATA CC-3′ (forward), 5′-AACCTTTGCTGGTCCCACAT-3′ (reverse); 5′-GCCTGTCAGCTGGTGCAT-3′ (forward), 5′-CCGCCAGGTCTTGATGTACT-3′ (reverse); and 5′-CA GTGCCAGCCTCGTCTCAT-3′ (forward), 5′-AGGGGCCATCCACAGTCTTC-3′ (reverse), respectively.
Statistical analysis
Differences between the two treatment groups were analyzed using unpaired Student’s t-test. Data analysis was completed using SPSS 22.0 (IBM Corporation, Armonk, NY, USA). P<0.05 was considered to indicate statistically significant difference.
Results
Sequence alignment
The results of TopHat alignment of clean reads with the human reference genome are shown in . In total, 9,485,360 (68.63%) and 7,843,445 (65.76%) of clean reads were mapped to the human reference genome for the two E2-treated U2OS cell samples; 8,129,145 (69.71%) and 8,531,536 (69.07%) cleans reads were mapped for the two control samples.
Table 1 Summary of clean reads alignment to the reference genome
DEGs and GO enrichment analysis
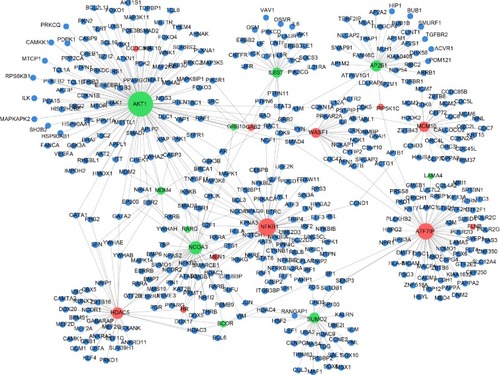
In total, 220 genes, including 128 upregulated and 92 downregulated genes, were identified as being significantly differently expressed between E2-treated U2OS osteosarcoma cells and the controls. According to the GO enrichment analysis, the top five GO terms of upregulated DEGs NFKB1, ATF7IP, HDAC5, MEN1, and EPC1 were significantly related to the regulation of gene expression and transcription (). Meanwhile, the top five GO terms of downregulated DEGs included Wnt receptor signaling through β-catenin involving RARG, TBL1X, and TCF7L2; axonogenesis involving ALCAM, NRP1, and SLC26A6; and cell cycle processes involving AKT1, DSN1, and POLD1 ().
Table 2 The top five GO terms of upregulated DEGs
Table 3 The top five GO terms of downregulated DEGs
Construction of the PPI network
In total, 1,185 nodes, including 91 upregulated DEGs, 55 downregulated DEGs, and 1,064 non-DEGs, were present in the PPI network (). The top five DEGs with the highest degree of connectivity in the network were AKT1 (117), NFKB1 (72), ATF7IP (64), NCOA3 (45), and HDAC5 (36).
Differences in NFKB1 and AKT1 expression levels
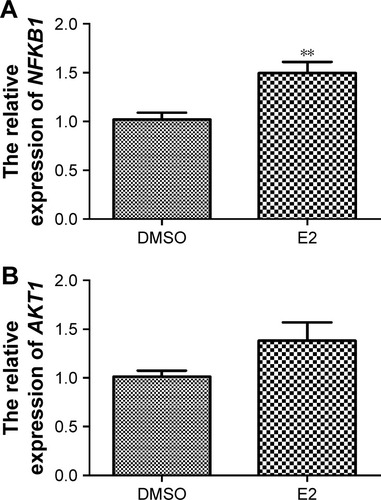
As shown in , NFKB1 expression levels significantly increased when U2OS osteosarcoma cells were treated with E2 (P=0.002), which confirmed the reliability of the bioinformatics method. Although AKT1 expression levels were increased when U2OS osteosarcoma cells were treated with E2, they were not significantly different (P>0.05; ).
Discussion
In the current study, RNA-seq was used to explore changes in the gene expression profile that are associated with E2 treatment of U2OS osteosarcoma cells. We found that E2 treatment induced the upregulation of genes related to the regulation of gene expression and transcription (eg, NFKB1, ATF7IP, and HDAC5) and downregulation of those involved in Wnt receptor signaling through β-catenin and morphogenesis (eg, TCF7L2, ALCAM, NRP1, SLC26A6, and AKT).
Our results demonstrated that NFKB1 was mainly enriched in the regulation of gene expression and transcription. NFKB1 belongs to the NF-κB family, which contains a group of proteins involved in carcinogenesis, immune response, cell adhesion, proliferation, angiogenesis, and apoptosis.Citation28 NF-κB is a transcription factor that participates in the regulation of viral and cellular genes.Citation29 Constitutive NF-κB activation has been observed in 67% of colorectal cancer cell lines and promoted tumor growth.Citation30 NFKB2, another member of the NF-κB family, can stimulate cell proliferation in U2OS osteosarcoma cells.Citation31 Furthermore, several studies have shown the relationship between NFKB1 and tumors. For example, a functional insertion/deletion polymorphism in the promoter region of NFKB1 increases the risk of nasopharyngeal carcinoma.Citation32 Riemann et alCitation33 discovered that the NFKB1 promoter polymorphism was a useful molecular marker for the risk of recurrence in superficial bladder cancer. However, although most studies have analyzed the correlation of the NFKB1 promoter polymorphism with tumors,Citation31,Citation34 those analyzing the effects of NFKB1 expression in osteosarcoma are limited. Thus, further experiments are needed to explore whether NFKB1 expression has any impact on osteosarcoma progression.
We also found that E2 treatment may repress the expression of genes, such as RARG, TBL1X, and TCF7L2, involved in Wnt receptor signaling through β-catenin in U2OS osteosarcoma cells. The activation of Wnt signaling and the accumulation of β-catenin have been reported in many carcinomas,Citation35,Citation36 including osteosarcoma.Citation37 TCF7L2 encodes the transcription factor TCF-4, which can be activated by dephosphorylated β-catenin via binding to a conserved N-terminal region in the nucleus, thereby initiating the expression of target genes, including the proto-oncogenes c-jun and fra-1.Citation38 Thus, E2 treatment may have an unfavorable effect on U2OS osteosarcoma cells.
Among the other downregulated DEGs, AKT1 was observed to have the highest degree in the PPI network. AKT, also known as protein kinase B (PKB; a serine/threonine kinase), is one of the most critical and versatile protein kinases involved in the mechanism of human physiology and disease.Citation39,Citation40 The activation of AKT pathways plays a central role in tumor metastasis.Citation41 Furthermore, Fukaya et alCitation42 have demonstrated the important role of AKT signaling in the pulmonary metastasis of osteosarcoma. The AKT family has three members: AKT1/PKBα, AKT2/PKBβ, and AKT3/PKBγ. AKT1 and AKT2 have been reported to be ubiquitously and similarly expressed in various tissues.Citation43 Recently, Zhu et alCitation44 have reported that elevated AKT2 expression is associated with poor outcomes in patients with osteosarcomas.
Here, AKT1 was enriched in the cell cycle process, which was closely related to tumor progression. A study by Ju et alCitation45 has revealed that AKT1 governed breast cancer progression in mice, whereas another study has indicated that AKT1 amplification regulates cisplatin (a chemotherapeutic agent) resistance in human lung cancer.Citation46 Collectively, these reports have demonstrated that AKT1 might be closely involved in osteosarcoma metastasis. In the current study, we predicted that AKT1 expression was downregulated. According to qRT-PCR results, although AKT1 expression levels were increased when U2OS osteosarcoma cells were treated with E2, they were not significantly different. However, the effect of AKT expression on U2OS osteosarcoma cells is controversial. For example, Nielsen-Preiss et alCitation47 have reported that the downregulation of AKT expression enhances osteosarcoma cell proliferation, whereas Díaz-Montero et alCitation48 have found that AKT expression is upregulated in anoikis-resistant human osteosarcoma SAOSar cells. Therefore, we speculated that E2 is involved in osteosarcoma metastasis, but the modulating mechanism is still unclear.
ALCAM was another gene downregulated by E2 treatment in U2OS osteosarcoma cells, which was speculated to function in axonogenesis, cell morphogenesis involved in neuron differentiation, cell cycle processes, and neuron projection morphogenesis. ALCAM encodes the CD166 antigen, which is a 100–105 kDa type-I transmembrane glycoprotein of the immunoglobulin protein superfamily. Similar to CD29, CD44, CD73, CD90, CD105, and CD106, it is known as a marker of the mesenchymal stem cell phenotype.Citation49,Citation50 Its overexpression has also been reported in colorectal carcinomaCitation51 and has been demonstrated to be associated with a poor prognosis of several tumors.Citation51–Citation53 Federman et al have reported that CD166 is highly expressed in the osteosarcoma cell lines HOS, KHOS, KHOS240s, and SJSA, but its expression status is not observed in U2OS osteosarcoma cells.Citation54 They further proposed that this gene is a potential candidate for the targeted therapy of osteosarcoma. Our findings suggested that E2 treatment decreases ALCAM expression in U2OS osteosarcoma cells, thereby playing an inhibitory role against osteosarcoma.
Taken together, we found that E2 treatment may mainly upregulate the expression of genes, such as NFKB1, ATF7IP, and HDAC5, related to the regulation of gene expression and transcription and downregulate that of genes involved in Wnt receptor signaling through β-catenin and morphogenesis (eg, TCF7L2, ALCAM, NRP1, SLC26A6, and AKT) in U2OS osteosarcoma cells. Thus, we proposed that E2 has an unfavorable effect against U2OS osteosarcoma cells. However, given that our findings were partly obtained using bioinformatics tools, they need to be further validated.
Acknowledgments
This study was funded by a special fund for the medical service of the Jilin Finance Department (Grant No SCZSY201507).
Disclosure
The authors report no conflicts of interest in this work.
References
- RyanKJBiochemistry of aromatase: significance to female reproductive physiologyCancer Res1982428 suppl3342s3344s7083198
- HoppéEMorelGBiverEMale osteoporosis: do sex steroids really benefit bone health in men?Joint Bone Spine201178S191S19622153669
- YangXQinLLiuJTianLQianH17β-estradiol protects the liver against cold ischemia/reperfusion injury through the Akt kinase pathwayJ Surg Res20121782996100222835949
- HartzAMMahringerAMillerDSBauerB17-β-estradiol: a powerful modulator of blood–brain barrier BCRP activityJ Cereb Blood Flow Metab201030101742175520216549
- MasonHDWillisDSBeardRWWinstonRMMargaraRFranksSEstradiol production by granulosa cells of normal and polycystic ovaries: relationship to menstrual cycle history and concentrations of gonadotropins and sex steroids in follicular fluidJ Clin Endocrinol Metab1994795135513607962330
- KelchRJennerMWeinsteinRKaplanSGrumbachMEstradiol and testosterone secretion by human, simian, and canine testes, in males with hypogonadism and in male pseudohermaphrodites with the feminizing testes syndromeJ Clin Invest19725148244259253
- HessRAEstrogen in the adult male reproductive tract: a reviewReprod Biol Endocrinol2003115212904263
- BarnabeiVMCochraneBBAragakiAKMenopausal symptoms and treatment-related effects of estrogen and progestin in the women’s health initiativeObstet Gynecol20051055, pt 11063107315863546
- WarmingLRavnPChristiansenCLevonorgestrel and 17β-estradiol given transdermally for the prevention of postmenopausal osteoporosisMaturitas2005502788515653003
- FinkHAEwingSKEnsrudKEAssociation of testosterone and estradiol deficiency with osteoporosis and rapid bone loss in older menJ Clin Endocrinol Metab200691103908391516849417
- MedinaDMammary developmental fate and breast cancer riskEndocr Relat Cancer200512348349516172188
- GunterMJHooverDRYuHInsulin, insulin-like growth factor-I, endogenous estradiol, and risk of colorectal cancer in postmenopausal womenCancer Res200868132933718172327
- TchafaAMZhongZMengRQuongJNQuongAAIncreased cellular invasion and proliferation via estrogen receptor after 17-β-estradiol treatment in breast cancer cells using stable isotopic labeling with amino acids in cell culture (SILAC)Adv Breast Cancer Res2013232
- TangNSongW-XLuoJHaydonRCHeT-COsteosarcoma development and stem cell differentiationClin Orthop Relat Res200846692114213018563507
- MarinaNGebhardtMTeotLGorlickRBiology and therapeutic advances for pediatric osteosarcomaOncologist20049442244115266096
- ZhouQShenLLiuCLiuCChenHLiuJThe effects of estradiol and glucocorticoid on human osteosarcoma cells: similarities and differencesAnticancer Res20163641683169127069146
- KallioAGuoTLamminenEEstrogen and the selective estrogen receptor modulator (SERM) protection against cell death in estrogen receptor alpha and beta expressing U2OS cellsMol Cell Endocrinol20082891384818455292
- LimaFVicoLLafage-ProustMHvan der SaagPAlexandreCThomasTInteractions between estrogen and mechanical strain effects on u2os human osteosarcoma cells are not influenced by estrogen receptor typeBone20043551127113515542038
- MaranAZhangMKennedyA2-methoxyestradiol induces interferon gene expression and apoptosis in osteosarcoma cellsBone200230239339811856647
- Góngora-CastilloEFedewaGYeoYChappellJDellaPennaDBuellCRGenomic approaches for interrogating the biochemistry of medicinal plant speciesMethods Enzymol201251713923084937
- TrapnellCPachterLSalzbergSLTopHat: discovering splice junctions with RNA-seqBioinformatics20092591105111119289445
- TrapnellCRobertsAGoffLDifferential gene and transcript expression analysis of RNA-seq experiments with TopHat and CufflinksNat Protoc20127356257822383036
- TrapnellCHendricksonDGSauvageauMGoffLRinnJLPachterLDifferential analysis of gene regulation at transcript resolution with RNA-seqNat Biotechnol2013311465323222703
- Huang daWShermanBTLempickiRASystematic and integrative analysis of large gene lists using DAVID bioinformatics resourcesNat Protoc2008414457
- PrasadTKGoelRKandasamyKHuman protein reference database – 2009 updateNucleic Acids Res200937suppl 1D767D77218988627
- SaitoRSmootMEOnoKA travel guide to Cytoscape pluginsNat Methods20129111069107623132118
- NewmanMEThe structure and function of complex networksSIAM Rev2003452167256
- AggarwalBBNuclear factor-kappaB: the enemy withinCancer Cell20046320320815380510
- OtaNNakajimaTShiraiYEmiMIsolation and radiation hybrid mapping of a highly polymorphic CA repeat sequence at the human nuclear factor kappa-beta subunit 1 (NFKB1) locusJ Hum Genet199944212913010083740
- SakamotoKMaedaSHikibaYConstitutive NF-kappaB activation in colorectal carcinoma plays a key role in angiogenesis, promoting tumor growthClin Cancer Res20091572248225819276252
- SchummKRochaSCaamanoJPerkinsNDRegulation of p53 tumour suppressor target gene expression by the p52 NF-kappaB subunitEMBO J200625204820483216990795
- ZhouBRaoLLiYA functional insertion/deletion polymorphism in the promoter region of NFKB1 gene increases susceptibility for nasopharyngeal carcinomaCancer Lett20092751727619006646
- RiemannKBeckerLStruweHRübbenHEisenhardtASiffertWInsertion/deletion polymorphism in the promoter of NFKB1 as a potential molecular marker for the risk of recurrence in superficial bladder cancerInt J Clin Pharmacol Ther200745842343017725175
- LinSCLiuCJYehWILuiMTChangKWChangCSFunctional polymorphism in NFKB1 promoter is related to the risks of oral squamous cell carcinoma occurring on older male areca (betel) chewersCancer Lett20062431475416387424
- LustigBBehrensJThe WNT signaling pathway and its role in tumor developmentJ Cancer Res Clin Oncol2003129419922112707770
- BrabletzTJungADagSHlubekFKirchnerTβ-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancerAm J Pathol199915541033103810514384
- HaydonRCDeyrupAIshikawaACytoplasmic and/or nuclear accumulation of the β-catenin protein is a frequent event in human osteosarcomaInt J Cancer2002102433834212402302
- BehrensJvon KriesJPKühlMFunctional interaction of β-catenin with the transcription factor LEF-1Nature199638265926386428757136
- DattaSRBrunetAGreenbergMECellular survival: a play in three AktsGenes Dev199913222905292710579998
- ManningBDCantleyLCAKT/PKB signaling: Navigating downstreamCell200712971261127417604717
- QiaoMShengSPardeeABMetastasis and AKT activationCell Cycle20087192991299618818526
- FukayaYIshiguroNSengaTA role for PI3K-AKT signaling in pulmonary metastatic nodule formation of the osteosarcoma cell line, LM8Oncol Rep200514484785216142341
- HanadaMFengJHemmingsBAStructure, regulation and function of PKB/AKT – a major therapeutic targetBiochim Biophys Acta20041697131615023346
- ZhuYZhouJJiYYuBElevated expression of AKT2 correlates with disease severity and poor prognosis in human osteosarcomaMol Med Rep201410273774224919955
- JuXKatiyarSWangCAKT1 governs breast cancer progression in vivoProc Natl Acad Sci U S A2007104187438744317460049
- LiuLZZhouXDQianGShiXFangJJiangBHAKT1 amplification regulates cisplatin resistance in human lung cancer cells through the mammalian target of rapamycin/p70S6K1 pathwayCancer Res200767136325633217616691
- Nielsen-PreissSMSilvaSRGilletteJMRole of PTEN and Akt in the regulation of growth and apoptosis in human osteoblastic cellsJ Cell Biochem200390596497514624456
- Díaz-MonteroCMWygantJNMcintyreBWPI3-K/Akt-mediated anoikis resistance of human osteosarcoma cells requires Src activationEur J Cancer20064210149116759849
- ParkB-WHahY-SKimDRKimJ-RByunJ-HOsteogenic phenotypes and mineralization of cultured human periosteal-derived cellsArch Oral Biol2007521098398917543271
- NadriSSoleimaniMKianiJAtashiAIzadpanahRMultipotent mesenchymal stem cells from adult human eye conjunctiva stromal cellsDifferentiation200876322323117825086
- WeichertWKnöselTBellachJDietelMKristiansenGALCAM/CD166 is overexpressed in colorectal carcinoma and correlates with shortened patient survivalJ Clin Pathol200457111160116415509676
- TachezyMEffenbergerKZanderHALCAM (CD166) expression and serum levels are markers for poor survival of esophageal cancer patientsInt J Cancer2012131239640521858815
- IshigamiSUenoSArigamiTClinical implication of CD166 expression in gastric cancerJ Surg Oncol20111031576120886585
- FedermanNChanJNagyJOEnhanced growth inhibition of osteosarcoma by cytotoxic polymerized liposomal nanoparticles targeting the alcam cell surface receptorSarcoma2012201211