Abstract
Background
Indoleamine 2,3-dioxygenase (IDO) catalyzes the rate-limiting step of tryptophan (Trp) degradation via the kynurenine (Kyn) pathway, which inhibits the proliferation of T cells and induces the apoptosis of T cells, leading to immune tolerance. Therefore, IDO has been considered as the most important mechanism for tumor cells to escape from immune response. Previous studies suggested that IDO might be involved in the progression of tumor and resistance to chemotherapy. Several preclinical and clinical studies have proven that IDO inhibitors can regulate IDO-mediated tumor immune escape and potentiate the effect of chemotherapy. Thus, the present study investigated the correlation between the clinical parameters, responses to chemotherapy, and IDO activity to provide a theoretical basis for the clinical application of IDO inhibitors to improve the suppression status and poor prognosis in cancer patients.
Methods
The serum concentrations of Trp and Kyn were measured by high-performance liquid chromatography in 252 patients with stage IIIB or IV non-small-cell lung cancer, and 55 healthy controls. The IDO activity was determined by calculating the serum Kyn-to-Trp (Kyn/Trp) ratio.
Results
The IDO activity was significantly higher in the lung cancer patients than in the controls (median 0.0389 interquartile range [0.0178–0.0741] vs 0.0111 [0.0091–0.0133], respectively; P<0.0001). In addition, patients with adenocarcinoma had higher IDO activity than patients with nonadenocarcinoma (0.0449 [0.0189–0.0779] vs 0.0245 [0.0155–0.0563], respectively; P=0.006). Furthermore, patients with stage IIIB disease had higher IDO activity than patients with stage IV disease (0.0225 [0.0158–0.0595] vs 0.0445 [0.0190–0.0757], respectively; P=0.012). The most meaningful discovery was that there was a significant difference between the partial response (PR) patients and the stable disease (SD) and progressive disease (PD) patients (0.0240 [0.0155–0.0381] vs 0.0652 [0.0390–0.0831] vs 0.0868 [0.0209–0.0993], respectively, P<0.0001).
Conclusion
IDO activity was increased in lung cancer patients. Higher IDO activity correlated with histological types and disease stages of lung cancer patients, induced the cancer cells’ resistance to chemotherapy, and decreased the efficacy of chemotherapy.
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide.Citation1 Non-small-cell lung cancer (NSCLC) comprises ~85% of all lung cancer patients.Citation2 Among patients with NSCLC, ~70% present with locally advanced nonresectable disease (stage IIIB)Citation3 or metastatic disease (stage IV),Citation4 which means that these patients will have a median survival time of 4–5 months since diagnosis and only 10% of them will survive for 1 year.Citation5,Citation6 For patients with advanced-stage NSCLC, chemotherapy with a platinum doublet offers the median overall survival (OS) of 10 months.Citation7 Recent introduction of molecularly targeted therapies for NSCLC resulted in clinically meaningful OS improvements. However, only selected patients whose tumors exhibit specific oncogene addiction can benefit from the targeted therapies.Citation8 Unfortunately, almost all patients eventually acquire resistance to targeted therapies.Citation9–Citation11 Therefore, lung cancer remains a disease with dismal prognosis, and novel therapies focused on new target are urgently needed.
In tumor immunology, the basic function of the host immune system is to differentiate between normal cells and cancer cells to protect the body from the damage caused by the cancer cells.Citation12 T cells play a key role in cell-mediated immunity. During the immune response, however, some of the transformed cancer cells employ several immune evasion strategies to generate an immunosuppressive microenvironment to overcome the immune response, resulting in tumor progression.Citation13–Citation17 The most important mechanism of immune tolerance by which cancer cells escape the immune system is to diminish local tryptophan (Trp) by indoleamine 2,3-dioxygenase (IDO).Citation18,Citation19
IDO is a key enzyme that catalyzes the initial and rate-limiting steps in the Trp degradation along the kynurenine (Kyn) pathway to form Kyn, which is converted into several metabolites through downstream enzymes.Citation20 For many years, IDO has been known as an innate defense mechanism, limiting the growth of viruses, bacteria, and intracellular pathogens by consuming Trp in the local microenvironment.Citation21,Citation22 IDO activity can be quantified by measuring the serum Kyn-to-Trp (Kyn/Trp) ratio using high-performance liquid chromatography (HPLC) because Kyn is the first product formed through catabolizing Trp, which is tightly regulated by IDO. Therefore, a high Kyn/Trp ratio reflects an enhanced IDO activity.Citation23–Citation26
Increased expression of IDO has been observed in wide range of types of human solid tumors, such as colorectal, breast, ovarian, and lung cancers and melanoma,Citation27–Citation31 as well as in hematological malignancies, including acute myeloid leukemiaCitation32 and lymphoma.Citation33 Within the tumor microenvironment, constitutive expression of IDO, which is expressed by cancer cells as well as by some tumor infiltrating cells, for example, dendritic cells (DCs),Citation18,Citation27,Citation34,Citation35 can be detected in the peritumoral stroma and in tumor-draining lymph nodes.Citation34,Citation36 IDO induces peripheral immunotolerance and immunosuppression by the following mechanisms: 1) reducing the local concentration of Trp and starving T cells from the important amino acid, resulting in the inhibition of T-cell proliferation,Citation37–Citation39 2) producing immunomodulatory toxic metabolites, such as Kyn (kynurenic acid [Kyna]), which efficiently suppress the function of T cells, render T cells more sensitive to apoptosis, and promote the differentiation of naive T cells into regulatory T cells (Tregs) that can directly inhibit the host immune response,Citation18,Citation20,Citation27,Citation40–Citation42 and 3) secreting immunosuppressive cytokines, such as IL-10 and TGF-β, to protect tumor form host immunity.Citation43,Citation44 To date, a large number of studies have shown that high expression of IDO is predictive of shorten survival in a variety of human malignancies, including solid tumors (lung,Citation34,Citation45,Citation46 colorectal,Citation47–Citation49 endometrial,Citation50,Citation51 ovarian,Citation52–Citation55 hepatocellular,Citation56 and breastCitation57–Citation60 cancers, malignant melanoma,Citation61,Citation62 gynecological,Citation63 cervical,Citation64,Citation65 esophageal,Citation66–Citation68 and pancreatic ductalCitation69 cancers, osteosarcoma,Citation70 and brain cancerCitation71) and various hematological tumors, such as myeloma,Citation72 leukemia,Citation73–Citation77 and lymphoma.Citation33,Citation78–Citation80
In several preclinical studies, the IDO-blocking agent 1-methyl-Trp (1-MT) has shown remarkable ability to inhibit IDO activity and cooperate with cytotoxic chemotherapeutic agents in inhibiting the growth of cancer cells in animal models.Citation81,Citation82
Although the above studies suggest critical relationship between IDO and disease progression in cancer, there have been no studies focusing on the relationship among the activity of IDO, clinical parameters, and response to chemotherapy in patients with stage IIIB or IV NSCLC. The present study measured the serum concentrations of Trp and Kyn by HPLC and estimated the IDO activity in advanced NSCLC patients. The object of our study is to clarify the correlation between the status of suppression in lung cancer patients and the clinical characteristics as well as the response to chemotherapy of the selected patients so that we can improve the poor prognosis of these patients.
Subjects and methods
Subjects
A total of 252 patients, of whom 155 were men and 97 were women, were enrolled at our institutions from May 2015 to September 2016. The main inclusion criteria were as follows: 1) cytologically or histologically confirmed primary NSCLC; 2) stage IIIB or IV according to the seventh TNM stage classification system; 3) no anticancer treatment before the enrollment of the study, including surgery, chemotherapy, target agents, and immunotherapy; 4) patients with a computed tomography (CT) or positron-emission tomography (PET) or magnetic resonance imaging (MRI) scan of evaluable primary lesions before the first cycle and third cycle of chemotherapy; and 5) Karnofsky Performance Status (KPS) scale of 60 and 100 and requirement of adequate organ, bone marrow, liver, and renal functions. The main exclusion criteria are as follows: 1) autoimmune disease, viral hepatitis, or acquired immunodeficiency syndrome (AIDS); 2) pregnant women; and 3) the other types of carcinoma. Histological types were classified according to the World Health Organization (WHO) criteria. The study subjects also included 55 healthy blood donors serving as a control group (32 men and 23 women). Healthy subjects with recent infection, immune system disease, and malignant tumors were excluded. This study was approved by the Ethics Committee of Shandong Cancer Hospital, who deemed written informed consent unnecessary as the experiments did not violate relevant rules of experimental ethics.
Treatment schedule
The chemotherapy regimens were used according to the histological types. Treatment schedules consisted of at least two cycles of pemetrexed (500 mg/m2) combined with cisplatin (75 mg/m2) or carboplatin (AUC =5–6) or nedaplatin (80 mg/m2) or lobaplatin (50 mg/m2); docetaxel (75 mg/m2) combined with cisplatin or carboplatin or nedaplatin; gemcitabine (1,250 mg/m2) combined with cisplatin or carboplatin or nedaplatin or lobaplatin; and etoposide (100 mg/m2) combined with cisplatin. Among them, some patients with lung adenocarcinoma were administered with bevacizumab (15 mg/kg) combined with chemotherapy agents.
Evaluation of response concerning the primary site
All the patients are required to have imaging examinations for the primary sites before the first cycle and third cycle of chemotherapy. Tumor responses were assessed as follows: complete response (CR) for the primary lesion was defined as the complete disappearance of all measurable and assessable target lesions for >4 weeks; partial response (PR) was defined as at least 30% decrease in the sum of diameters of target lesions; progressive disease (PD) was defined as a ≥20% enlargement of the tumor or the appearance of a new tumor lesion; and stable disease (SD) was defined as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD.
Measurements of serum Trp and Kyn
For the patients with advanced NSCLC, venous blood samples were collected from peripheral vein after at least 12 hours of fasting before the first cycle of chemotherapy. For the healthy subjects, blood samples were collected from peripheral vein after at least 12 hours of fasting. Blood samples were centrifuged, and the separated serum samples were deep frozen and stored at -80°C immediately until further analysis. After thawing at room temperature, 200 µL of samples were acidified with 200 µL perchloric acid. Then, the samples were centrifuged for 10 minutes (10,000 rpm, 4°C), and the supernatants were centrifuged under the same conditions again for the purpose of removing precipitated proteins completely. Analyses were separated by an isocratic elution of the injected samples. Eluted Trp and Kyn was subjected to HPLC (Shimadzu LC-20A HPLC system: C18 reverse-phase column) and quantified fluorometrically (Shimadzu RF-20A fluorescence detector). Concentrations of Trp and Kyn were determined by HPLC as previously described.Citation54 To estimate the IDO activity, the Kyn/Trp ratio was calculated.Citation83
Statistical analysis
All continuous variables were assessed for normality of the distribution using the P–P plot. For the data that fitted normal distribution, it was presented as the mean value and standard deviation and analyzed by independent-samples t-test or one-way ANOVA. In case of skewed distributions, the median and interquartile ranges (IQRs, 25th–75th percentile) were presented and statistical analysis was performed using the nonparametric Kruskal–Wallis test or Mann–Whitney U test. Spearman rank correlation analysis was applied to assess correlations. P<0.05 was considered as statistically significant. Analyses were performed using the software package, version 23.0 for Windows (IBM Corporation, Armonk, NY, USA).
Results
Clinical characteristics
A group of 252 cytologically or histologically proven NSCLC patients with a mean age of 57.9±10.4 years and 55 healthy subjects with a mean age of 60.1±10.5 years was evaluated. The clinical characteristics of the subjects are listed in . No age and gender differences were observed between healthy controls and lung cancer patients (P>0.05). Among the patients, 54 patients were diagnosed at stage IIIB and 198 patients were diagnosed at stage IV. A total of 185 patients had lung adenocarcinoma, and the others had nonadenocarcinoma (52 squamous cell carcinoma, 8 large cell carcinoma, 4 atypical carcinoid, and 3 adenosquamous cell carcinoma). As for the response to chemotherapy, no one reached CR, 122 patients reached PR and 109 patients had SD, whereas 21 patients progressed after two cycles of chemotherapy.
Table 1 Clinical characteristics of patients with non-small-cell lung cancer and healthy controls
Serum Trp and Kyn and IDO activity in patients and controls
The serum concentrations of Trp and Kyn and the IDO activity are presented in . Trp concentrations in patients were significantly lower compared to those in healthy individuals (P<0.0001). Lung cancer patients had significantly higher Kyn concentrations (P<0.0001) and IDO activity (P<0.0001) than healthy controls.
Table 2 Serum concentrations of Trp and Kyn and IDO activity in patients and controls
Correlation of serum Trp and Kyn and IDO activity with clinical characteristics
Patients with lung adenocarcinoma had significantly lower concentration of Trp (P=0.017) and higher IDO activity (P=0.006) with slightly higher concentration of Kyn but not significantly different (P=0.268) than the patients with nonadenocarcinoma (). However, we did not explore the difference between the patients with nonadenocarcinoma due to the limited sample size. Interestingly, comparing patients with stage IIIB disease, significant decreases in serum concentrations of Trp (P=0.018) and increases in IDO activity (P=0.012) were found in patients with stage IV disease, whereas the Kyn concentration did not reach statistical significance ().
Table 3 Serum concentrations of Trp and Kyn and IDO activity in the different groups of histological types
Table 4 Serum concentrations of Trp and Kyn and IDO activity in the different groups of disease stages
We also explored the differences between the smoking status and KPS scale with the concentrations of Trp and Kyn and IDO activity; no significant differences were observed. In addition, no correlation between patient’s age and concentrations of Trp or Kyn as well as the IDO activity was observed.
Correlation of serum Trp and Kyn and IDO activity with responses to chemotherapy
The PR patients had significantly lower concentrations of Trp with significantly higher concentrations of Kyn, resulting in higher IDO activity compared to the SD patients (P<0.0001) and PD patients (P<0.0001). However, there are no differences between the SD patients and the PD patients concerning the concentrations of Trp and Kyn and IDO activity ( and ).
Figure 1 Comparison of serum concentrations of Trp (A) and Kyn (B) and IDO activity (C) between PR patients, SD patients, and PD patients.
Abbreviations: Kyn, kynurenine; IDO, indoleamine 2,3-dioxygenase; Max, maximum; Min, minimum; PD, progressive disease; PR, partial response; SD, stable disease; Trp, tryptophan.
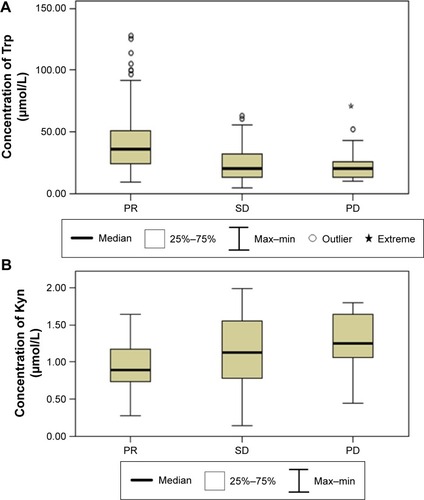
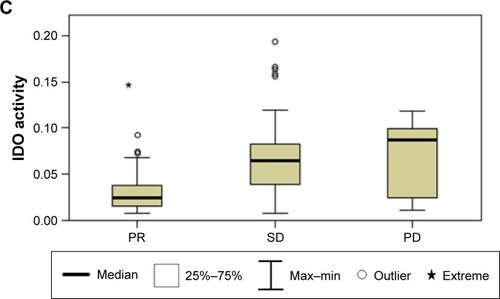
Table 5 Serum concentrations of Trp and Kyn and IDO activity in the different groups of responses to chemotherapy
Discussion
Our study, for the first time, measured the pretreatment serum concentrations of Trp and Kyn by HPLC and estimated the activity of IDO through calculating Kyn/Trp ratio in patients with locally advanced or metastatic NSCLC. This study showed an accelerated Trp catabolism in NSCLC patients than in healthy controls, which was consistent with previous observations in lung cancer,Citation45,Citation84,Citation85 gynecologic cancer,Citation86 breast cancer,Citation87 malignant melanoma,Citation61 colorectal cancer,Citation49 thyroid cancer,Citation88 leukemia,Citation75,Citation76 esophageal cancer,Citation66,Citation89 and ovarian cancer.Citation90 There are several explanations for this result. The most important reason is that the IDO expressed constitutively by cancer cells and some other cells starve T cells from the important amino acid, Trp, causing them to be incapable of performing an appropriate immune response.Citation18 The other potential reason for this phenomenon is that patients with cancer reduce dietary intake of this essential amino acid, whereas the cancer cells still consume Trp in the tumor microenvironment to protect them from the immunosurveillance.
Sagan et alCitation91 found that Kyna level in the serum of patients with adenocarcinoma was significantly higher than that in the serum of patients with squamous cell cancer (P<0.05). Kyna is the end-stage product of the transamination side branch in the Kyn pathway.Citation18,Citation92,Citation93 Therefore, the level of Kyna can reflect the activity of Kyn pathway; in other words, it can reflect the activity of IDO. Previous studies have shown that lung adenocarcinoma is characterized by more aggressive development, resulting in poorer prognosis compared to other histological types of NSCLC.Citation94–Citation96 This characteristic of invasiveness of lung adenocarcinoma may be attributed to the properties of the tumor itself, as well as to impaired antitumor immune response.Citation97,Citation98 Supporting the above theory, our data showed that the degradation of Trp and high level of IDO activity were observed in patients with adenocarcinoma than in patients with nonadenocarcinoma.
We also found that patients with metastatic lung cancer had significantly higher IDO activity than those with locally advanced lung cancer. Similarly, Suzuki et alCitation45 reported that an increased IDO activity was discovered in advanced stages of lung cancer than in early stages of lung cancer. However, Karanikas et alCitation46 found no significant correlation between disease stages and mRNA IDO expression by tumor tissues in 28 patients with NSCLC. This conflict may be partially attributed to the different sample sizes and methods to assess IDO expression or activity between these studies. Our results were also consistent with previous studies, which found that high expression of IDO has been associated with high frequencies of metastasis in patients with hepatocellular carcinoma,Citation56 endometrial tumors,Citation51 and colorectal cancer.Citation47 These results indicate that IDO activity is enhanced in patients with a larger tumor burden.
Our study discovered that there was a significant difference between the PR patients and the SD and PD patients, whereas no significant difference was found between the SD patients and the PD patients. Previous studiesCitation81 suggested that IDO might be associated with resistance to chemotherapeutic agents, which was consistent with another report showing that IDO was involved in paclitaxel resistance in ovarian cancer.Citation52
Consequently, IDO may serve as an important target for anticancer agents. To our knowledge, several small-molecule IDO inhibitors, such as 1-MT, show effective antitumor activity in animal models, especially when they are combined with cytotoxic chemotherapeutic agents, such as platinum compounds, taxane derivatives, and cyclophosphamide. The IDO inhibitors reactivate the T cells by suppressing the consumption of Trp and the production of Kyn to block the immune escape without increased toxicity.Citation81,Citation82,Citation99,Citation100 Furthermore, 1-MT has been evaluated in clinical trials to disrupt tumor tolerance in cancer patients to improve the immunosuppression status and postpone the growth of the tumor.Citation101
Study limitations
However, there are several limitations of the present study, which should be discussed. First, the small sample size might impair the effectiveness of our results and a more robust sample size may have improved the power to detect significant differences between the levels of Trp and Kyn and IDO activity and the clinical parameters as well as the response to chemotherapy. Second, we did not examine the concentrations of the Trp and Kyn and IDO activity after standard treatment. Therefore, we cannot figure out whether IDO activity changed by chemotherapy. In addition, this study did not perform survival analysis. As a result, we cannot give the conclusion whether levels of Trp and Kyn and IDO activity can serve as prognostic factors for stage IIIB or IV NSCLC patients.
Conclusion
The present study presented that patients with stage IIIB or IV NSCLC had lower serum concentration of Trp and higher serum concentration of Kyn, resulting in more enhanced IDO activity than healthy controls, and that increased IDO activity related to the histological types and disease stages. The most important discovery of our study was that IDO activity differed significantly between the PR patients and the SD and PD patients. The result suggested that patients with an increased immunosuppression status induced by higher IDO activity might decrease the efficacy of chemotherapy. Taken together with previous studies, the combined treatment of IDO inhibitor and chemotherapy is effective in improving the immunosup-pression status and has potential clinical prospect.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statisticsCA Cancer J Clin201666173026742998
- GovindanRPageNMorgenszternDChanging epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results databaseJ Clin Oncol200624284539454417008692
- AuperinALe PechouxCRollandEMeta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancerJ Clin Oncol201028132181219020351327
- BesseBAdjeiABaasP2nd ESMO Consensus Conference on Lung Cancer: non-small-cell lung cancer first-line/second and further lines of treatment in advanced diseaseAnn Oncol20142581475148424669016
- PageDBBourlaABDaniyanATumor immunology and cancer immunotherapy: summary of the 2014 SITC primerJ Immunother Cancer2015325
- SchreiberRDOldLJSmythMJCancer immunoediting: integrating immunity’s roles in cancer suppression and promotionScience201133160241565157021436444
- ScagliottiGVParikhPvon PawelJPhase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancerJ Clin Oncol200826213543355118506025
- JackmanDMMillerVACioffrediLAImpact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small celllung cancer patients: results of an online tumor registry of clinical trialsClin Cancer Res200915165267527319671843
- SicaASaccaniABottazziBAutocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophagesJ Immunol2000164276276710623821
- KobayashiSBoggonTJDayaramTEGFR mutation and resistance of non-small-cell lung cancer to gefitinibN Engl J Med2005352878679215728811
- AwadMMKatayamaRMcTigueMAcquired resistance to crizotinib from a mutation in CD74eROS1N Engl J Med2013368252395e240123724914
- DaviesMNew modalities of cancer treatment for NSCLC: focus on immunotherapyCancer Manag Res20146637524520205
- ZouWImmunosuppressive networks in the tumor environment and their therapeutic relevanceNat Rev Cancer20055426327415776005
- ZouWRegulatory T cells, tumor immunity and immunotherapyNat Rev Immunol20066429530716557261
- RabinovichGAGabrilovichDSotomayorEMImmunosuppressive strategies that are mediated by tumor cellsAnnu Rev Immunol20072526729617134371
- DunnGPOldLJSchreiberRDThe three Es of cancer immunoeditingAnnu Rev Immunol20042232936015032581
- GermenisAEKaranikasVImmunoepigenetics: the unseen side of cancer immunoeditingImmunol Cell Biol2007851555917130900
- MunnDHMellorALIndoleamine 2,3-dioxygenase and tumor-induced toleranceJ Clin Invest200711751147115417476344
- PrendergastGCImmune escape as a fundamental trait of cancer: focus on IDOOncogene200827283889390018317452
- TakikawaOYoshidaRKidoRHayaishiOTryptophan degradation in mice initiated by indoleamine 2,3-dioxygenaseJ Biol Chem19862618364836532419335
- PfefferkornERInterferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophanProc Natl Acad Sci U S A19848139089126422465
- OzakiYEdelsteinMPDuchDSInduction of indoleamine 2,3- dioxygenase: a mechanism of the antitumor activity of interferon gammaProc Natl Acad Sci U S A1988854124212463124115
- SchefoldJCZedenJPFotopoulouCIncreased indoleamine 2,3-dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: a possible link between chronic inflammation and uraemic symptomsNephrol Dial Transplant20092461901190819155537
- PawlakKBrzoskoSMysliwiecMPawlakDKynurenine, quinolinic acid – the new factors linked to carotid atherosclerosis in patients with end-stage renal diseaseAtherosclerosis2009204256156619027117
- KatoASuzukiYSudaTRelationship between an increased serum kynurenine/tryptophan ratio and atherosclerotic parameters in hemodialysis patientsHemodial Int201014441842420673309
- LaichANeurauterGWidnerBFuchsDMore rapid method for simultaneous measurement of tryptophan and kynurenine by HPLCClin Chem200248357958111861457
- UyttenhoveCPilotteLTheateIEvidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenaseNat Med20039101269127414502282
- KimJWNamKHAhnSHPrognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancerGastric Cancer2016191425225424150
- TheateIvan BarenNPilotteLExtensive profiling of the expression of the indoleamine 2,3-dioxygenase 1 protein in normal and tumoral human tissuesCancer Immunol Res20153216117225271151
- ChevoletISpeeckaertRHaspeslaghMPeritumoral indoleamine 2,3-dioxygenase expression in melanoma: an early marker of resistance to immune control?Br J Dermatol2014171598799524814041
- VanderstraetenALuytenCVerbistGTuyaertsSAmantFMapping the immunosuppressive environment in uterine tumors: implications for immunotherapyCancer Immunol Immunother201463654555724658839
- ArpinatiMCurtiAImmunotherapy in acute myeloid leukemiaImmunotherapy2014619510624341888
- ChoeJYYunJYJeonYKIndoleamine 2,3-dioxygenase (IDO) is frequently expressed in stromal cells of Hodgkin lymphoma and is associated with adverse clinical features: a retrospective cohort studyBMC Cancer20141433524886161
- AstigianoSMorandiBCostaREosinophil granulocytes account for indoleamine 2,3-dioxygenase-mediated immune escape in human non-small cell lung cancerNeoplasia20057439039615967116
- MellorAMunnDIDO expression by dendritic cells: tolerance and tryptophan catabolismNat Rev Immunol200441076277415459668
- FribergMJenningsRAlsarrajMIndoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejectionInt J Cancer2002101215115512209992
- MellorALMunnDHTryptophan catabolism and T-cell tolerance: immunosuppression by starvation?Immunol Today1999201046947310500295
- MunnDHShafzadehEAttwoodJTBondarevIPashineAMellorALInhibition of T cell proliferation by macrophage tryptophan catabolismJ Exp Med199918991363137210224276
- HwuPDuMXLapointeRDoMTaylorMWYoungHAIndoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferationJ Immunol200016473596359910725715
- CurtiAPandolfiSValzasinaBModulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25- into CD25+ T regulatory cellsBlood200710972871287717164341
- ChenWLiangXPetersonAJMunnDHBlazarBRThe indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generationJ Immunol200818185396540418832696
- ChungDJRossiMRomanoEIndoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cellsBlood2009114355556319465693
- DubinettSSharmaSTowards effective immunotherapy for lung cancer: simultaneous targeting of tumor-initiating cells and immune pathways in the tumor microenvironmentImmunotherapy20091572172520636013
- HalakBKMaguireHCJrLattimeECTumor-induced interleukin-10 inhibits type 1 immune responses directed at a tumor antigen as well as a non-tumor antigen present at the tumor siteCancer Res199959491191710029084
- SuzukiYSudaTFuruhashiKIncreased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancerLung Cancer201067336136519487045
- KaranikasVZamanakouMKerenidiTIndoleamine 2,3-dioxygenase (IDO) expression in lung cancerCancer Biol Ther2007681258126217700060
- BrandacherGPerathonerALadurnerRPrognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cellsClin Cancer Res20061241144115116489067
- WeissGKronbergerPConradFBodnerEWachterHReibneggerGNeopterin and prognosis in patients with adenocarcinoma of the colonCancer Res19935322602658417818
- HuangAFuchsDWinderBGloverCHendersonDCAllen-MershTGSerum tryptophan decrease correlates with immune activation and impaired quality of life in colorectal cancerBr J Cancer200286111691169612087451
- InoKYoshidaNKajiyamaHIndoleamine 2,3-dioxygenase is a novel prognostic indicator for endometrial cancerBr J Cancer200695111555156117117179
- InoKYamamotoEShibataKInverse correlation between tumoral indoleamine 2,3-dioxygenase expression and tumor-infltrating lymphocytes in endometrial cancer: its association with disease progression and survivalClin Cancer Res2008142310231718413819
- OkamotoANikaidoTOchiaiKIndoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cellsClin Cancer Res200511166030603916115948
- TakaoMOkamotoANikaidoTIncreased synthesis of indoleamine-2,3-dioxygenase protein is positively associated with impaired survival in patients with serous-type, but not with other types of, ovarian cancerOncol Rep20071761333133917487387
- ReibneggerGHetzelHFuchsDClinical significance of neopterin for prognosis and follow-up in ovarian cancerCancer Res19874718497749813621185
- InabaTInoKKajiyamaHRole of the immunosuppressive enzyme indoleamine 2,3-dioxygenase in the progression of ovarian carcinomaGynecol Oncol2009115218519219665763
- PanKWangHChenMSExpression and prognosis role of indoleamine 2,3-dioxygenase in hepatocellular carcinomaJ Cancer Res Clin Oncol2008134111247125318438685
- MurrCBergantAWidschwendterMHeimKSchrocksnadelHFuchesDNeopterin is an independent prognostic variable in females with breast cancerClin Chem199945111998200410545071
- ChenJYLiCFKuoCCTsaiKKHouMFHungWCCancer/stroma interplay via cyclooxygenase-2 and indoleamine 2,3-dioxygenase promotes breast cancer progressionBreast Cancer Res201416441025060643
- YuJDuWYanFMyeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancerJ Immunol201319073783379723440412
- YuJSunJWangSEUpregulated expression of indoleamine 2,3-dioxygenase in primary breast cancer correlates with increase of infiltrated regulatory T cells in situ and lymph node metastasisClin Dev Immunol2011201146913522110525
- WeinlichGMurrCRichardsenLWinklerCFuchsDDecreased serum tryptophan concentration predicts poor prognosis in malignant melanoma patientsDermatology2007214181417191041
- SpeeckaertRVermaelenKvan GeelNIndoleamine 2,3-dioxygenase, a new prognostic marker in sentinel lymph nodes of melanoma patientsEur J Cancer201248132004201122033321
- SchroecksnadelKWinklerCFuithLCFuchsDTryptophan degradation inpatients with gynecological cancer correlates with immune activationCancer Lett2005223232332915896467
- ReibneggerGJBichlerAHDapuntONeopterin as a prognostic indicator in patients with carcinoma of the uterine cervixCancer Res19864629509553940654
- InabaTInoKKajiyamaHIndoleamine 2,3-dioxygenase expression predicts impaired survival of invasive cervical cancer patients treated with radical hysterectomyGynecol Oncol2010117342342820350764
- LiuJLuGTangFLiuYCuiGLocalization of indoleamine 2,3-dioxygenase in human esophageal squamous cell carcinomasVirchows Arch2009455544144819844741
- ZhangGLiuWLZhangLInvolvement of indoleamine 2, 3-dioxygenase in impairing tumor-infiltrating CD8 T-cell functions in esophageal squamous cell carcinomaClin Dev Immunol2011201138472622013481
- JiaYWangHWangYLow expression of Bin1, along with high expression of IDO in tumor tissue and draining lymph nodes, are predictors of poor prognosis for esophageal squamous cell cancer patientsInt J Cancer201513751095110625683635
- WitkiewiczAWilliamsTKCozzitortoJExpression of indoleamine 2,3-dioxygenase in metastatic pancreatic ductal adenocarcinoma recruits regulatory T cells to avoid immune detectionJ Am Coll Surg2008206584985418471709
- UrakawaHNishidaYNakashimaHShimoyamaYNakamuraSIshiguroNPrognostic value of indoleamine 2,3-dioxygenase expression in high grade osteosarcomaClin Exp Metastasis20092681005101219802733
- WainwrightDABalyasnikovaIVChangALIDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survivalClin Cancer Res201218226110612122932670
- BonannoGMariottiAProcoliAIndoleamine 2,3-dioxygenase 1 (IDO1) activity correlates with immune system abnormalities in multiple myelomaJ Transl Med20121024723232072
- ChamuleauMEvan de LoosdrechtAAHessCJHigh INDO (indoleamine 2,3-dioxygenase) mRNA level in blasts of acute myeloid leukemic patients predicts poor clinical outcomeHaematologica200893121894189819050070
- FolgieroVGoffredoBMFilippiniPIndoleamine 2,3-dioxygenase 1 (IDO1) activity in leukemia blasts correlates with poor outcome in childhood acute myeloid leukemiaOncotarget2014582052206424903009
- CormSBerthonCImbenotteMIndoleamine 2,3-dioxygenase activity of acute myeloid leukemia cells can be measured from patients’ sera by HPLC and is inducible by IFN-gammaLeuk Res200933349049418639339
- HoshiMItoHFujigakiHIndoleamine 2,3-dioxygenase is highly expressed in human adult T-cell leukemia/lymphoma and chemotherapy changes tryptophan catabolism in serum and reduced activityLeuk Res2009331394518639341
- MasakiAIshidaTMaedaYPrognostic significance of trypto-phan catabolism in adult T-cell leukemia/lymphomaClin Cancer Res201521122830283925788494
- YoshikawaTHaraTTsurumiHSerum concentration of L-kynurenine predicts the clinical outcome of patients with diffuse large B-cell lymphoma treated with R-CHOPEur J Haematol201084430430919995374
- NinomiyaSHaraTTsurumiHIndoleamine 2,3-dioxygenase in tumor tissue indicates prognosis in patients with diffuse large B-cell lymphoma treated with R-CHOPAnn Hematol201190440941620938662
- LiuXQLuKFengLLUpregulated expression of indoleamine 2,3-dioxygenase 1 in non-Hodgkin’s lymphoma correlates with increase of infiltrated regulatory T cellsLeuk Lymphoma20135540541423682557
- MullerAJDu HadawayJBDonoverPSSutanto-WardEPrendergastGCInhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapyNat Med200511331231915711557
- HouDYMullerAJSharmaMDInhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responsesCancer Res200767279280117234791
- HuberCBatchelorJRFuchsDImmune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gammaJ Exp Med198416013103166429267
- EnginABOzkanYFuchsDYardim-AkaydinSIncreased tryptophan degradation in patients with bronchus carcinomaEur J Cancer Care2010196803808
- XieQWangLZhuBWangYGuJChenZThe expression and significance of indoleamine -2,3-dioxygenase in non-small cell lung cancer cellZhongguo Fei Ai Za Zhi200811111511920727279
- de JongRANijmanHWBoezenHMSerum tryptophan and kynurenine concentrations as parameters for indoleamine 2,3-dioxygenase activity in patients with endometrial, ovarian, and vulvar cancerInt J Gynecol Cancer2011211320132721720257
- LyonDEWalterJMStarkweatherARSchubertCMMcCainNLTryptophan degradation in women with breast cancer: a pilot studyBMC Res Notes2011415621615916
- SakuraiKFujisakiSNagashimaSStudy of indoleamine 2,3-dioxygenase expression in patients of thyroid cancerGan To Kagaku Ryoho201138121927192922202241
- SakuraiKEnomotoKAmanoSStudy of indoleamine 2,3-dioxygenase expression in patients of esophageal squamous cell carcinomaGan To Kagaku Ryoho200431111780178215553713
- Sperner-UnterwegerBNeurauterGKlieberMEnhanced tryptophan degradation in patients with ovarian carcinoma correlates with several serum soluble immune activation markersImmunobiology2011216329630120822828
- SaganDKockiTKockiJSxumiloJSerum kynurenic acid: possible association with invasiveness of non-small cell lung cancerAsian Pac J Cancer Prev20121394241424423167321
- MunnDHSharmaMDHouDExpression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodesJ Clin Invest200411428029015254595
- MellorALKeskinDBJohnsonTChandlerPMunnDHCells expressing indoleamine 2,3-dioxygenase inhibit T cell responsesJ Immunol200216883771377611937528
- CookeDTNguyenDVYangYSurvival comparison of adenosquamous, squamous cell, and adenocarcinoma of the lung after lobectomyAnn Thorac Surg201090394394820732522
- MaedaRYoshidaJIshiiGRisk factors for tumor recurrence in patients with early-stage (stage I and II) non-small cell lung cancer: patient selection criteria for adjuvant chemotherapy according to the 7th edition TNM classificationChest20111401494150221622548
- TomaszekSCKimYCassiviSDBronchial resection margin length and clinical outcome in non-small cell lung cancerEur J Cardiothorac Surg20114051151115621450488
- DaiFLiuLCheGThe number and microlocalization of tumor-associated immune cells are associated with patient’s survival time in non-small cell lung cancerBMC Cancer20101022020487543
- WatanabeKEmotoNHamanoEGenome structure-based screening identified epigenetically silenced microRNA associated with invasiveness in non-small-cell lung cancerInt J Cancer2011130112580259021702040
- OuXCaiSLiuPEnhancement of dendritic cell-tumor fusion vaccine potency by indoleamine-pyrrole 2,3-dioxygenase inhibitor, 1-MTJ Cancer Res Clin Oncol2008134552553317909857
- ZengJCaiSYiYPrevention of spontaneous tumor development in a ret transgenic mouse model by ret peptide vaccination with indoleamine 2,3-dioxygenase inhibitor 1-methyl tryptophanCancer Res20096993963397019383920
- JacksonEMintonSEIsmail-KhanRA Phase I Study of 1-Methyl-D-Tryptophan in Combination with Docetaxel in Metastatic Solid Tumors2012 ASCO Annual Meeting suppl; abstr TPS26202012 Available from: http://meetinglibrary.asco.org/content/95284-114Accessed June 21, 2017
