Abstract
The molecular triad involving receptor activator of nuclear factor kβ (RANK)/RANK ligand (RANKL)/osteoprotegerin cytokine system has been well implicated in several physiological and pathological processes including bone metabolism, mammary gland development, regulation of the immune function, tumorigenesis and metastasis of cancer stem cell, thermoregulation, and vascular calcification. However, this review aimed to summarize several original and up-to-date articles focusing on the role of this signaling system in cancer cell development and metastasis as well as potential therapeutic agents targeting any of the three tumor necrotic factor super family proteins and/or their downstream signaling pathways. The RANK/RANKL axis has direct effects on tumor cell development. The system is well involved in the development of several primary and secondary tumors including breast cancer, prostate cancer, bone tumors, and leukemia. The signaling of this triad system has also been linked to tumor invasiveness in the advanced stage. Bone is by far the most common site of cancer metastasis. Several therapeutic agents targeting this system have been developed. Among them, a monoclonal antibody, denosumab, was clinically approved for the treatment of osteoporosis and cancer-related diseases.
Introduction
Overview of receptor activator of nuclear factor kβ (RANK)/RANK ligand (RANKL)/osteoprotegerin (OPG) signaling system
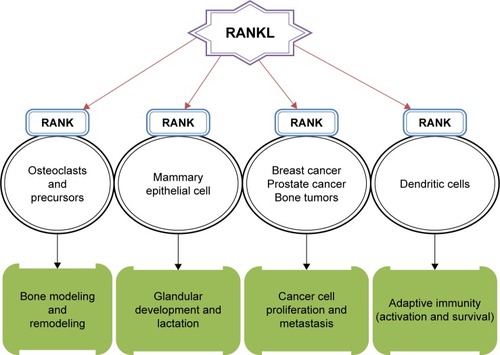
The molecular triad involving RANK/RANKL/OPG cytokine system has been observed to influence various physiological and pathological processes throughout the body. These include bone modeling and remodeling, mammary gland development, tumor cell development and migration, and modulation of adaptive immunity ().Citation1–Citation4 The role of this signaling system has been well emphasized in bone where RANKL/RANK signaling mediates osteoclastogenesis and bone resorption via paracrine signaling between osteoblast (RANKL) and osteoclast (RANK) cells. OPG produced by osteoblast and stromal cells acts as a soluble decoy receptor for RANKL and hence prevents osteoclast differentiation and activation by interfering with RANKL–RANK interaction.Citation1 RANK is also constitutively expressed in mammary epithelial tissues where RANKL works through RANK to provide proliferative and survival signals and thereby promotes the final stages of lactating mammary gland development.Citation5 It has also been shown to be involved in thermoregulation signaling in females (linked with ovarian sex hormones) possibly via the COX2-PGE2/EP3R pathway, vascular calcification, and atherosclerosis by cross talking with the renin–angiotensin system.Citation6–Citation10
Methods
In this review, both open access scholarly sources and subscription based journals were utilized for the study. Legitimate databases and indexing services including directory of open access journals, PubMed, PubMed Central, Medline, Scopus, and ProQuest were considered for the collection of articles related to the topic of interest. Other supplementary sources such as Google Scholar, WorldCat, and ResearchGate were also used for extensively collecting updated and sufficient information on this topic. For subscription based journals, however, Hinari: World Health Organization for developing countries was used to access such journals (eg, Elsevier journals, subscribed journals in PubMed, Science Direct, etc.). The primary key terms used during data collection were “RANK,” “RANKL,” “OPG,” “RANK/RANKL/OPG system,” “cancer,” “tumor,” and “therapeutic.” In each of the databases and web directories, Boolean logic (AND, OR) was routinely applied to connect the key terms. What is more, truncation and alternative terms were also employed to expand the chance of obtaining related articles to the topics of interest. Based on these key terms and following in-depth screening of the relevance of each and every article for the topic, only articles that are highly relevant to this review were included and duplicated articles were excluded from the study in advance. Excluding the background, 73 references were filtered for the body of this review. Coming to the timing distribution of references cited, the majority of references, 52 (71.23%), were published from 2010 to 2017. Data collection was conducted from September 2016 to April 2017.
Overview of RANK/RANKL/OPG system and cancer
Primary tumors will commonly metastasize into bone. Tumors such as breast and prostate cancers typically have a greater chance of inducing secondary cancers within bone.Citation11 Based on Stephen Paget’s seed and soil theory, the bone microenvironment provides a conducive area (fertile soil) in which the seeds (secondary tumors) can easily grow in. RANK and RANKL have been known to be involved in cancer cell migration and development in bone.Citation12 Secondary tumors within the bone will secrete several growth factors and cytokines which stimulate osteoblast cells. Upon stimulation, osteoblast cells increase the expression and release of RANKL which in turn acts either on the RANK receptor found on the osteoclast cells or soluble decoy receptor (OPG) that circulates in body fluids. RANKL–RANK interaction activates osteoclasts to further release growth factors such as tumor growth factor β and insulin-like growth factor 1 from the bone matrix which in turn stimulate parathyroid hormone-related peptide production and promote tumor growth. This interaction between tumor cells and the bone microenvironment results in a vicious cycle of bone destruction and tumor growth. Generally, skeletal-related events (SREs) are used to describe a collection of adverse events associated with bone metastases. SREs include pathologic fractures that require surgery or chemotherapy, spinal cord compression, and less frequently malignant hypercalcemia.Citation11,Citation13,Citation14 RANKL was also observed to be more accurate than conventional markers in the breast cancer subgroup, and was a better predictor of bone progression than N-terminal telopeptides of type I collagen. The study suggested that RANKL could serve as an accurate marker of bone response in metastatic patients. High RANKL levels may identify patients with a shift in bone homeostasis toward bone resorption.Citation15
The role of RANK/RANKL/OPG system in solid cancers
Primary bone tumors
Malignant tumors of the bone can be either primary tumors or secondary (metastasized) tumors. Primary tumors include osteosarcoma, multiple myeloma, and giant cell tumor of the bone (). Osteosarcoma is the most common primary malignant tumor of the bone.Citation16
Table 1 The role of RANKL/RANK/OPG system in primary malignant tumors of the bone
This triad system also plays a significant role in various solid malignancies that are capable of metastasizing to bone. These include breast cancer, prostate cancer, and lung cancer, among others.Citation22 This triad system is by far strongly implicated in the physiology of mammary gland development and pathogenesis of breast cancer cells.Citation5,Citation23,Citation24 The role of the RANK/RANKL/OPG triad system goes beyond involvement of breast cancer pathogenesis. The second most common target is prostate cancer. It has also been implicated in the pathogenesis of several and rare malignant tumors such as lung cancer, renal cell carcinoma, hepatocellular carcinoma, and melanoma ().Citation22,Citation25
Table 2 The role of RANK/RANKL/OPG system in the development and metastasis of solid cancers
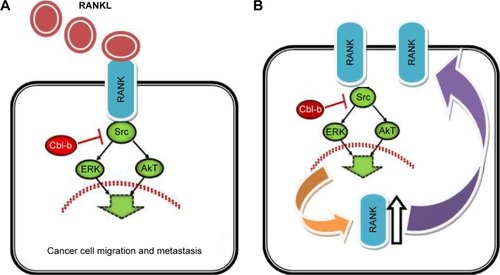
The regulatory function of RANKL is one of the key factors in progesterone-induced proliferation of the breast. Progesterone has a direct action on progesterone receptor (PR) expressing cells but PR-negative cells are affected indirectly through RANKL-induced paracrine actions leading to the proliferation of mammary epithelial PR-negative cells. RANK induces epithelial to mesenchymal transition and stemness in human mammary epithelial cells and promotes tumorigenesis and metastasis.Citation26 RANKL stimulation of RANK expressing cells increased multidrug resistance protein 1, breast cancer resistance protein, and lung resistance protein 1 expression and decreased Bim expression through various signaling molecules. These results indicate that the RANK/RANKL system induces chemoresistance through the activation of multiple signal transduction pathways.Citation27 What is more, expression of RANKL was observed during pregnancy. This evidence suggests that RANKL can be used as a potential breast cancer therapeutic target particularly in young women and pregnancy-associated tumors. RANKL/RANK has also been shown to control breast cancer 1 gene mutation-driven mammary tumors. Besides, the E3 ubiquitin ligase Cbl-b protein has been shown to improve the prognosis of RANK+ breast cancer patients via inhibiting RANKL-induced cancer cell migration and metastasis ().Citation28–Citation31
Figure 2 The role of Cbl-b in RANKL-induced breast cancer cell migration and metastasis.
Abbreviations: ERK, extracellular signal regulated kinase; RANK, receptor activator of nuclear factor kβ; RANKL, RANK ligand.
Other solid cancers
RANKL, RANK, and OPG were variably expressed in tumors of the thyroid, including papillary carcinomas, medullary carcinomas, and macrovascular adenomas.Citation60 Increased serum OPG has also been correlated with poor prognosis in gastric carcinomaCitation61 and bladder carcinoma.Citation62 RANK overexpression was also considered as a novel esophageal cancer marker.Citation63
Nonsolid cancers
Beyond its role in the pathogenesis of several primary and secondary solid malignant tumors, this signaling system has been known to be involved in certain nonsolid cancers such as chronic lymphocytic leukemia and acute myeloid leukemia (AML).
Leukemia
Expression of RANKL was also observed in chronic lymphocytic leukemia and has been known to induce inflammatory cytokines involved in the disease pathogenesis (tumor necrosis factor [TNF], interleukin [IL]-6, and IL-8). A novel Fc-engineered RANK fusion protein was shown to induce natural killer (NK) cell-mediated antitumor immunity against RANKL expressing targets.Citation12 Besides, RANKL influences the interaction of NK and AML cells by mediating a feedback loop that involves the release of factors by the latter which upregulates RANK on the former. In addition to the immediate inhibitory effects of RANKL-induced factors, RANK is readily available to interact with RANKL expressed by AML cells. This process leads to the activation of a bidirectional signal transduction cascade that causes the delivery of RANK-mediated inhibitory signals to NK cells and perpetuates RANKL reverse signaling process in AML cells.Citation64
The adhesion of the freshly isolated lymphoma B cells to bone marrow stromal cells or freshly isolated lymphoma stromal cells inhibited B cell spontaneous apoptosis in culture. This inhibition of apoptosis correlated with decreased cleavage of caspase-3/8 and increased activation of canonical and noncanonical nuclear factor kappa B signaling pathway.Citation65 Prognostic analysis revealed a higher probability of overall survival in cases with lower RANKL expression (<1.6 and ≥1.6, 15.6 vs 12.2 months, P=0.008, hazard ratio 0.36, P=0.008). The study revealed that RANKL is a promising marker to forecast patients’ prognosis in AML.Citation66
Potential therapeutic approaches
Considering this molecular triad system as a hot spot in the area of oncology research, several therapeutic agents have been developed over the last decade. These include humanized monoclonal antibodies, herbal medicines, RNA interference technology, and proteolytic enzymes, among others ().
Table 3 Potential therapeutic agents targeting this signaling system
Conclusion
The role of the RANK/RANKL/OPG system is well emphasized in this review article. Beyond regulation of many biological functions throughout the body, the system has a key role in the pathophysiology of various disorders. This triad system can be considered as a hot spot in the area of experimental oncology. This review revealed that the system is strongly implicated in the development of mammary gland structures and tumorigenesis of lobuloalveolar cells of the breast. It has also been shown to take part in the pathogenesis of several solid and nonsolid cancer types including prostate cancer, lung cancer, renal cell carcinoma, melanoma, and leukemia. What is more, the system is highly associated with tumor invasiveness and metastasis. Considering the role of this triad system in cancer cell development and metastasis, scientists are striving to discover therapeutic agents targeting these TNF-related proteins (RANK, RANKL, and OPG) and their downstream signaling pathways for better treatment of cancer and osteoporosis (bone osteolysis) in the near future. Some of these agents include denosumab, enteropeptidase enzymes, small hairpin RNAs, and Jolkinolide B.
Author contributions
All authors contributed toward conception of the original idea, data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work. MS also prepared the final manuscript for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
- HofbauerLCHeufelderAERole of receptor activator of nuclear factor-κB ligand and osteoprotegerin in bone cell biologyJ Mol Med2001795–624325311485016
- WongBRJosienRLeeSYVologodskaiaMSteinmanRMChoiYThe TRAF family of signal transducers mediates NF-κB activation by the TRANCE receptorJ Biol Chem19982734328355283599774460
- BoyceBFXingLBiology of RANK, RANKL, and osteoprotegerinArthritis Res Ther2007911
- ChengMLFongLEffects of RANKL-targeted therapy in immunity and cancerFront Oncol2014332924432249
- FataJEKongYYLiJThe osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland developmentCell20001031415011051546
- HanadaRLeibbrandtAHanadaTCentral control of fever and female body temperature by RANKL/RANKNature2009462727250550919940926
- HanadaRThe new function of RANKL/RANK system in the central nervous systemsNeurosci Res201171e22
- BucayNSarosiIDunstanCROsteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcificationGenes Dev1998129126012689573043
- NdipAWilliamsAJudeEBThe RANKL/RANK/OPG signaling pathway mediates medial arterial calcification in diabetic Charcot neuroarthropathyDiabetes20116082187219621659498
- OsakoMKNakagamiHShimamuraMCross-talk of receptor activator of nuclear factor-κB ligand signaling with renin–angiotensin system in vascular calcificationArterioscler Thromb Vasc Biol20133361287129623580147
- ColemanREMetastatic bone disease: clinical features, pathophysiology and treatment strategiesCancer Treat Rev200127316517611417967
- SchmiedelBJScheibleCANueblingTRANKL expression, function, and therapeutic targeting in multiple myeloma and chronic lymphocytic leukemiaCancer Res201373268369423139212
- DeleaTEMcKiernanJBrandmanJImpact of skeletal complications on total medical care costs among patients with bone metastases of lung cancerJ Thorac Oncol20061657157617409919
- LewisMAHendricksonAWMoynihanTJOncologic emergencies: pathophysiology, presentation, diagnosis, and treatmentCA Cancer J Clin201161528731421858793
- IbrahimTRicciMScarpiEBongiovanniARicciRRivaNRANKL: a promising circulating marker for bone metastasis responseOncol Lett2016122970297527698885
- HerzogCEOverview of sarcomas in the adolescent and young adult populationJ Pediatr Hematol Oncol200527421521815838394
- GrimaudESoubigouLCouillaudSReceptor activator of nuclear factor κB ligand (RANKL)/osteoprotegerin (OPG) ratio is increased in severe osteolysisAm J Pathol200316352021203114578201
- LamoureuxFRichardPWittrantYTherapeutic relevance of osteoprotegerin gene therapy in osteosarcoma: blockade of the vicious cycle between tumor cell proliferation and bone resorptionCancer Res200767157308731817671200
- ChenYDi GrappaMAMolyneuxSDRANKL blockade prevents and treats aggressive osteosarcomasSci Transl Med20157317317ra197
- PearseRNSordilloEMYaccobySMultiple myeloma disrupts the TRANCE/osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progressionProc Natl Acad Sci U S A20019820115811158611562486
- LewinJThomasDDenosumab: a new treatment option for giant cell tumor of boneDrugs Today (Barc)2013491169370024308016
- SantiniDPerroneGRoatoIExpression pattern of receptor activator of NFκB (RANK) in a series of primary solid tumors and related bone metastasesJ Cell Physiol2011226378078420857484
- DougallWCMolecular pathways: osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasisClin Cancer Res201218232633522031096
- Gonzalez-SuarezEJacobAPJonesJRANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesisNature2010468732010310720881963
- AlbanyCHahnNMNovel bone-targeting agents in prostate cancerProstate Cancer Prostatic Dis201417211211824662963
- KieselLKohlARole of the RANK/RANKL pathway in breast cancerMaturitas201686101626921922
- TsubakiMTakedaTYoshizumiMUedaEItohTImanoMRANK–RANKL interactions are involved in cell adhesion-mediated drug resistance in multiple myeloma cell linesTumour Biol20163779099911026762414
- NolanEVaillantFBranstetterDRANK ligand as a potential target for breast cancer prevention in BRCA1-mutation carriersNat Med201622893393927322743
- AzimHAPeccatoriFABrohéeSRANK-ligand (RANKL) expression in young breast cancer patients and during pregnancyBreast Cancer Res20151712425849336
- SiglVOwusu-BoaiteyKJoshiPARANKL/RANK control Brca1 mutation-driven mammary tumorsCell Res201626776177427241552
- ZhangLTengYFanYThe E3 ubiquitin ligase Cbl-b improves the prognosis of RANK positive breast cancer patients by inhibiting RANKL-induced cell migration and metastasisOncotarget2015626229182293326087197
- SchramekDLeibbrandtASiglVOsteoclast differentiation factor RANKL controls development of progestin-driven mammary cancerNature201046873209810220881962
- EmeryJGMcDonnellPBurkeMBOsteoprotegerin is a receptor for the cytotoxic ligand TRAILJ Biol Chem19982732314363143679603945
- HolenICrossSSNeville-WebbeHLOsteoprotegerin (OPG) expression by breast cancer cells in vitro and breast tumours in vivo – a role in tumour cell survival?Breast Cancer Res Treat200592320721516155791
- OwenSYeLSandersAJMasonMDJiangWGExpression profile of receptor activator of nuclear-κB (RANK), RANK ligand (RANKL) and osteoprotegerin (OPG) in breast cancerAnticancer Res201333119920623267146
- WeichhausMChungSTConnellyLOsteoprotegerin in breast cancer: beyond bone remodelingMol Cancer201514111726054853
- TanWZhangWStrasnerATumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signallingNature2011470733554855321326202
- PalafoxMFerrerIPellegriniPRANK induces epithelial–mesenchymal transition and stemness in human mammary epithelial cells and promotes tumorigenesis and metastasisCancer Res201272112879288822496457
- TsubakiMKomaiMFujimotoSIActivation of NF-κB by the RANKL/RANK system up-regulates snail and twist expressions and induces epithelial-to-mesenchymal transition in mammary tumor cell linesJ Exp Clin Cancer Res2013326224011086
- BlakeMLTometskoMMillerRJonesJCDougallWCRANK expression on breast cancer cells promotes skeletal metastasisClin Exp Metastasis201431223324524272640
- PfitznerBMBranstetterDLoiblSRANK expression as a prognostic and predictive marker in breast cancerBreast Cancer Res Treat2014145230731524737168
- FortnerRTSarinkDSchockHOsteoprotegerin and breast cancer risk by hormone receptor subtype: a nested case-control study in the EPIC cohortBMC Med2017152628173834
- CuyàsECorominas-FajaBMartínMMBRCA1 haplo insufficiency cell-autonomously activates RANKL expression and generates denosumab-responsive breast cancer-initiating cellsOncotarget2017821350193503228388533
- WidschwendterMBurnellMFraserLOsteoprotegerin (OPG), the endogenous inhibitor of receptor activator of NF-κB ligand (RANKL), is dysregulated in BRCA mutation carriersBioMedicine201521013311339
- ReyesMEFujiiTBranstetterDPoor prognosis of patients with triple-negative breast cancer can be stratified by RANK and RANKL dual expressionBreast Cancer Res Treat20171641576728417335
- ChenGSircarKAprikianAPottiAGoltzmanDRabbaniSAExpression of RANKL/RANK/OPG in primary and metastatic human prostate cancer as markers of disease stage and functional regulationCancer2006107228929816752412
- HolenICroucherPIHamdyFCEatonCLOsteoprotegerin (OPG) is a survival factor for human prostate cancer cellsCancer Res20026261619162311912131
- LuoJLTanWRiconoJMNuclear cytokine-activated IKKα controls prostate cancer metastasis by repressing MaspinNature2007446713669069417377533
- Odero-MarahVAWangRChuGReceptor activator of NF-κB ligand (RANKL) expression is associated with epithelial to mesenchymal transition in human prostate cancer cellsCell Res200818885887018645583
- MillerRERoudierMJonesJArmstrongACanonJDougallWCRANK ligand inhibition plus docetaxel improves survival and reduces tumor burden in a murine model of prostate cancer bone metastasisMol Cancer Ther2008772160216918606716
- SiampanopoulouMEl Mantani OrdoulidisSMoustakasGThe role of serum osteoprotegerin in metastatic prostate cancer – a case control studyHippokratia201620213313828416910
- TakayamaKInoueTNaritaSInhibition of the RANK/RANKL signaling with osteoprotegerin prevents castration-induced acceleration of bone metastasis in castration-insensitive prostate cancerCancer Lett201739710311028373003
- ChenLMKuoCHLaiTYRANKL increases migration of human lung cancer cells through intercellular adhesion molecule-1 up-regulationJ Cell Biochem2011112393394121328467
- Curioni-FontecedroAHusmannLSoldiniDStahelRAPrimary non-small cell lung cancer response upon treatment with denosumabLung Cancer201382350650824075124
- MillerREJonesJCTometskoMBlakeMLDougallWCRANKL inhibition blocks osteolytic lesions and reduces skeletal tumor burden in models of non-small-cell lung cancer bone metastasesJ Thorac Oncol20149334535424496001
- MikamiSKatsubeKIOyaMIncreased RANKL expression is related to tumour migration and metastasis of renal cell carcinomasJ Pathol2009218453053919455604
- SasakiAIshikawaKHaraguchiNReceptor activator of nuclear factor-κB ligand (RANKL) expression in hepatocellular carcinoma with bone metastasisAnn Surg Oncol20071431191119917195907
- JonesDHNakashimaTSanchezOHRegulation of cancer cell migration and bone metastasis by RANKLNature2006440708469269616572175
- KhanISMouchessMLZhuMLEnhancement of an anti-tumor immune response by transient blockade of central T cell toleranceJ Exp Med2014211576176824752296
- HeymannMFRietALe GoffBBattagliaSPaineauJHeymannDOPG, RANK and RANK ligand expression in thyroid lesionsRegul Pept20081481–3465318367263
- ItoRNakayamaHYoshidaKExpression of osteoprotegerin correlates with aggressiveness and poor prognosis of gastric carcinomaVirchows Arch2003443214615112838418
- MizutaniYMatsubaraHYamamotoKPrognostic significance of serum osteoprotegerin levels in patients with bladder carcinomaCancer200410181794180215386310
- CuiXPengHJinJRANK overexpression as a novel esophageal cancer marker: validated immunohistochemical analysis of two different ethnicitiesInt J Clin Exp Pathol2015822249225825973136
- SchmiedelBJGrosse-HovestLSalihHRA “vicious cycle” of NK-cell immune evasion in acute myeloid leukemia mediated by RANKL?Oncoimmunology201325e2385023762785
- SuTLiJMengMBone marrow stromal cells induced activation of nuclear factor κB signaling protects non-Hodgkin’s B lymphoma cells from apoptosisTumour Biol2016378107451075226873486
- SchmohlJUNueblingTWildJKroellTKanzLSalihHRExpression of RANK-L and in part of PD-1 on blasts in patients with acute myeloid leukemia correlates with prognosisEur J Haematol201697651752727096305
- KostenuikPJNguyenHQMcCabeJDenosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKLJ Bone Miner Res200924218219519016581
- PeddiPLopez-OlivoMAPrattGFSuarez-AlmazorMEDenosumab in patients with cancer and skeletal metastases: a systematic review and meta-analysisCancer Treat Rev20133919710422898302
- PalmeriniEChawlaNSFerrariSDenosumab in advanced/unresectable giant-cell tumour of bone (GCTB): for how long?Eur J Cancer20177611812428324746
- van der HeijdenLDijkstraPDSBlayJYGelderblomHGiant cell tumour of bone in the denosumab eraEur J Cancer201777758328365529
- NolanEVaillantFBranstetterDRANK ligand as a potential target for breast cancer prevention in BRCA1-mutation carriersNat Med201622893393927322743
- HeathDJVanderkerkenKChengXAn osteoprotegerin-like peptidomimetic inhibits osteoclastic bone resorption and osteolytic bone disease in myelomaCancer Res200767120220817210700
- KimHChoiHKShinJHSelective inhibition of RANK blocks osteoclast maturation and function and prevents bone loss in miceJ Clin Invest2009119481382519258703
- TéletchéaSStresingVHervouetSNovel RANK antagonists for the treatment of bone-resorptive disease: theoretical predictions and experimental validationJ Bone Miner Res20142961466147724390798
- ZhaoYJinMMaJInhibition effect of enteropeptidase on RANKL–RANK signalling by cleavage of RANKFEBS Lett2013587182958296423954298
- NaiduVGBabuKRThwinMMSatishRLKumarPVGopalakrishnakonePRANKL targeted peptides inhibit osteoclastogenesis and attenuate adjuvant induced arthritis by inhibiting NF-κB activation and down regulating inflammatory cytokinesChem Biol Interact2013203246747923333834
- MaRXuJDongBKautherMDJägerMWedemeyerCInhibition of osteoclastogenesis by RNA interference targeting RANKBMC Musculoskelet Disord20121315422913338
- BrennanTCRybchynMSGreenWAtwaSConigraveADMasonRSOsteoblasts play key roles in the mechanisms of action of strontium ranelateBr J Pharmacol200915771291130019563530
- XiuYXuHZhaoCChloroquine reduces osteoclastogenesis in murine osteoporosis by preventing TRAF3 degradationJ Clin Invest2014124129731024316970
- MaXLiuYZhangYYuXWangWZhaoDJolkinolide B inhibits RANKL-induced osteoclastogenesis by suppressing the activation NF-κB and MAPK signaling pathwaysBiochem Biophys Res Commun2014445228228824491533
- HaHShimKSKimTWater extract of Acer tegmentosum reduces bone destruction by inhibiting osteoclast differentiation and functionMolecules20141943940395424694651
- IhnHJKimJABaeYCShinHIBaekMCParkEKAfatinib ameliorates osteoclast differentiation and function through downregulation of RANK signaling pathwaysBMB Rep201750315015528256196