Abstract
Background
Toll-like receptors (TLRs) play a critical role in cancer, yet the clinical relevance of TLR7/8 expression in melanoma remains unclear. This study aimed to evaluate the prognostic value of TLR7/8 mRNA levels in melanoma and their correlation with immune biomarkers relevant to disease progression.
Methods
Normalized gene expression and corresponding clinical data of patients with skin cutaneous melanoma were obtained from two public databases: the Cancer Genome Atlas and GSE19234. Log rank (Mantel–Cox) tests were used to perform survival analysis. Multivariate survival analysis was performed on a Cox-regression hazard model. Spearman correlation analyses were used to address the relationship between the expressions of TLR7/8 levels and immune biomarkers in melanoma tumors.
Results
Survival analysis suggested that high levels of TLR7 or TLR8 expression predicted better clinical outcome for melanoma patients (TLR7: HR =1.734, P<0.0001; TLR8: HR =2.072, P<0.0001). Moreover, multivariate survival analysis implicated TLR7 as a prognostic factor independent of age, gender, or pathological stage. Further analysis demonstrated that expression levels of TLR7/8 strongly correlated with that of dendritic cell markers and chemokines/chemokine receptors, including CCR2, CCR5, CCL3, and CCL5. Importantly, expression levels of both TLR7 and TLR8 were also highly correlated with the expressions of CD8 and other functional markers of CD8+ T cells.
Conclusion
High gene expression of TLR7 and TLR8 in melanoma tumors is associated with high expression levels of functional markers of immune cells, which predicts longer overall survival of patients with melanoma. Our results not only provide an important reference for the clinical prognosis of melanoma but also present new implications for the design of melanoma immunotherapy.
Introduction
Toll-like receptors (TLRs) are signaling molecules that recognize pathogen-associated molecular patterns derived from bacteria, virus, or fungi.Citation1 Activation of TLRs triggers several signaling pathways and regulates downstream expression of a variety of genes. TLRs are, therefore, crucial for both innate immunity and adaptive immune responses.Citation2 Aberrant regulation of TLRs in human skin could, in part, lead to chronic inflammation and contribute to melanoma tumorigenesis. TLR7 and TLR8 (TLR7/8) are expressed in immune cells, including dendritic cells (DCs), as well as in keratinocytes and melanoma cells.Citation3 TLR7 binds to single-stranded RNA (ssRNA) of viruses or bacteria. TLR8 is phylogenetically related to TLR7 and, like TLR7, binds to viral ssRNAs and foreign bacteria.Citation4 Specific agonists of TLR7 and TLR8 have been used as targeted therapies in a variety of cancers, including pancreatic and bladder cancers, basal cell carcinoma, and melanoma.Citation1,Citation3,Citation5–Citation7
Activation of TLRs using agonists can modulate the signaling pathways of MyD88/NF-kB and interferon (IFN) regulatory factors (IRF) 3/7. TLR7/8 activation may thus result in the upregulation of chemokines and their receptors, either positively or negatively affecting tumor infiltration of immune cells.Citation8,Citation9 Although TLR7 agonists have been used to treat cancer patients, their ability to generate tumor-specific immunity and to kill tumor cells directly by activating innate immune responses remains controversial.Citation10,Citation11 Moreover, it has been theorized that TLR7 agonists may provoke inflammation and promote tumorigenesis in some cancer types.Citation12 Though few studies have addressed the clinical relevance of TLR7/8 in terms of its prognostic value in cancer and its association with immunological biomarkers, it is imperative to address the precise role of TLR7/8 during cancer progression.
In this study, we performed a systematic analysis of tumor TLR7/8 expression levels and survival data from a large cohort of melanoma patients provided by the publicly available databases, The Cancer Genome Atlas (TCGA) and GSE19234. Our data highlight the prognostic value of TLR7/8 in melanoma and we address its association with the expression of immune cell markers.
Methods
Skin cutaneous melanoma database query and processing
The calculated and normalized gene expression (level 3) data, and corresponding clinical data, of 473 melanoma samples were acquired from TCGA through the UCSC cancer browser.Citation13 We excluded duplicate samples from same patients and samples from patients without available survival data, with a final cohort of 427 patients for survival analysis. The clinical information of skin cutaneous melanoma (SKCM) patients used in this study included age, gender, and pathological TNM (tumor, node, and metastases) stage. Among the cohort of 427 patients, there were ~10% of patients who received radiation therapy (21/214 for TLR7 high vs 20/213 for TLR7 low) prior to the sample collection. For GSE19234 database sampling,Citation14 gene expression and clinical data were downloaded from the Gene Expression Omnibus (GEO) and processed using the R package “GEOquery”.
Survival analysis
To evaluate the prognostic value of TLR7/8, we categorized the patients into two groups, high and low, based on the median of TLR7 or TLR8 gene expression, respectively. Survival analysis between the “high” group and the “low” group in each analysis was assessed by Kaplan–Meier survival analysis and compared by two-sided log-rank test. To determine whether the prognostic value of TLR7/8 was independent of other clinical variables (eg, age, gender, and tumor stage), multivariate Cox regression was performed on each dataset by using the R package “survival”, with survival as the dependent variable and TLR7 expression and other clinical variables as explanatory variables. Hazard ratios (HR) and 95% confidence intervals (CI) were calculated.
Correlation analysis
Pearson’s correlation tests were used to study the association between the expressions of TLR7/8 and genes of interest in tumor samples. HRs and 95% CIs were then calculated. All graphical presentations were done using GraphPad Prism 6.0.
Results
High levels of TLR7/8 expression predict better prognoses in patients with melanoma
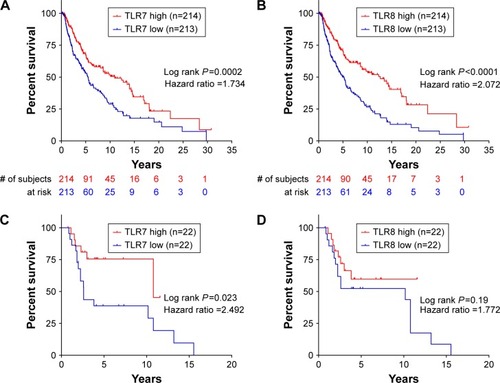
Taking advantage of the publicly available database TCGA, we accessed the normalized tumor gene expression levels and corresponding clinical data of 473 SKCM patients. As shown in , our survival analysis indicated that high TLR7 expression levels predict longer overall survival times (HR =1.734, P=0.0002) for these patients. Similar results were observed in the survival analysis stratified with TLR8 expression levels (HR =2.072, P<0.0001, ). To validate these results, we analyzed a second dataset, GSE 19234,Citation14 with gene expression levels and clinical data of 44 melanoma patients. Despite the smaller patient cohort, our survival analysis with the GSE19234 database indicated that high levels of TLR7 expression also predict longer overall survival times (HR =2.492, P=0.023, ). A similar trend was observed in our survival analysis stratified with TLR8 expression levels (HR =1.772, P=0.19, ).
Figure 1 Transcriptional levels of TLR7 and TLR8 predict the clinical outcome of patients with melanoma. Survival analysis of melanoma patients (TCGA database) is shown with high and low expressions of TLR7 (A) and TLR8 (B). Survival analysis of melanoma patients (GSE19234) is shown with high and low expressions of TLR7 (C) and TLR8 (D). The patients were divided into two groups based on the median mRNA expression levels of TLR7 and TLR8.
Both TLR7 and TLR8 genes are located in human chromosome Xp22.2.Citation4 We, therefore, sought to assess the association between TLR7 and TLR8 expression levels. Our results indicated a strong correlation between the expression levels of TLR7 and TLR8 (r=0.8903, P<0.0001, ). Next, we performed multivariate survival analysis and found that high TLR7 expression levels were significantly associated with better prognosis independent of other variables (). These results suggest that TLR7 is a prognostic factor of melanoma independent of age, gender, and TNM stage.
Table 1 Correlation analysis of TLR7/8 and genes of interest
Table 2 Multivariate cox analyses applied to survival model
TLR7/8 expression levels are correlated with the expression of CD11b and CD11c
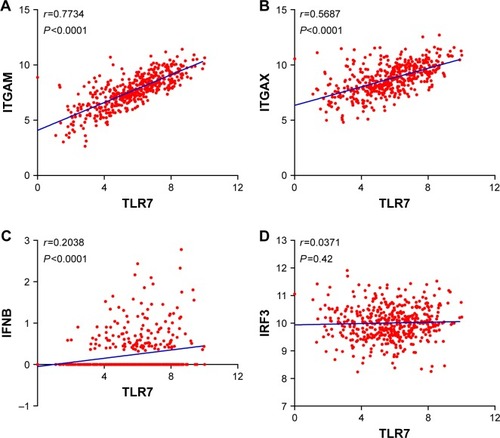
TLR7/8 has been found to be expressed in macrophages, monocytes, and DCs. We, therefore, assessed the correlation between the expressions of TLR7/8 levels and DC-specific markers. We found a strong correlation between the expressions of TLR7 levels and CD11b (ITGAM) and CD11c (ITGAX) levels ( and ). Expression levels of TLR7 correlated well with that of FLT3G, an important marker for a subset of DCs, but not with the levels of IFNβ (IFNB1) or IRF3. These findings confirm the relationship between TLR7/8 expression and DC infiltration, though the role TLR7 plays in activating downstream signaling requires further functional analysis.
Figure 2 Correlation analysis between expressions of TLR7 and factors associated with dendritic cells (DC). Correlation analysis between (A) TLR7 and ITGAM, (B) TLR7 and ITGAX, (C) TLR7 and IFNB, and (D) TLR7 and IRF3.
Abbreviations: TLR, Toll-like receptor; IRF3, interferon regulatory factor 3; IFNB, interferon B.
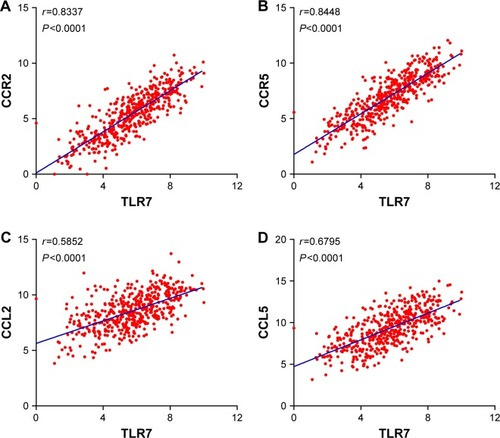
Expression levels of TLR7/8 are correlated with that of chemokines and their receptors
To test whether TLR7/8 is involved in immune cell infiltration and trafficking, we performed correlation analysis between the expression levels of TLR7/8 and chemokines and their receptors. Our data showed that the levels of TLR7 strongly correlated with that of CCR2 (r=0.8337, P<0.0001) and CCR5 (r=0.8448, P<0.0001) ( and ). There was also a correlation between the expression levels of TLR7/8 and chemokine ( and ).
Expression levels of TLR7/8 are correlated with that of CD8+ T-cell functional markers
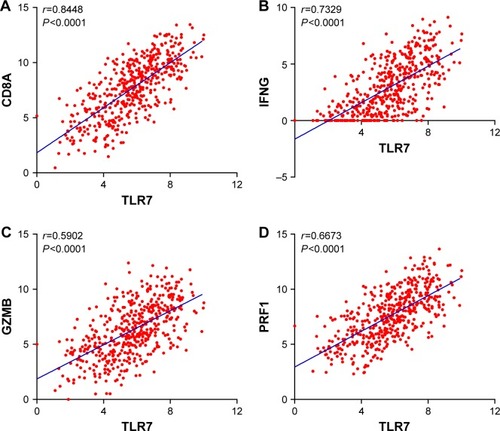
Because high levels of CD8+ T-cell infiltration have been shown to be associated with better survival rates in patients with melanoma,Citation15 we sought to investigate the correlation between the expression levels of TLR7 and CD8+ T-cell response-related genes. As shown in and , we found that TLR7 expression levels are significantly associated with CD8A expression (r=0.7329, P<0.0001). More importantly, expression levels of TLR7/8 also correlated with genes such as perforin (PRF1), granzyme B (GZMB), and IFNg (IFNG), which are well-known indicators of CD8+ T-cell cytotoxic function. These findings suggest that TLR7/8 expression is associated with CD8+ T-cell tumor infiltration and functions.
Figure 4 Correlation analysis between expression levels of TLR7 and CD8+ cytotoxic T-cell markers. Correlation analysis between (A) TLR7 and CD8A, (B) TLR7 and IFNG, (C) TLR7 and GZMB, and (D) TLR7 and PRF1.
Abbreviations: TLR, Toll-like receptor; IFNG, interferon gamma.
Discussion
TLRs are important components of innate immunity in skin tissues and their dysregulation has been shown to contribute to the development of melanoma.Citation1,Citation16 The role of TLRs in cancer is controversial, as increased expression and activation of these proteins may result in pro- or antitumorigenic effects depending on the cellular context. Ligand activation of TLRs on DCs triggers type-I IFN expression and contributes to killing and removing cancer cells.Citation17 It was previously reported that elevated TLR9 expression levels are associated with better overall survival in patients with breast cancer.Citation18 However, some studies also have reported that aberrant activation of TLR enhances inflammation and promoted tumorigenesis.Citation16,Citation17 Noteworthy, a recent study that immunohistologically analyzed TLR7 expression levels in a small number (n=30) of cutaneous malignant melanoma patients did not establish a significant correlation between TLR7 protein expression and relapse-free survival.Citation12 Thus, it is imperative to clarify the prognostic value of TLR7 using a larger patient cohort. In our current study, we analyzed the TCGA SKCM database, with more than 400 patients’ data, and revealed the clinical prognostic value of TLR7/8 in melanoma. Here, we observed that patients with high tumor expression levels of TLR7/8 had longer overall survival rates. Our results were validated using another published dataset, GSE19234. Moreover, according to multivariate analysis, high expression level of TLR7/8 protein was a prognostic factor independent of age, gender, or TNM stage. To the best of our knowledge, this is the first report to demonstrate a positive prognostic value of TLR7/8 in melanoma.
TLRs are expressed on the surface of immune cells, such as DCs, which are antigen-presenting cells. In agreement, our data show a strong correlation between the expressions of TLR7/8 and DC-specific markers, such as CD11c. The binding of endogenous ligands or synthetic agonists to TLRs triggers a cascade of signaling events and the upregulation of several chemokines and their receptors.Citation16 Indeed, intratumoral administration of a TLR7/8 agonist generated systemic antitumor immunity against B16.F10 melanoma tumors accompanied by an increase in the levels of CCL2 and infiltration of M1 phenotype–shifted macrophages.Citation19 TLR7/8 activation also promotes tumor eradication by activating a combination of immune cells, chemokines, and chemokine receptors at the tumor site.Citation20,Citation21 In parallel, our data indicate that expression levels of TLR7/8 are strongly correlated with that of CCR2, CCR5, and their respective ligands, CCL2, CCL3, CCL4, and CCL5. We speculate that high levels of TLR7/8 in melanoma induce a chemoattracting microenvironment, ultimately resulting in the recruitment of immune cells to the tumor site.
TLR activation has been shown to promote DC maturation and the production of cytokines, potentially resulting in the activation of specific type of T-cell immunity, involving the recruitment of CD8+ T cells.Citation22,Citation23 Tumor-infiltrating CD8+ T cells are an important prognostic factor for patients with a variety of cancer types.Citation15 An increased presence of CD8+ T cells at the tumor site is a key to obtaining a therapeutic response using immunotherapy (eg, PD-1/PD-L1 antibodies).Citation24 In the present study, we demonstrated a strong correlation between the expression levels of TLR7/8 and CD8A. Moreover, we found that expression levels of TLR7/8 were associated with that of CD8+ T cell-specific markers, including perforin, granzyme B, and IFNg. Taken together, these data suggest that TLR7/8 expression is closely associated with CD8+ T-cell immunity. Interestingly, we found that the expression of those genes that had a strong positive correlation with TLR7/8 expression (r>0.5) also predicted better patient outcomes. These genes included CCR2, CCR5, CD8A, IFNG, GZMB, and PRF1. These results further validate the correlation between TLR7/8 expression and DC/T-cell infiltration and trafficking in melanoma. Ultimately, these findings may provide new insights for the development of novel immunotherapies to treat melanoma and other types of cancer.
We recognize that there exist several limitations to our study. First, our analysis was primarily performed using an open public database with a large cohort of patients and RNAseq gene data. The gene expression data we collected were for tumor tissues, which contained not just tumor cells but also stromal cells and immune cells. The contribution of each cell type remains unclear and requires further analysis, such as immunohistochemical analysis of TLR protein expression. Second, while our correlation analysis provided important results, functional experiments are required to further validate them.
Conclusion
We report that TLR7/8 expression represents an independent prognostic factor in patients with melanoma. Our data demonstrate that expression levels of TLR7/8 highly correlated with that of chemokines and their receptors. We also show a strong association between TLR7/8 expression and CD8+ T-cell immunity. Our findings not only provide an important reference for the clinical prognosis of melanoma but also present a new therapeutic modality for the development of melanoma immunotherapy.
Acknowledgments
This work was supported by the Zhejiang Provincial Medical and Health Project (2016KYA163). The funders had no role in study design, data collection, and analysis; decision to publish this article; or in the preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- BurnsEMYusufNToll-like receptors and skin cancerFront Immunol2014513524744758
- AkiraSHemmiHRecognition of pathogen-associated molecular patterns by TLR familyImmunol Lett2003852859512527213
- SongPIParkYMAbrahamTHuman keratinocytes express functional CD14 and toll-like receptor 4J Invest Dermat20021192424432
- BauerSPigischSHangelDKaufmannAHammSRecognition of nucleic acid and nucleic acid analogs by Toll-like receptors 7, 8 and 9Immunobiology20082133–431532818406377
- SantoniMAndrikouKSotteVToll like receptors and pancreatic diseases: from a pathogenetic mechanism to a therapeutic targetCancer Treat Rev201541756957626036357
- OchiAGraffeoCSZambirinisCPToll-like receptor 7 regulates pancreatic carcinogenesis in mice and humansJ Clin Invest2012122114118412923023703
- AspordCTramcourtLLeloupCImiquimod inhibits melanoma development by promoting pDC cytotoxic functions and impeding tumor vascularizationJ Invest Dermatol2014134102551256124751730
- CraftNBruhnKWNguyenBDThe TLR7 agonist imiquimod enhances the anti-melanoma effects of a recombinant Listeria monocytogenes vaccineJ Immunol200517531983199016034143
- GoldingerSMDummerRBaumgaertnerPMihic-ProbstSMSchwarzSMHammann-HaenniSMNano-particle vaccination combined with TLR-7 and -9 ligands triggers memory and effector CD8(+) T-cell responses in melanoma patientsEur J Immunol201242113049306122806397
- ArandaFLlopizDDiaz-ValdesNAdjuvant combination and antigen targeting as a strategy to induce polyfunctional and high-avidity T-cell responses against poorly immunogenic tumorsCancer Res20117193214322421402711
- DrobitsBHolcmannMAmbergNImiquimod clears tumors in mice independent of adaptive immunity by converting pDCs into tumor-killing effector cellsJ Clin Invest2012122257558522251703
- EiroNOviesCFernandez-GarciaBExpression of TLR3, 4, 7 and 9 in cutaneous malignant melanoma: relationship with clinicopathological characteristics and prognosisArch Dermatol Res20133051596723179584
- GoldmanMCraftBSwatloskiTThe UCSC Cancer Genomics Browser: update 2013Nucleic Acids Res201341Database issueD949D95423109555
- BogunovicDO’NeillDWBelitskaya-LevyIImmune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survivalProc Natl Acad Sci U S A200910648204292043419915147
- FridmanWHPagesFSautes-FridmanCGalonJThe immune contexture in human tumours: impact on clinical outcomeNat Rev Cancer201212429830622419253
- DajonMIribarrenKCremerIToll-like receptor stimulation in cancer: a pro- and anti-tumor double-edged swordImmunobiology201722218910027349597
- RidnourLAChengRYSwitzerCHMolecular pathways: toll-like receptors in the tumor microenvironment – poor prognosis or new therapeutic opportunityClin Cancer Res20131961340134623271799
- Gonzalez-ReyesSMarinLGonzalezLStudy of TLR3, TLR4 and TLR9 in breast carcinomas and their association with metastasisBMC Cancer20101066521129170
- SinghMKhongHDaiZEffective innate and adaptive anti-melanoma immunity through localized TLR7/8 activationJ Immunol201419394722473125252955
- ChaturvediAPierceSKHow location governs toll-like receptor signalingTraffic200910662162819302269
- SmitsELPonsaertsPBernemanZNVan TendelooVFThe use of TLR7 and TLR8 ligands for the enhancement of cancer immunotherapyOncologist200813885987518701762
- GauzziMCDel CornoMGessaniSDissecting TLR3 signalling in dendritic cellsImmunobiology20102159–1071372320561711
- SchreibeltGTelJSliepenKHToll-like receptor expression and function in human dendritic cell subsets: implications for dendritic cell-based anti-cancer immunotherapyCancer Immunol Immunother201059101573158220204387
- ArinaACorralesLBronteVEnhancing T cell therapy by overcoming the immunosuppressive tumor microenvironmentSemin Immunol2016281546326872631