Abstract
Background
Interleukin (IL)-35 is a novel inhibitory cytokine and has recently been implicated in tumor immunity. However, the role of IL-35 in prostate cancer (PCa) has not been elucidated.
Objective
To evaluate the role of plasma IL-35 in the diagnosis and prognosis of PCa in Chinese patients undergoing initial prostate biopsy.
Materials and methods
Using ELISA, plasma IL-35 levels were measured in 180 patients, who underwent a prostate biopsy. The clinical correlation of IL-35 with clinicopathological parameters was also evaluated. Univariate and multivariate logistic regression and receiver operating characteristic (ROC) curve analysis were performed to establish the role of IL-35 as a clinical biomarker.
Results
Seventy-five (41.6%) of patients were histopathologically confirmed to have PCa. Plasma IL-35 levels were significantly higher in PCa patients (134.48±78.48 pg/mL) compared to non-PCa patients (67.22±24.08 pg/mL). ROC analysis showed that IL-35 was an independent predictor of PCa. Furthermore, IL-35 was found to be a significantly independent predictor of PCa in a group of patients with prostate-specific antigen levels between 4 and 10 ng/mL; was also able to predict advanced PCa from localized PCa and bone metastasis positive PCa from negative PCa.
Conclusion
Our data suggest for the first time that plasma IL-35 levels are correlated with PCa and is the independent predictor of PCa progression and metastasis. Thus, IL-35 could be utilized as a potential biomarker for diagnosis and prognosis of PCa, could also aid in decision making and predict the stage of the disease.
Introduction
Prostate carcinoma (PCa) is one of the most common cancers among men, and there has been a significant increase in the incidence of PCa over the recent decades.Citation1 The early detection and management of early-stage PCa are particularly important and challenging issues. Currently-used screening tests for PCa mainly include serum prostate-specific antigen (PSA) levels, digital rectal examination (DRE) and transrectal ultrasonography (TRUS),Citation2 which play a significant role in the detection and staging of patients suffering from the disease.Citation3 TRUS-guided biopsy of the prostate in patients with an elevated PSA and an abnormal finding on DRE, remains the gold standard for the detection of PCa and at least 10–12 biopsy cores are required.Citation2 However, the sensitivity and specificity of TRUS-guided biopsy in the detection of PCa is low. Therefore, more reliable biomarkers that have a high specificity for the detection, diagnosis, and prognosis of PCa are desirable.
More recently, evidence showed that the development and progression of urologic cancers are associated with an imbalance in the self-related immunity.Citation4–Citation6 Interleukin (IL)-35 belongs to IL-12 family of cytokines, which is a novel, responsive anti-inflammatory cytokine.Citation7 IL-35 is a heterodimer comprised of the p35 subunit of IL-12 and EBI3 subunit of IL-27.Citation8 IL-35 exhibits immunosuppressive effects and is primarily expressed by Foxp3+ regulatory T cells (Tregs).Citation9 Recent studies have revealed that IL-35 is widely distributed and mediate its effects both in vivo and in vitro including an ability to suppress T-cell proliferation.Citation7 Furthermore, IL-35 induces the conversion of conventional T-cells into an IL-35-expressing inducible Treg (iTr35 cells), which downregulate the development and differentiation of Th17 cell,Citation10 and by which it suppress the progression of inflammatory diseases and autoimmune diseases. Recent studies have also discovered the role of IL-35 in inducing IL-10-producing regulatory B-cells (Breg-cells) and promote their conversion to IL-35+ Breg cells, which has a role in autoimmune diseases and cancer.Citation11
Recently, gene expression analyses have suggested a broader tissue distribution of IL-35, including expression in cancer cells and Tregs.Citation12 Further, the immunohistochemical analysis also revealed that EBI3 and p35 are highly expressed in placental trophoblasts and tumor tissues from lung cancer, colon cancer, esophageal carcinoma, hepatocellular carcinoma, and cervical carcinoma.Citation13,Citation14 Taken together, these findings suggest that IL-35 plays a critical role in tumor development and progression. In tumor microenvironment, Foxp3+ Tregs and other Tregs are considered to be the primary producer of IL-35,Citation15 and IL-35 has also been found to promote the suppression of Th1, Th17 and Th2 cells.Citation10 The IL-35 expression has been associated with immunosuppression, tumor progression, and poor prognosis. Previous studies have also shown the correlation of EBI3 expression with poor prognosis of human lung cancer.Citation16 Moreover, by enhancing myeloid cells accumulation in the tumor microenvironment, tumor-cell derived IL-35 promotes tumor growth, as well as tumor angiogenesis.Citation17 Though, Olson et al reported that human prostate tumor antigen-specific Tregs can be inhibited by IL-35 blockade.Citation18 However, to the best of our knowledge, the clinical correlation of IL-35 and clinicopathological parameters of PCa has not been established.
The present study was initiated to investigate the role of plasma IL-35 levels in distinguishing PCa from non-PCa, and also to determine plasma IL-35 levels in patients who underwent initial prostate biopsy. Further, the plasma IL-35 levels were analyzed and correlated with clinicopathological parameters to evaluate its role as a potential biomarker in PCa.
Materials and methods
Study subjects
One hundred and eighty patients who underwent initial TRUS-guided prostate biopsy at the First Affiliated Hospital of Soochow University, from January 2015 to December 2015 were enrolled for the present study. Patients with autoimmune or inflammatory diseases, secondary or multiple malignancies and drug treatment for benign prostatic hyperplasia (BPH) were excluded from the study. Written informed consent was obtained from all patients. The study was approved by the Ethics Committees of Soochow University.
Sample collection and processing
Blood samples (3 mL) were collected early morning after an overnight (12 h) fast using EDTA tubes, before any treatment. None of the patients had received biopsy, surgery, chemotherapy, or radiotherapy before blood collection. All blood samples were processed within 2 h of collection. Plasma samples were isolated by centrifugation for 15 min at 1,200 g, then transferred to new tubes and stored at −80°C.
ELISA analysis
The plasma levels of IL-35 in patients’ samples were analyzed using human IL-35 ELISA kit (Cloud-Clone Corp, Wuhan, People’s Republic of China), according to manufacturer’s protocol. Samples were assayed in duplicate.
Transrectal ultrasound guided prostate biopsy
Ultrasound consisted of grayscale and color Doppler imaging using a Sequoia 512 unit with a convex 8 MHz transrectal probe (Siemens, Munich, Germany). All prostate biopsies were performed following a 12-core scheme using 18G automatic core biopsy needle (CR Bard Inc., Govington, GA, USA). When a hypoechoic area or suspicious lesion was noted under ultrasound, one or two additional cores were also collected. Clinicopathological characteristics of the patients including PSA, total prostate volume, prostate specific antigen density, tumor node metastasis (TNM) stage, histological grade and age at diagnosis were obtained from medical records.
Statistical analysis
All the statistical analyses were performed using SPSS V19.0 software (IBM Corporation, Armonk, NY, USA) and GraphPad5 (GraphPad Software, La Jolla, CA, USA). Data are presented as the mean ± standard deviation (SD). Using χ2 test, the differences between the clinicopathological characteristics of the PCa and the non-PCa group were compared. Continuous variables were measured by Kolmogorov–Smirnov for testing the fitting of data to normal distribution. Student’s t-test was used for parametric data and Mann–Whitney test for non-parametric data. Significance between groups was evaluated by ANOVA. Two-sided P-values were calculated, and P<0.05 was considered as statistically significant. Important variables were then subjected to multivariate logistic regression analyses. Then a receiver operating characteristic (ROC) curve analysis was applied and area under curve (AUC) was calculated to determine the predictive value of each independent variable.
Results
Basic clinicopathological characteristics of patients
According to the histopathologic examinations, 75 (41.6%) of 180 patients were diagnosed with PCa and 105 (58.4%) patients were non-PCa. The comparisons between the two groups are presented in . Plasma IL-35 levels were significantly higher in PCa patients than non-PCa patients (134.48±78.48 vs 67.22±24.08 pg/mL, P<0.001; ). In addition, the significant differences of serum PSA levels (51.94±8.61 vs 13.46±1.01 ng/µL, P<0.001) and age (70±7.49 vs 63±7.96 years, P<0.001), were observed between the two groups.
Figure 1 (A) Plasma IL-35 levels between PCa and non-PCa patients. (B) Plasma levels of IL-35 among non-PCa patients, localized PCa and advanced PCa (C) Plasma IL-35 levels in bone metastasis positive patients (M1) and negative patients (M0). (D) Plasma IL-35 levels between HG and non-HG PCa patients.
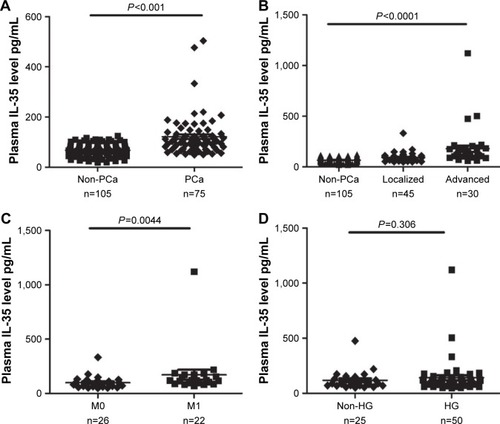
Table 1 Clinical characteristics of PCa patients and non-PCa patients
Correlation of IL-35 and clinicopathological characteristics
The significantly associated clinicopathological parameters including PSA, plasma IL-35 levels and age between the PCa and non-PCa patients are presented in . These parameters were further analyzed using logistic regression analysis. The result of univariate analysis showed that PCa was significantly associated with IL-35 (P<0.001), PSA (P<0.001) and age (P<0.001). The result of multivariate analysis showed that IL-35, PSA, and age were independent factors in predicting the presence of PCa (P<0.05). Area under ROC curve (AUC) of IL-35 was significantly higher than PSA (AUC =0.820 vs 0.703, P<0.05; ), the sensitivity was 77.3%, specificity was 73.3%, and diagnostic accuracy was 78.3%. When we combined IL-35 and PSA as PCa predictor, diagnostic efficiency had a remarkable improvement (AUC =0.879 vs 0.820 or 0.703, P<0.05; ), the sensitivity was 80.3%, specificity was 85.7%.
Figure 2 (A) ROC curve analysis of IL-35 and PSA between PCa and non-PCa patients. (B) ROC curve analysis of IL-35 and PSA patients with PSA levels of 4 to 10 ng/mL. (C) ROC curve analysis of IL-35 and PSA, to discriminate advanced PCa patients from localized PCa patients. (D) ROC curve analysis using IL-35 and PSA, to discriminate positive bone metastasis patients from negative bone metastasis patients.
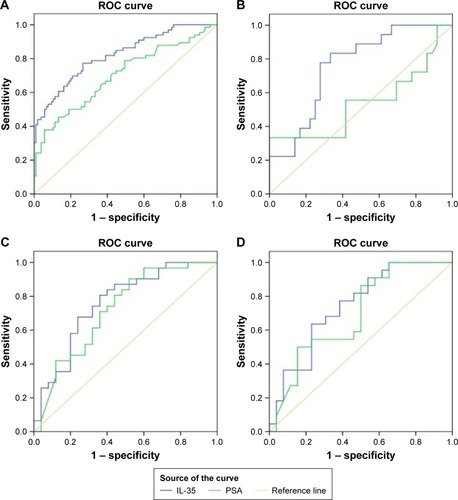
Figure 3 IL-35+ PSA combination as prostate cancer predictor.
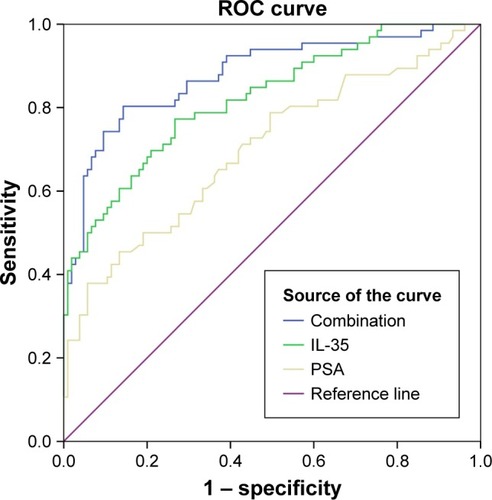
Table 2 Univariate and multivariate analysis for prognostic factors in patients with PCa
Moreover, patients with PSA levels of 4–10 ng/mL were also classified into two groups. In Group I of 57 men, a significant difference was found between IL-35 and PSA for the detection of PCa. At the best the cut-off values of 78.7 pg/mL, the sensitivity, specificity and diagnostic accuracy of IL-35 were 71.4%, 66.4% and 83.9%, respectively. A statistically significant difference was observed between IL-35 and PSA in the diagnosis of PCa (AUC 0.78 vs 0.54, P<0.05; ).
Association of IL-35 and PCa stage
Based on the standard for TNM stage, PCa patients were classified into two groups: localized PCa patients (stage I–II, n=45) and advanced PCa patients (stage III–IV, n=30). As illustrated in , plasma IL-35 levels in advanced PCa patients were significantly higher than in the localized PCa (164.57±116.82 vs 95.38±53.52 pg/mL, P<0.01). ROC analysis showed that plasma IL-35 level was the better predictor of advanced PCa than PSA (AUC 0.757 vs 0.709, P<0.05; ). At the cutoff value of 88.9 pg/mL, IL-35 could effectively discriminate advanced PCa with a sensitivity of 80.6%, a specificity of 64% and a diagnostic accuracy of 73.9%.
In the present study, a total of 48 PCa patients underwent bone scan analysis, and 22 (45.83%) of them were found to be positive for bone metastasis, and as expected, the plasma IL-35 levels were significantly higher in bone metastasis positive patients than the negative bone metastasis patients (172.36 146.02 vs 99.65±57.52 pg/mL; ), and the ROC analysis showed that plasma IL-35 levels (AUC 0.741) were the better predictor than PSA (AUC 0.694; ).
Correlation of IL-35 and Gleason score of PCa
On the basis of the Gleason score, PCa patients were again classified into two groups, high grade (HG; Gleason score ≥8, n=50) and non-high grade (non-HG, Gleason score ≤7, n=25). Interestingly, no significant difference was observed for IL-35 (P=0.306; ) when PSA showed a significant difference between the two groups (P<0.05).
Discussion
IL-35, the most recently identified member of the IL-12 family, and similar to other members of the IL-12 family (including IL-12, IL-23, and IL-27), IL-35 is composed of a heterodimer of α and β chains.Citation7 IL-12 comprised of p35 and p40 subunits, while, IL-27 has been identified to consist of EBI3 subunits and p28 subunits. IL-35 plays important role in anti-inflammatory responses and has been associated with reduced graft-versus-host diseases (GVHDs) from allogeneic hematopoietic stem cell transplantation (allo-HSCT) and autoimmune diseases.Citation19,Citation20 More recently, IL-35 has been recognized to play a significant role in the patho genesis of tumors, progression and prognosis and tumor derived IL-35 has been confirmed to promote tumor progression in two tumor models, including plasmacytoma and B16 melanoma.Citation17 In addition, the expression of IL-35 has been associated with tumor progression as well as poor prognosis. However, the role of IL-35 in prostate tumorigenesis, progression, and prognosis, remains to be elucidated.
To the best of our knowledge, the present study provides the first report on the significant association of increased plasma IL-35 levels in histopathologically confirmed patients of PCa in comparison to non-PCa patients. The ROC curve indicated that high plasma IL-35 levels (81.3 pg/mL) could effectively predict the presence of PCa. ROC curves were compared to significantly discriminate the effect of PSA and plasma IL-35 levels. The results indicated that plasma levels of IL-35 are a better predictor than serum PSA levels in discriminating PCa patients especially when the levels of PSA is between 4 and 10 ng/mL.
Moreover, we found that in advanced PCa patients, the concentration of IL-35 in plasma was significantly higher than in localized PCa patients, which indicate IL-35 a better predictor in discriminating advanced from localized PCa patients. However, plasma levels of IL-35 were not found to be associated with Gleason scores, and only a trend towards higher Gleason scores was observed, which may be attributed to a lower number of PCa patients in the study.
Previous studies have suggested that IL-35 is a member of Tregs cytokine family and is required for their function. In vivo, IL-35 is mainly expressed by Tregs and promotes the differentiation of naïve T cells into IL-35 induced Tregs (iTr35 cells).Citation7,Citation9,Citation10 Furthermore, gene expression analysis revealed that IL-35 have a broader tissue distribution. EBI3 was detected in Hodgkin lymphoma cells and acute myeloid leukemia cells.Citation21,Citation22 Thus, we believe that IL-35 not only mediates the biological function of Tregs but also contributes to the tumor microenvironment. More recently, human prostate tumor antigen-specific CD8+ Treg cells were found to be inhibited by CTLA-4 or IL-35 blockade.Citation18 Turnis et alCitation23 further suggested that IL-35 limits anti-tumor immunity. However, contradictory results have also been reported.Citation13 Therefore, further studies on the immunological mechanisms of IL-35 in PCa are desired.
In the present study, plasma IL-35 levels were found to be significantly positively associated with tumor stage and bone metastasis, two important indicators of poor outcomes. Given the roles of IL-35 in protecting tumor cells against immunity, these evidences further support our hypothesis that IL-35 might promote the progression of PCa. Furthermore, higher levels of IL-35 in the tumor microenvironment are likely to play a significant role in PCa progression and metastasis. This is consistent with the previous findings on other carcinomas.Citation24–Citation27 Therefore, monitoring IL-35 levels could aid in medical decision making among Chinese men undergoing a prostate biopsy and may save unnecessary biopsies.
The present study does have its own limitations and strengths. The major limitation of the present study is the small sample size. Also, lack of tissues samples for the examination of IL-35 made it difficult to elucidate the mechanism of IL-35 in the tumor microenvironment. However, the present study was first to show the association of plasma IL-35 with PCa progression and metastasis.
Conclusion
Plasma levels of IL-35 were significantly higher in PCa patients, also higher plasma IL-35 levels were positively associated with advanced stage of PCa, indicating that plasma IL-35 is a predictable indicator which could aid in decision making among patients undergoing an initial prostate biopsy.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grants 81500572 and 81272839).
Disclosure
The authors report no conflicts of interest in this work.
References
- FerlayJShinHRBrayFFormanDMathersCParkinDMEstimates of worldwide burden of cancer in 2008: GLOBOCAN 2008Int J Cancer2010127122893291721351269
- HeidenreichAAusGBollaMEAU guidelines on prostate cancerEur Urol20085316880 Available from: https://www.ncbi.nlm.nih.gov/pubmed/?term=17920184Accessed June 22, 201717920184
- PallweinLMitterbergerMPelzerAUltrasound of prostate cancer: recent advancesEur Radiol200818470771517938936
- SankhwarMSankhwarSNAbhishekARajenderSClinical significance of the VEGF level in urinary bladder carcinomaCancer Biomark201515434935525792473
- MontironiRSantoniMSotteVEmerging Immunotargets and Immunotherapies in Prostate CancerCurr Drug Targets201617777778226898309
- LeclercBGCharleboisRChouinardGCD73 Expression Is an Independent Prognostic Factor in Prostate CancerClin Cancer Res201622115816626253870
- CollisonLWWorkmanCJKuoTTThe inhibitory cytokine IL-35 contributes to regulatory T-cell functionNature2007450716956656918033300
- DevergneOBirkenbachMKieffEEpstein-Barr virus-induced gene 3 and the p35 subunit of interleukin 12 form a novel heterodimeric hematopoietinProc Natl Acad Sci USA1997942212041120469342359
- NiedbalaWWeiXQCaiBIL-35 is a novel cytokine with therapeutic effects against collagen-induced arthritis through the expansion of regulatory T cells and suppression of Th17 cellsEur J Immunol200737113021302917874423
- CollisonLWChaturvediVHendersonALIL-35-mediated induction of a potent regulatory T cell populationNat Immunol201011121093110120953201
- WangRXYuCRDambuzaIMInterleukin-35 induces regulatory B cells that suppress autoimmune diseaseNat Med201420663364124743305
- LiXMaiJVirtueAIL-35 is a novel responsive anti-inflammatory cytokine – a new system of categorizing anti-inflammatory cytokinesPLoS One201273e3362822438968
- LongJZhangXWenMIL-35 over-expression increases apoptosis sensitivity and suppresses cell growth in human cancer cellsBiochem Biophys Res Commun2013430136436923154182
- MaoHGaoWMaCHuman placental trophoblasts express the immunosuppressive cytokine IL-35Hum Immunol201374787287723619469
- LiyanageUKMooreTTJooHGPrevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinomaJ Immunol200216952756276112193750
- NishinoRTakanoAOshitaHIdentification of Epstein-Barr virus-induced gene 3 as a novel serum and tissue biomarker and a therapeutic target for lung cancerClin Cancer Res201117196272628621849417
- WangZLiuJQLiuZTumor-derived IL-35 promotes tumor growth by enhancing myeloid cell accumulation and angiogenesisJ Immunol201319052415242323345334
- OlsonBMJankowska-GanEBeckerJTVignaliDABurlinghamWJMcNeelDGHuman prostate tumor antigen-specific CD8+ regulatory T cells are inhibited by CTLA-4 or IL-35 blockadeJ Immunol2012189125590560123152566
- LiuYWuYWangYIL-35 mitigates murine acute graft-versus-host disease with retention of graft-versus-leukemia effectsLeukemia201529493994625363669
- OlsonBMSullivanJABurlinghamWJInterleukin 35: a key mediator of suppression and the propagation of infectious toleranceFront Immunol2013431524151492
- NiedobitekGPazoltDTeichmannMDevergneOFrequent expression of the Epstein-Barr virus (EBV)-induced gene, EBI3, an IL-12 p40-related cytokine, in Hodgkin and Reed-Sternberg cellsJ Pathol2002198331031612375263
- PoleganovMABachmannMPfeilschifterJMuhlHGenome-wide analysis displays marked induction of EBI3/IL-27B in IL-18-activated AML-derived KG1 cells: critical role of two kappaB binding sites in the human EBI3 promotorMol Immunol200845102869288018336908
- TurnisMESawantDVSzymczak-WorkmanALInterleukin-35 Limits Anti-Tumor ImmunityImmunity201644231632926872697
- ZhangYSunHWuHTanQXiangKInterleukin 35 is an independent prognostic factor and a therapeutic target for nasopharyngeal carcinomaContemp Oncol (Pozn)201519212012426034389
- JinPRenHSunWXinWZhangHHaoJCirculating IL-35 in pancreatic ductal adenocarcinoma patientsHum Immunol2014751293324121041
- ZengJCZhangZLiTYAssessing the role of IL-35 in colorectal cancer progression and prognosisInt J Clin Exp Pathol2013691806181624040445
- GuXTianTZhangBElevated plasma interleukin-35 levels predict poor prognosis in patients with non-small cell lung cancerTumour Biol20153642651265625480413
