Abstract
Background
Growing evidence has demonstrated that Ki-67/MIB-1 has an effect on the clinical progression and prognosis in cancers. However, the diagnostic and prognostic values of Ki-67/MIB-1 in thyroid cancer remain unclear.
Materials and methods
The meta-analysis was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Studies were retrieved from PubMed, EBSCO, EMBASE, ISI Web of Science, China National Knowledge Infrastructure, WanFang and Chinese VIP databases. MetaDiSc and STATA12.0 were used to analyze the meta-analysis. Fixed-effect analysis and random-effect analysis were applied to pool the relative ratio based on heterogeneity in this meta-analysis.
Results
In the meta-analysis, 51 eligible studies were included. The pooled sensitivity of Ki-67/MIB-1 was 0.61 (95% confidence interval [CI]: 0.59–0.63) and specificity was 0.75 (95% CI: 0.74–0.77) in thyroid cancer. The pooled positive likelihood ratio was 3.19 (95% CI: 2.30–4.42) and negative likelihood ratio was 0.43 (95% CI: 0.35–0.54). In the diagnosis of thyroid cancer, the pooled diagnostic odds ratio of Ki-67/MIB-1 was 8.54 (95% CI: 5.03–14.49). The area under the symmetric receiver operating characteristic curve was 0.804 (standard error =0.031). Our results showed that there were statistical associations between Ki-67/MIB-1 and age (odds ratio [OR] =1.71, 95% CI: 1.14–2.57, P=0.010), tumor size (OR =1.86, 95% CI: 1.17–2.96, P=0.008), lymph node metastasis (OR =2.49, 95% CI: 1.42–4.39, P=0.002), metastasis status (OR =6.96, 95% CI: 2.46–19.69, P<0.001), tumor node metastasis stage (OR =6.56, 95% CI: 3.80–11.34, P<0.001) and extrathyroid extension (OR =1.91, 95% CI: 1.27–2.87, P=0.002). Furthermore, thyroid cancer patients with a high level of Ki-67/MIB-1 had a worse disease-free survival as compared to patients with a low level of Ki-67/MIB-1 (hazard ratio =5.19, 95% CI: 3.18–8.46, P<0.001). Also, Ki-67/MIB-1 was found to be associated with increased risk of mortality (hazard ratio =3.56, 95% CI: 1.17–10.83, P=0.025).
Conclusion
Our results demonstrated that Ki-67/MIB-1 might act as a potential factor in diagnosing thyroid cancer in Chinese. Also, the meta-analysis indicated that Ki-67/MIB-1 might have an effect on prognosis in non-Chinese thyroid cancer patients.
Introduction
Thyroid carcinoma, accounting for nearly 1% of all the cancers, is the most common malignancy in the endocrine organs.Citation1 Traditionally, thyroid carcinomas are classified into papillary thyroid cancer (PTC), follicular thyroid cancer, medullary thyroid cancer, poorly differentiated thyroid cancer and anaplastic thyroid cancer, based on histopathology.Citation2 In China, it has been reported that 90 per 100,000 people were diagnosed with thyroid cancer and 6.8 people died among these patients.Citation3 In USA, it has been estimated that 1,980 people died of the disease in 2016Citation4 and there will be 64,300 new patients of thyroid cancer. The diagnosis of thyroid cancer is often based on magnetic resonance imaging, ultrasound, computed tomography, fine needle aspiration and radionuclide imaging. Although various methods are well applied in clinics, patients often have poor outcome. Reports have shown that the patients’ age when being diagnosed, sex, tumor size, lymph node, distant metastases and pathologic differentiation of the cancer are the risk factors of prognosis in thyroid cancer.Citation5–Citation8
However, no studies showed the markers had prognostic value in thyroid cancer. Cell proliferative activity is an important factor in cancer biologic behavior. Ki-67, considered as a nuclear antigen, is expressed in all cell nuclei, except those in the G0 phase. Further, MIB-1 acts as a monoclonal antibody which increases against Ki-67. In recent years, Ki-67 has been studied in many cancers, including cervical cancer,Citation9 lung cancer,Citation10 breast cancerCitation11 and thyroid cancer.Citation12 It has been reported that Ki-67 is an independent prognostic factor in thyroid cancer patients.Citation13 A study predicted that patients with Ki-67 labeling index (LI) >3% would show a worse cause-specific survival than those with Ki-67 LI <3%.Citation12 However, Gnemmi et alCitation14 reported that Ki-67 LI (≥4%) is an independent factor and predictor of cause-specific survival.
Though many studies have demonstrated Ki-67/MIB-1 is expressed in thyroid cancer, there is no systematic literature assessing the relationship between Ki-67/MIB-1 expression and clinical factors in thyroid cancer patients. So, the significance of Ki-67/MIB-1 for prognostication of thyroid cancer remains uncertain. Thus, a literature-based meta-analysis study was performed to evaluate the diagnostic and prognostic values of Ki-67/MIB-1 in thyroid cancer.
Materials and methods
Studies selection
Studies were selected to demonstrate the diagnostic and prognostic values of Ki-67/MIB-1 for thyroid cancer. Electronic literatures were searched in PubMed, EBSCO, EMBASE, ISI Web of Science, WanFang, China National Knowledge Infrastructure and Chinese VIP databases from April 1, 1989 to July 31, 2016. The following keywords were used to identify the related publications: “thyroid cancer”, “Ki-67”, “MIB-1”, “proliferative marker”, “proliferative index”, “diagnosis”, “prognostic”, “survival”. The eligible studies were selected in accordance with the following criteria: 1) studies should be published in full assays; 2) the goal of the publication was to illustrate the clinical significance of Ki-67/MIB-1 in primary thyroid cancer; 3) sufficient data were used to determine the connection between Ki-67/MIB-1 and clinicopathologic parameters; 4) when the same patient cohort was reported in different publications, only the most complete and recent study was selected in the meta-analysis.
Also, we screened the references from the reviews and identified articles.
Data extraction and assessment of study quality
Three authors (Deng-hua Pan, Dong-yue Wen and Yi-huan Luo) read the studies carefully and independently. The information of the publications was collected from each study: first author’s name, publication date, the number of patients, patient age, country, follow-up time, antibody of testing Ki-67/MIB-1, the method of detecting Ki-67/MIB-1 expression and threshold used for assessing Ki-67/MIB-1 expression positively. True positive, true negative, false positive and false negative were extracted to construct a diagnostic contingency table. Disease-free survival (DFS) or mortality or distant recurrences-free survival was used to measure the effect of Ki-67/MIB-1 expression on survival in thyroid cancer patients. The following clinical parameters were extracted to evaluate the connection between Ki-67/MIB-1 and thyroid cancer aggressiveness: age, tumor size, lymph node metastasis, metastasis status, extrathyroid extension, tumor node metastasis stage. Minimal size of patients and minimal follow-up time are not defined in this meta-analysis. Studies that met the following criteria were excluded: 1) reviews, conference papers, case reports, expertise public opinion, letters, zoopery were not included; 2) studies without sufficient information to calculate the impact of diagnosis, survival and prognosis of Ki-67/MIB-1 in primary thyroid cancer were excluded; 3) studies with duplicated data from similar or the same population were excluded. QUADAS-2 was used to assess the quality of the studies for diagnosis.Citation15 Newcastle–Ottawa scale (NOS) was used to assess the quality of the studies for prognosis.Citation16 The study with NOS scores ≥6 was identified as a high-quality study and the study with NOS scores <6 was considered as a low-quality study.
Statistical methods
According to the cut-off values, Ki-67/MIB-1 expression was divided into positive and negative groups. The pooled sensitivity and specificity, positive likelihood ratio (LR+), negative likelihood ratio (LR−), diagnostic odds ratio (DOR) and the area under the symmetric receiver operating characteristic curve were used to measure the diagnosis of Ki-67/MIB-1 in thyroid cancer. The odds ratio (OR) and 95% confidence intervals (CIs) were used to estimate the relationship between Ki-67/MIB-1 and clinicopathologic parameters in thyroid cancer patients. When the OR was >1, it indicated that high Ki-67/MIB-1 was a risk factor in thyroid cancer. Hazard ratio (HR) and 95% CI were calculated to measure the effect of Ki-67/MIB-1 on prognosis. Also, when the HR was >1, it indicated that high level of Ki-67/MIB-1 was related to worse survival in thyroid cancer patients.
Further, Cochran’s Q-test was performed to measure heterogeneity. Also, I2 was calculated to assess the inconsistency of the studies. When I2 was over 50% or chi-squared P-value was >0.1, fixed-effect meta-analysis was performed; otherwise, random-effect meta-analysis was used when there was less or no heterogeneity (when I2 was less than 50% or chi-squared P-value was <0.1). MetaDiSc was used to measure the diagnosis of Ki-67/MIB-1 in thyroid cancer. STATA12.0 was used to calculate the progression and prognosis of Ki-67/MIB-1 in thyroid cancer. The potential publication bias was investigated through funnel plot and by computation of Begg’s test. When the P-value was <0.05, it was considered significant.
Results
Description of studies
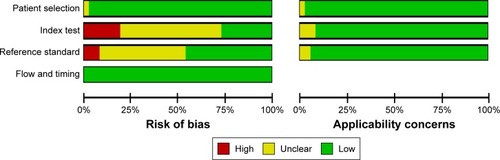
The flow chart of search process is presented in . One thousand one hundred and sixty-two relevant studies were identified. After reviewing the abstracts and full text, only 51 studiesCitation1,Citation12,Citation14,Citation17–Citation64 were found to be eligible and were included in the meta-analysis. The characteristics of these articles are summarized in –. gives the main information of the studies included in diagnosis. Thirty-seven studies detecting the diagnostic value of Ki-67/MIB-1 in thyroid cancer were included in our analysis, including 4,818 samples (2,601 cases and 2,217 controls). The quality assessment of the included studies in diagnosis is shown in . The risks of bias in “index test” and “reference standard” were unclear in this meta-analysis. shows the correlation between clinicopathologic parameters and Ki-67/MIB-1, including 4,375 samples (1,317 cases and 3,058 controls). As shown in , a total of 10 studies reporting the relation between Ki-67/MIB-1 and thyroid cancer patient survival were included, of which three studies had data on DFS, seven on mortality and only one on distant recurrences-free survival (DRFS). The studies included in survival analysis had 1,083 samples. The age of patients ranged from 45 to 64.8 years, and the median follow-up time ranged from 68 months to 20 years. Immunohistochemistry (IHC) was used to detect the expression of Ki-67/MIB-1 in all the included studies. The cut-off value ranged from 1% to 10%. The quality assessment of the studies included in prognosis is shown in .
Table 1 Characteristics of studies included in diagnosis
Table 2 The relationships between Ki-67/MIB-1 expression and clinicopathologic parameters
Table 3 The features of the studies relating Ki-67/MIB-1 to patients’ prognosis
Table 4 Quality assessment of included studies according to NOS
Effect of Ki-67/MIB-1 on diagnosis
Due to heterogeneity, random-effect model was used to calculate the overall performance of Ki-67/MIB-1 in thyroid tissues in diagnosing thyroid cancer. Ki-67/MIB-1 was found to be a valuable diagnostic factor for thyroid cancer. The pooled sensitivity was 0.61 (95% CI: 0.59–0.63; ) and specificity was 0.75 (95% CI: 0.74–0.77; ). The pooled LR+ was 3.19 (95% CI: 2.30–4.42; ) and the pooled LR− was 0.43 (95% CI: 0.35–0.54; ). The pooled DOR of Ki-67/MIB-1 was 8.54 (95% CI: 5.03–14.49; ). The area under the symmetric receiver operating characteristic curve was 0.804 (standard error =0.031; ). For Ki-67/MIB-1, the summary indexes of 37 studies are displayed in forest plots. High heterogeneity was found in sensitivity (I2=94.5%, P<0.001) and specificity (I2=94.7%, P<0.001). Also, subgroup analysis was performed to identify the possible reasons for heterogeneity. There were 12 studies with Ki-67/MIB-1 cut-off value ≤5%, which revealed a pooled sensitivity of 0.70 (95% CI: 0.67–0.73), specificity of 0.80 (95% CI: 0.77–0.83) and DOR of 7.49 (95% CI: 3.61–15.52). Fifteen studies had Ki-67/MIB-1 cut-off value of 10%, a pooled sensitivity of 0.56 (95% CI: 0.53–0.59), specificity of 0.67 (95% CI: 0.63–0.70) and DOR of 7.73 (95% CI: 2.68–22.30). There were eight studies with a cut-off value >10%, and the pooled sensitivity, specificity and DOR were 0.78 (95% CI: 0.75–0.81), 0.79 (95% CI: 0.74–0.84) and 15.40 (95% CI: 3.41–69.62), respectively. In the subgroup analysis of PTC, the results showed that the pooled sensitivity, specificity and DOR were 0.63 (95% CI: 0.60–0.65), 0.74 (95% CI: 0.72–0.76) and 8.22 (95% CI: 4.08–16.56), respectively. In the subgroup analysis of papillary thyroid microcarcinoma (PTMC), the results showed that the pooled sensitivity, specificity and DOR were 0.45 (95% CI: 0.37–0.52), 0.86 (95% CI: 0.77–0.92) and 4.76 (95% CI: 2.48–9.17), respectively ().
Figure 3 Forest plots for the accuracy of Ki-67/MIB-1 for the diagnosis of thyroid cancer.
Abbreviations: CI, confidence interval; df, degrees of freedom; LR, likelihood ratio.
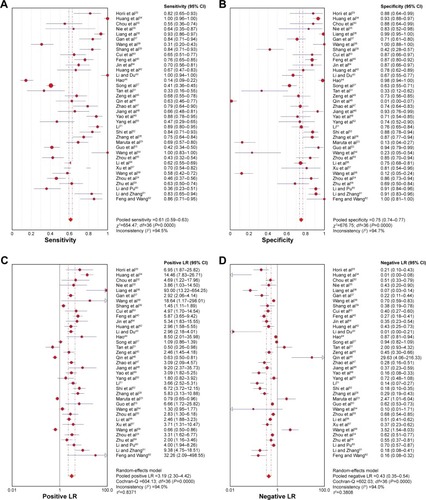
Figure 4 Forest plot of DOR of Ki-67/MIB-1 for the diagnosis of thyroid cancer.
Abbreviations: CI, confidence interval; df, degrees of freedom; DOR, diagnostic odds ratio; OR, odds ratio.
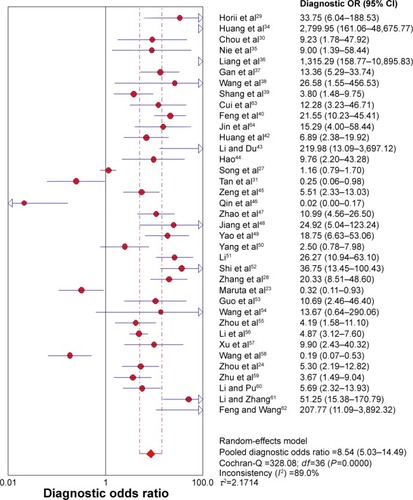
Figure 5 SROC curve for the accuracy of Ki-67/MIB-1 in the diagnosis of thyroid cancer.
Abbreviations: AUC, area under the curve; SE, standard error; SROC, symmetric receiver operating characteristic.
Associations of Ki-67/MIB-1 with clinicopathologic parameters
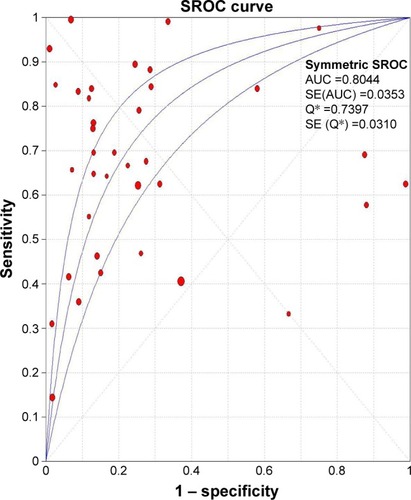
shows the main results of the effects value of Ki-67/MIB-1 on clinicopathologic parameters in thyroid cancer patients. The results suggested that older patients (>45 years old) had high level of Ki-67/MIB-1 with a pooled OR of 1.71 (95% CI: 1.14–2.57, P=0.010; I2=0.00%, P=0.806; ). The level of Ki-67/MIB-1 was higher in large tumor size (>4 cm) than in small-sized tumor (<4 cm; pooled OR =1.86, 95% CI: 1.17–2.96, P=0.008; I2=2.00%, P=0.395; ). Overall, 15 studies had data to estimate the relationship between Ki-67/MIB-1 and lymph node metastasis. The pooled OR estimated from 15 studies indicated that Ki-67/MIB-1 was lower in negative lymph node metastasis than in positive lymph node metastasis (pooled OR =2.49, 95% CI: 1.42–4.39, P=0.002; I2=50.20%, P=0.014; ). Also, the association between Ki-67/MIB-1 and metastasis status was calculated and the combined OR was 6.96 (95% CI: 2.46–19.69, P<0.001; ). The combined OR for tumor node metastasis stage was 6.56 (95% CI: 3.80–11.33, P<0.001; I2=0.00%, P=0.960), suggesting that overexpression of Ki-67/MIB-1 was significantly correlated with advanced stage (). In addition, four studies provided insufficient information to estimate the effect of extrathyroid extension. The pooled OR was 1.91 (95% CI: 1.27–2.87, P=0.002; ).
Figure 6 Forest plots of Ki-67/MIB-1 and clinicopathologic parameters in thyroid cancer patients.
Abbreviations: CI, confidence interval; OR, odds ratio; TNM, tumor node metastasis.

Impact of Ki-67/MIB-1 expression on survival in thyroid cancer
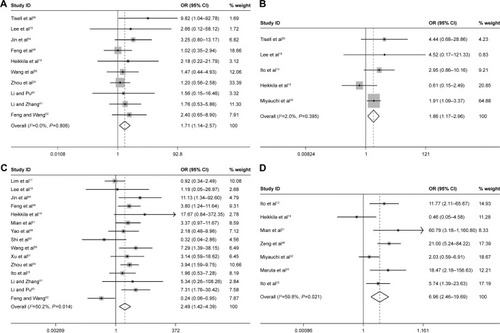
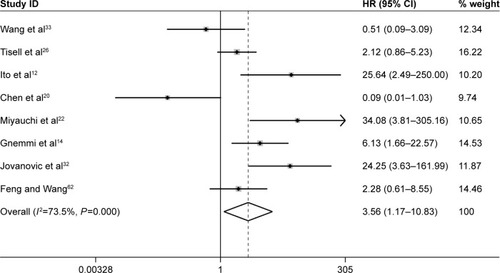
Furthermore, three studies assessing the association of Ki-67/MIB-1 expression on DFS were identified in this meta-analysis. The combined HR was 5.19. It was demonstrated that overexpression of Ki-67/MIB-1 was associated with worse DFS in thyroid cancer by fixed-effect model (95% CI: 3.18–8.46, P<0.001; I2=36.1%, P=0.209; ). The meta-analysis showed overexpression of Ki-67/MIB-1 had effect on mortality (HR =3.56, 95% CI: 1.17–10.83, P=0.025) in eight studies, with heterogeneity (I2=73.5%, P<0.001; ). Worsened mortality was found among patients with Ki-67/MIB-1 cut-off value >10% (HR =34.08, 95% CI: 3.81–305.00, P=0.002) by subgroup analysis. Nevertheless, when Ki-67/MIB-1 was more than 5%, the patients were afflicted with poor mortality (HR =28.06, 95% CI: 6.68–117.87, P<0.001) in thyroid cancer. No connection was found between Ki-67/MIB-1 and thyroid cancer when Ki-67/MIB-1 was less than 5% (HR =1.98, 95% CI: 0.65–6.06, P=0.230). In subgroup analysis, no significant association was found between medullary thyroid cancer and mortality (HR =0.96, 95% CI: 0.24–2.16). In addition, no significant relationship was observed between PTC and mortality (HR =1.47, 95% CI: 2.23–5.18; ).
Figure 7 Meta-analysis evaluating the association between Ki-67/MIB-1 and DFS (fixed-effect analysis).
Abbreviations: CI, confidence interval; DFS, disease-free survival; HR, hazard ratio.
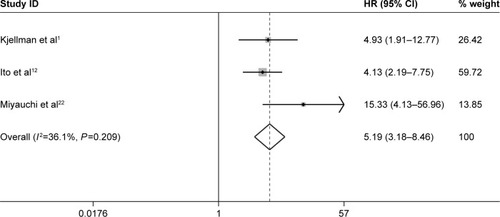
Figure 8 Meta-analysis evaluating the association between Ki-67/MIB-1 and mortality (random-effect analysis).
Abbreviations: CI, confidence interval; HR, hazard ratio.
Publication bias
The Begg’s test and funnel plot showed that there was no evidence of publication bias present among all the analyses in our meta-analysis (all P>0.05; and ).
Discussion
Cell proliferative activity is regarded as an important factor for evaluating the biologic behavior of cancer cells. It is a key process in the development of tumors when the balance between cell death and proliferation is destroyed. We used IHC to detect cell cycle-specific antigens and this method can assess the proliferative activity of cells. Ki-67/MIB-1 is the most widely used marker for assessing the proliferative capacity of tumor cells. Increased expression of Ki-67/MIB-1 has been linked to increased invasiveness in many cancers.Citation10,Citation65–Citation67 Many meta-analyses have shown that high expression of Ki-67/MIB-1 contributed to poor survival in many tumors.Citation68–Citation72 However, there is no consensus on the association between high level of Ki-67/MIB-1 and thyroid cancer at present. Meta-analysis is a systematical method applied widely to evaluate the prognostic indicators in different trials. Thus, in this meta-analysis, we explored the diagnosis and prognosis of Ki-67/MIB-1 expression in thyroid cancer.
In our study, we found the valuable predicting effect of Ki-67/MIB-1 for the diagnosis of thyroid cancer with a high accuracy for Chinese. In clinical practice, detecting Ki-67/MIB-1 expression may contribute to diagnosing thyroid cancer when combined with clinical symptoms, laboratory examinations and other radiologic imaging. Our analysis demonstrated that patients with overexpression of Ki-67/MIB-1 seemed to have a poor survival in thyroid cancer. Also, Ki-67/MIB-1 was found to be associated with tumor size, lymph node metastasis, metastasis status, extrathyroid extension and other clinicopathologic parameters. However, the mechanism of Ki-67/MIB-1 in thyroid cancer is still unclear. Ki-67/MIB-1 as a cellular marker has a positive effect on cell proliferation. Ki-67/MIB-1 expression detected by IHC can evaluate tumor cell proliferation. A previous study confirmed that Sp1 plays an important role in regulation of Ki-67/MIB-1 gene expression.Citation73 Another study pointed out that E2F2 transcription factor was positively correlated with Ki-67/MIB-1 expression in human glioblastomaCitation74 and E2F1–3 factors are the transcriptional activator in tumor progression and the retinoblastoma tumor suppressor pathway regulates E2F1–3 factors which could control cellular proliferation.Citation75
In this meta-analysis, heterogeneity existed among the studies. Heterogeneity was still a potential problem which affected the meta-analysis results, though random-effect models were used to analyze the data. Meanwhile, in order to reduce heterogeneity, only the studies with the method of IHC to detect Ki-67/MIB-1 were included in this meta-analysis. However, evaluation standards, study location, number of patients, sex and age of patients were different, which contributed to the heterogeneity. Also, various cut-off values were used to define thyroid cancer with Ki-67/MIB-1 positive expression by different investigators, which potentially contributed to the heterogeneity. So, it is difficult to apply a standard critical value in clinical practice. Spyratos et alCitation76 found that few tumors with low proliferation rate were under misclassification when the cut-off value of Ki-67/MIB-1 was 10%, and it is acceptable to identify a highly proliferative tumor with a Ki-67/MIB-1 cut-off value of 25%. In this meta-analysis, the cut-off values of Ki-67/MIB-1 ranged from 0.5% to 25%. Therefore, different Ki-67/MIB-1 cut-offs may cause heterogeneity. Higher sensitivity, specificity and DOR were found in patients with cut-off value >10% by diagnostic subgroup analysis. Furthermore, those patients may have worse mortality. Given the small size of studies included in mortality analysis, further research with larger sample size would be needed to explore the impact of KI-67/MIB-1 on mortality. Besides, HRs were extracted from survival curves or calculated from data which might be less than another variance. Most of the studies included in diagnosis were from China; however, most of the studies related to DFS and mortality were from outside of China. We found that Ki-67/MIB-1 had a diagnostic value in Chinese. However, we did not have enough data to calculate the relationship between Ki-67/MIB-1 and mortality for Chinese. So, regional variation may become a score of heterogeneity. Besides, due to several types of thyroid cancer dealt with in the same study, we conducted subgroup analysis in this meta-analysis. Ki-67/MIB-1 had diagnostic effect on different thyroid cancer types. We did not find significant association between Ki-67/MIB-1 and different thyroid cancer types.
Despite the above limitations, the current meta-analysis proves the associations between high Ki-67/MIB-1 and tumor deterioration, poor DFS and increased mortality in patients with thyroid cancer. In conclusion, we showed that high expression of Ki-67/MIB-1 was significantly connected with tumor size, lymph metastasis, metastasis status, extrathyroid extension and poor prognosis of thyroid cancer in this study.
Conclusion
Our meta-analysis shows that Ki-67/MIB-1 may be a biomarker for clinical deterioration in Chinese and has an effect on prognosis in thyroid cancer among non-Chinese. Therefore, detection of Ki-67/MIB-1 in the clinic will be beneficial to the treatment and prognostic assessment for thyroid cancer patients. However, well-designed prospective studies are necessary to further confirm our results.
Acknowledgments
The study was supported by funds from the Guangxi Scientific Research and Technology Development Plan (1598011-4). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the paper.
Supplementary materials
Table S1 Subgroup analysis evaluating the diagnostic value of Ki-67/MIB-1 in thyroid cancer
Table S2 Subgroup analysis evaluating the prognostic value of Ki-67/MIB-1 for mortality in thyroid cancer
Disclosure
The authors report no conflicts of interest in this work.
References
- KjellmanPWallinGHoogAAuerGLarssonCZedeniusJMIB-1 index in thyroid tumors: a predictor of the clinical course in papillary thyroid carcinomaThyroid200313437138012812214
- SofiadisATaniEFoukakisTDiagnostic and prognostic potential of MIB-1 proliferation index in thyroid fine needle aspiration biopsyInt J Oncol200935236937419578751
- ChenWZhengRBaadePDCancer statistics in China, 2015CA Cancer J Clin201666211513226808342
- TorreLASauerAMChenMSJrKagawa-SingerMJemalASiegelRLCancer statistics for Asian Americans, Native Hawaiians, and Pacific Islanders, 2016: Converging incidence in males and femalesCA Cancer J Clin201666318220226766789
- ItoYMiyauchiAItoMPrognosis and prognostic factors of differentiated thyroid carcinoma after the appearance of metastasis refractory to radioactive iodine therapyEndocr J201461882182424871888
- ItoYTomodaCUrunoTUltrasonographically and anatomo-pathologically detectable node metastases in the lateral compartment as indicators of worse relapse-free survival in patients with papillary thyroid carcinomaWorld J Surg200529791792015951927
- ItoYTomodaCUrunoTPrognostic significance of extrathyroid extension of papillary thyroid carcinoma: massive but not minimal extension affects the relapse-free survivalWorld J Surg200630578078616411013
- ItoYKakudoKHirokawaMBiological behavior and prognosis of familial papillary thyroid carcinomaSurgery2009145110010519081481
- YuJQZhouQZhengYFBaoYExpression of vimentin and Ki-67 proteins in cervical squamous cell carcinoma and their relationships with clinicopathological featuresAsian Pac J Cancer Prev201516104271427526028085
- GhanimBKlikovitsTHodaMAKi67 index is an independent prognostic factor in epithelioid but not in non-epithelioid malignant pleural mesothelioma: a multicenter studyBr J Cancer2015112578379225633038
- HaoSHeZXYuKDYangWTShaoZMNew insights into the prognostic value of Ki-67 labeling index in patients with triple-negative breast cancerOncotarget2016717248242483127050075
- ItoYMiyauchiAKakudoKHirokawaMKobayashiKMiyaAPrognostic significance of ki-67 labeling index in papillary thyroid carcinomaWorld J Surg201034123015302120703465
- PascualJBercianoJAn open trial of buspirone in migraine prophylaxis. Preliminary reportClin Neuropharmacol19911432452502070365
- GnemmiVRenaudFDo CaoCPoorly differentiated thyroid carcinomas: application of the Turin proposal provides prognostic results similar to those from the assessment of high-grade featuresHistopathology201464226327324164362
- WhitingPFRutjesAWWestwoodMEQUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studiesAnn Intern Med2011155852953622007046
- StangACritical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analysesEur J Epidemiol201025960360520652370
- LimDJBaekKHLeeYSClinical, histopathological, and molecular characteristics of papillary thyroid microcarcinomaThyroid200717988388817956162
- LeeYSHaSAKimHJMinichromosome maintenance protein 3 is a candidate proliferation marker in papillary thyroid carcinomaExp Mol Pathol201088113814219818763
- HeikkilaASiironenPHagstromJFollicular thyroid neoplasm: clinicopathologic features suggesting malignancyAPMIS20101181184685420955457
- ChenJHFaquinWCLloydRVNoseVClinicopathological and molecular characterization of nine cases of columnar cell variant of papillary thyroid carcinomaMod Pathol201124573974921358618
- MianCPennelliGBarolloSCombined RET and Ki-67 assessment in sporadic medullary thyroid carcinoma: a useful tool for patient risk stratificationEur J Endocrinol2011164697197621422198
- MiyauchiAKudoTHirokawaMKi-67 labeling index is a predictor of postoperative persistent disease and cancer growth and a prognostic indicator in papillary thyroid carcinomaEur Thyroid J201321576424783039
- MarutaJHashimotoHYamashitaHValue of thyroid specific peroxidase and Ki-67 stains in preoperative cytology for thyroid follicular tumorsDiagn Cytopathol201543320220925195571
- ZhouYJiangHGLuNLuBHChenZHExpression of ki67 in papillary thyroid microcarcinoma and its clinical significanceAsian Pac J Cancer Prev20151641605160825743839
- ItoYUrunoTTakamuraYPapillary microcarcinomas of the thyroid with preoperatively detectable lymph node metastasis show significantly higher aggressive characteristics on immunohistochemical examinationOncology2005682–3879615886500
- TisellLEOdenAMuthAThe Ki67 index a prognostic marker in medullary thyroid carcinomaBr J Cancer200389112093209714647143
- SongQWangDLouYDiagnostic significance of CK19, TG, Ki67 and galectin-3 expression for papillary thyroid carcinoma in the northeastern region of ChinaDiagn Pathol2011612622188859
- ZhangYMengZZhangMImmunohistochemical evaluation of midkine and nuclear factor-kappa B as diagnostic biomarkers for papillary thyroid cancer and synchronous metastasisLife Sci20141181394525283079
- HoriiAYoshidaJSakaiMKi-67 positive fractions in benign and malignant thyroid tumours: application of flow cytometryActa Otolaryngol1999119561762010478606
- ChouSJChenCMHarnHJChenCJLiuYCIn situ detection of hTERT mRNA relates to Ki-67 labeling index in papillary thyroid carcinomaJ Surg Res2001991758311421607
- TanAEtitDBayolUAltinelDTanSComparison of proliferating cell nuclear antigen, thyroid transcription factor-1, Ki-67, p63, p53 and high-molecular weight cytokeratin expressions in papillary thyroid carcinoma, follicular carcinoma, and follicular adenomaAnn Diagn Pathol201115210811621315633
- JovanovicRKostadinova-KunovskaSJanevskaVNovel RET mutations in macedonian patients with medullary thyroid carcinoma: genotype-phenotype correlationsPril (Makedon Akad Nauk Umet Odd Med Nauki)20153619310726076779
- WangWJohanssonHBergholmUWilanderEGrimeliusLApoptosis and expression of the proto-oncogenes bcl-2 and p53 and the proliferation factor Ki-67 in human medullary thyroid carcinomaEndocr Pathol199671374512114678
- HuangXZhangPChenJGaolLingYExpression of p21ras protein, treanforming growth factorβ1 and Ki-67 in papillary thyroid carcinomaJ Fujian Med University20002129131
- NieMDuMLiXExpression of cytokeratin19 and Ki67 in papillary thyroid carcinomaHeilongjiang Med J2005293168169
- LiangFFuQDaiCWangGLiJZhaoMPathological study of papillary thyroid carcinoma and papillary thyroid typerplasiaCancer Res Clin20061811755756
- GanXZhuWZhangXWanLThe expression and significance of Ki-67 and CK-19 in papillary thyroid carcinomaZhejiang Practical Med2007126391392
- WangCSunJTengMHuanDExpression of RET, HBME-1 and Ki67 in thyroid benign and malignant tumorsPractical Oncol J2007215427429
- ShangLYangYLiDWeiDChenXExpressions of CD26, Ki67 and EGFR proteins in primany thyroid neoplasms and their value in differential diagnosisChinese J Endocrinol Meta2008242174177
- FengWCaoYZhangJExpression of Ki-67 and survivin in thyroid cacner and their significanceJ Practical Med2009251932153218
- WangLXiFYangYExpression of survivin and Ki-67 and their significance in thyroid tumorsShandong Med J20094928911
- HuangHMeiJXuLChenRExpression of CK19, Ki67 and VEGF and their clinical significances in papillary thyroid carcinomaJ Nanchang University (Medical Sciences)201050112427
- LiNDuJExpression of HBME-1, CK19, Gal-3 and Ki-67 in papillary thyroid lesionsJ Harbin Med University2010446575578
- HaoGThe expresion p53, p21 and Ki-67 in papillary thyroid carcinomaChina Modern Med201023838
- ZengXYangXWangTExpression and significance of tumor-related factors COX-2 and Ki-67 in thyroid neoplasm tissuePractical Preventive Med2011184612614
- QinLNiuJLiuSHeJXuYSignificance and Expression of MCM3 and Ki-67 in Normal Thyroid, Nodular Goiter and CarcinomaJournal of Shihezi University (Natural Science)2012303356360
- ZhaoLLinJShiBLinQHuaiYWangKExpression and clinical pathology significance of P53, ki-67, galectin-3, HBME-1, 34βE12 and CK19 in papillary thyroid carcinomasBasic Amp Clin Med2012321012021206
- JiangWWangYChenMZhuCExpression and relationship of XIAP, P53 and Ki67 in papillary thyroid carcinomaBMJ20123411971974
- YaoHWeiZLiangXThe expressin and prognosis of tumor metastasis gene KISS-1 in the tissues of thyroid cancerChinese Remedies Clinics2012121114081411
- YangRTengXDingWSunKExpressions of MCM2, Ki67, CyclinD1 in Follicular Carcinoma and Follicular Adenoma of the Thyroid and the Comparison with Galectin-3 and CK19J Basic Clin Oncol20136464468
- LiXExpression and clinial significance of Receptor for Activated Protein C Kinase1 and Ki67 in papillary thyroid carcinomaJ Dalian Med University2013
- ShiLZhangALuoYZhaoSTianHYangYAbnormal expressions of positive cell cycle control factors and thyroid carcinoma occurrence and progressionJ Southern Med University201333710311035
- GuoMXingYChenWZhangJMengDBcl-1, P53 and ki-67 expression in the thyroid carcinoma and its clinical significanceJ Modern Oncol201422612941297
- WangYWangHZhuHExpression and diagnosistic value of CK19, HBME-1 and MIB-1 between hyalinizing trabecular tumor and thyroid papillary carcinomaPractical Oncol J20144326330
- ZhouYJiangHLuBChenZLuNYLiJExpression and Clinical Significance of Ki67, CK19, Galectin-3 and HBME-1 in Papillary Thyroid Carcinoma ZhejiangPractical Med2014196395397
- LiTWuGCaiDClinical significance of four immunohistochemical markers in the diagnosis of the papillary thyroid carcinomaShanghai Med Pharm J201435102427
- XuYLiXYuJThe relationship between BRAF gene mutation and Ki67 protein expression in papillary thyroid carcinomaJ Nanchang University (Medical Sciences)201454101214
- WangLSunJHouHPengJThe expression and significance of C-erB-2 and Ki-67 in thyroid papillary carcinomasJ Chinese Physician20141617678
- ZhuCShuaiJShaoQHuGDiscuss application of antibody marker MC combining with CK19, CK19, TPO, Galectin-3, KI-67 in thyroid papillary lesionsWorld Latest Med Info2015531112
- LiGPuYExpression of CD147 and Ki-67 in papillary thyroid cacner and significanceJ Modern Oncol2015231318181820
- LiYZhangJClinical Significance of the ProtientExpression of MCM7,CDK2 and Ki-67 in Thyroid CancerPractical J Cancer201503359361
- FengJWangJExpression and clinical significance of Ki67 and calcitonin in medullary thyroid carcinomaLin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi2014282419211924 Chinese25895305
- CuiWChenXLiuCZhangWExpressions of Gal-3, PTTG and Ki-67 in primany thyroid neoplasms and their value in differential diagnosisShaanxi Medical J2009386670672
- JinDLiLLiuXLiangSXiFGaoJExpression of survivin and Ki-67 and their significance in thyroid tumorsChinese J of Clin and Experimental Pathol200925154157
- ZhangTZhaoCLuoLZhaoHChengJXuFThe expression of Mcl-1 in human cervical cancer and its clinical significanceMed Oncol20122931985199121674276
- JosephMGShibaniAPanjwaniNUsefulness of Ki-67, mitoses, and tumor size for predicting metastasis in carcinoid tumors of the lung: a study of 48 cases at a tertiary care centre in CanadaLung Cancer Int2015201554560126770831
- AhmedSRashedHHegazyAMohamedAMElmesallamyWPrognostic value of ALDH1, EZH2 and Ki-67 in astrocytic gliomasTurk Patoloji Derg2016322708127136105
- TianYMaZChenZClinicopathological and prognostic value of Ki-67 expression in bladder cancer: a systematic review and meta-analysisPLoS One2016117e015889127410033
- PetrelliFVialeGCabidduMBarniSPrognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patientsBreast Cancer Res Treat2015153347749126341751
- LuoYZhangXMoMHigh Ki-67 immunohistochemical reactivity correlates with poor prognosis in bladder carcinoma: a comprehensive meta-analysis with 13,053 patients involvedMedicine (Baltimore)20169515e333727082587
- LuoYRenFLiuYClinicopathological and prognostic significance of high Ki-67 labeling index in hepatocellular carcinoma patients: a meta-analysisInt J Clin Exp Med201587102351024726379815
- PanDWeiKLingYSuSZhuMChenGThe prognostic role of Ki-67/MIB-1 in cervical cancer: a systematic review with meta-analysisMed Sci Monit20152188288925807305
- TianHQianGWLiWA critical role of Sp1 transcription factor in regulating the human Ki-67 gene expressionTumour Biol201132227328320963645
- JinQZhangWQiuXGGene expression profiling reveals Ki-67 associated proliferation signature in human glioblastomaChin Med J (Engl)2011124172584258822040407
- WuLTimmersCMaitiBThe E2F1–3 transcription factors are essential for cellular proliferationNature2001414686245746211719808
- SpyratosFFerrero-PousMTrassardMCorrelation between MIB-1 and other proliferation markers: clinical implications of the MIB-1 cutoff valueCancer20029482151215912001111