Abstract
Desmosomes, which are intercellular adhesive complexes, are essential for the maintenance of epithelial homeostasis. They are located at the cell membrane, where they act as anchors for intermediate filaments. Downregulation of desmosome proteins in various cancers promotes tumor progression. However, the role of desmosomes in carcinogenesis is still being elucidated. Recent studies revealed that desmosome family members play a crucial role in tumor suppression or tumor promotion. This review focuses on studies that provide insights into the role of desmosomes in carcinogenesis and address their molecular functions.
Introduction
Desmosomes are intercellular junctions that, in association with intermediate filaments, mechanically link cells and stabilize tissue architecture.Citation1 Desmosome structure was first observed in 1864 by Bizzozero, an Italian pathologist; its structure has since been analyzed by techniques such as electron microscopy (EM) to reveal a complex structure and organization. The desmosomal components comprise three main protein families: transmembrane cadherin family (desmogleins [DSGs] and desmocollins [DSCs]), armadillo (ARM) protein family (plakoglobin [PKG], plakophilins [PKPs], and β-catenin), and plakin protein family (desmoplakin [DSP]). Genes encoding desmosomal constituents were found mutated, which can have effects on tissue integrity; but they are not only simple static adhesive structures, increased evidences show that desmosomes also act as tumor suppressors or oncogenes in various cancers, regulating cell proliferation, differentiation, migration, apoptosis, and treatment sensitivity.Citation2 The following sections of this review describe the structure of the desmosome family members and functional characteristics of the major desmosomal proteins.
Desmosomal structure
Desmosomal cadherin family
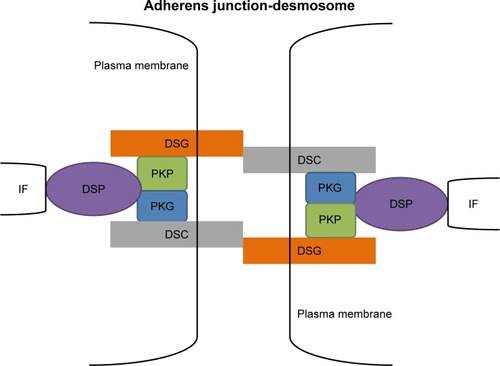
DSGs (Dsg1–4) and DSCs (Dsc1–3) are desmosomal cadherin family members found in humans. DSGs and DSCs are required for strong cell–cell adhesionCitation3 via their interaction with each other across the intercellular space (), in a homophilic and/or heterophilic manner; the difference between the two types of interactions remains unclear. These desmosomal cadherins show complex developmental and differentiation patterns of expression.Citation1,Citation4 Dsg1/3 and Dsc1/3 are present in stratified epithelia, and Dsg4 is found in stratified epithelia and hair.Citation5–Citation7 Dsg2 and Dsc2 are the primary isoforms in simple epithelia and are present at low levels in the basal layer of stratified epithelia.Citation6 All three DSC1-3 gene products undergo alternative splicing, resulting in the generation of the Dsc “a” form and a shorter Dsc “b” form of the proteins, which differ in the length of their respective carboxy-terminal domains.Citation8,Citation9 The DSC extracellular (EC) domains can be divided into a number of subdomains, including four cadherin-like EC domains and an extracellular anchor (EA) domain. DSG EC domains are organized in a similar fashion. Within the cell, both DSC “a” and “b” proteins possess an intracellular anchor (IA) domain, but only “a” form proteins have an intracellular cadherin-like sequence (ICS) domain. DSG cytoplasmic tails also have IA and ICS domains. DSC and DSG ICS domains provide binding sites for other desmosomal constituents.Citation10
ARM family
ARM family members are mainly β-catenin, PKG (or γ-catenin), and PKPs (PKP1–3).Citation11 They are characterized by the presence of a central domain, containing repeating units of a 42 amino acid sequence homology domain,Citation12 and they mediate the cytoplasmic associations with the cadherins. β-Catenin consists of several very characteristic repeats, each ~40 amino acids long. All these β-catenin elements fold together into a single, rigid protein domain with an elongated shape, called an ARM domain. PKG, which contains 12 arm repeats, exhibits dual localization in desmosomes and adherens junctions. In addition, β-catenin contributes to desmosomes only in PKG-negative organisms. PKPs contain 9 arm repeats with a flexible insert between repeats 5 and 6 that introduces a major bend in the overall structure.Citation13 There are two isoforms of PKPs 1 and 2, a shorter “a” form and a longer “b” form, generated by alternative splicing. PKP1a and 1b differ by the insertion of 21 amino acids between arm repeats 3 and 4, whereas PKP2a and 2b differ by the insertion of 44 amino acids between repeats 2 and 3.Citation14,Citation15
Plakin family
There are several plakin proteins, including DSP, plectin, envoplakin, and periplakin. DSP, which is the most abundant component of the plakin family, interacts with other desmosomal family members, such as PKG, PKPs, and intermediate filaments, providing the link in the chain from the plasma membrane to the cytoskeleton.Citation16 The DSP gene is located on chromosome 6p24.3, containing 24 exons and spanning ~45 kDa of genomic DNA.Citation17 There are two predominant isoforms; the first, known as “DPI”, has molecular weight 332 kDa (2,871 amino acids) and the second, known as “DPII”, has molecular weight 260 kDa (2,272 amino acids). These isoforms are identical except for the shorter rod domain in DPII. DPI is the predominant isoform expressed in cardiac muscle.Citation18 Although DPI and DPII are functionally redundant, loss of the C-terminal tail domain from DPI/DPII has devastating consequences on skin integrity and results in early neonatal death in lethal acantholytic epidermolysis bullosa.Citation19
The role of desmosome in cancer
During the past 15–20 years, studies revealed the role of desmosome in human diseases, especially in heart and skin diseases. Although the role of desmosome proteins in cancer development and progression is not clear, some recent progress has been made.Citation20 Recently, a body of evidence shows that they may influence epithelial cell invasion and metastasis since an important function of desmosomes related to cancer is their ability to inhibit cell motility.Citation21
Desmosomal cadherins and cancer
Recently, studies have shown that desmosomal proteins have both tumor-promoting and tumor-suppressive functions in different types of cancers.Citation22 For example, DSG2 is found to be overexpressed in skin cancer,Citation23 and overexpression of DSG2 promotes lung cancer cell growth through regulation of p27 and CDK2.Citation24 DSG3 was upregulated in head and neck cancer and lung cancer.Citation25,Citation26 Brown et alCitation27 showed that DSG3 promotes cancer cell migration and invasion by regulating AP-1 and PKC in head and neck cancer. By contrast, loss of DSC2 contributes to the growth of colorectal cancer cells by regulating Akt/β-catenin signaling.Citation28 DSC3 ablation increased the incidence of Ras-induced skin tumors in mice;Citation29 furthermore, it is found to be downregulated in breast, lung, and colorectal cancers due to promoter hypermethylation.Citation10,Citation30,Citation31 We found that ectopic expression of DSC3 by introducing a DSC3 expression vector into lung cancer cells successfully suppressed lung cancer cell growth and motility through inactivation of the EGFR/ERK signaling pathway.Citation30 Desmosomal cadherin proteins have also been considered as prognostic markers in various cancer types. For example, decreased DSG3 expression was associated with poor prognosis in lung cancer.Citation26 Our studies showed that DSC1 may be a marker for tumor differentiation, DSC3 has a potential diagnostic value in sub-classification of non-small-cell lung cancer (NSCLC) into squamous cell carcinoma (SCC) and adenocarcinoma (ADC), and furthermore, DSC1 and DSC3 may be prognostic markers for lung cancer.Citation32
ARM proteins and signaling pathway
ARM proteins also mediate important signal transduction pathways in human cancer. β-Catenin, which is widely expressed in many tissues, is involved in regulation and coordination of cell–cell adhesion and gene transcription. Meanwhile, it acts as an intracellular signal transducer in the Wnt signaling pathway. The Wnt signaling pathway plays an essential role in embryonic development and stemness and has also been described in carcinogenesis.Citation33 When the Wnt signaling pathway is activated by the binding of Wnt ligands to the Frizzled receptors and the low-density lipoprotein co-receptors, the degradation complex is inactivated, resulting in the stabilization of β-catenin. This leads to the translocation of β-catenin into the nucleus where it associates with the lymphoid enhancer factor/T-cell factor (LEF/TCF) family of transcription factors to activate many downstream target genes.Citation34 More details of the Wnt/β-catenin signaling pathway can be found elsewhere.Citation33
PKG, which is closely related to β-catenin, is another important member of ARM family. It interacts with similar molecules, such as β-catenin. Miravet et alCitation35 found that PKG could reduce transcription of Wnt target genes through binding to adjacent sites on Tcf-4 with β-catenin and inhibiting binding of Tcf-4 to DNA. Thus, PKG is a negative regulator of Wnt/β-catenin signaling and acts as a tumor/metastasis suppressor in various cancers. For example, in ovarian cancer, exogenous expression of PKG or knockdown of N-cadherin is more effective than expression of E-cadherin in inhibiting the growth, migratory, and invasive properties of ES-2 cells.Citation36 PKG-mediated HAI-1 regulation offers a promising novel strategy to inhibit the c-MET signaling pathway in lung cancer.Citation37 Silencing PKG in esophageal cancer cells causes defects in cell–cell adhesion and a concomitant increase in cell migration.Citation38
PKPs, which have been characterized as desmosomal plaque proteins, stabilize desmosomal cadherins at the plasma membrane and interact with the cytoskeletal linker protein DSP. They are predominantly expressed in epithelial cells with distinct expression patterns. PKP 1 is found in suprabasal layers of epithelia, while PKPs 2 and 3 localize to desmosomes from simple epithelia.Citation39 Like β-catenin, PKPs have diverse non-desmosomal functions. Loss of PKPs 1–3 is found in some tumors.Citation40–Citation42 Reduced PKP 3 expression is correlated with desmosome instability, increased cell migration, and poor prognoses for patients. Mechanistically, it is transcriptionally repressed by E-cadherin repressor ZEB1 in tumor cells, which suggests a common regulation for adherens junctions and desmosomes during tumor progression.Citation43 Surprisingly, in other tumors, PKP 1 or 3 is overexpressed, such as head and neck tumors,Citation44 lung cancer,Citation26 and Ewing sarcoma.Citation45
DSP and Wnt signaling pathway
DSP proteins are widely expressed in numerous tissues.Citation46,Citation47 Loss of expression of DSP promotes increased local tumor invasion in a mouse model of pancreatic neuroendocrine carcinogenesis.Citation48 Interestingly, in keratinocytes, decreased expression of DSP could increase cell proliferation associated with elevated phospho-ERK1/2 and phospho-Akt levels.Citation49 In our studies, we found that DNA methylation contributes to the downregulation of DSP in lung cancer. Additionally, ectopic expression of DSP enhanced expression of PKG (γ-catenin), which is a component modulating the Wnt signaling pathway, resulting in decreased TCF/LEF-dependent transcriptional activity and reduced expression of the Wnt/β-catenin target genes, Axin2 and matrix metalloproteinase MMP14. The epigenetic regulation of DSP and its ability to increase the sensitivity to anticancer drug-induced apoptosis has potential implications for clinical application.Citation50
Conclusion
Although a substantial amount of evidence is available to support the idea that desmosomes are involved in progression of cancer, unlike the role of desmosomes in adherens junctions, our understanding of the role of desmosomes and how they are involved in cancer and metastasis is still evolving. Additional studies are necessary to explore the complete function of desmosomes in human cancer. A better understanding of the regulatory mechanisms of the expression changes of desmosomes and their role as mediators of intracellular signal transduction will be important, especially for personalized therapeutic strategies. Progression on the mechanistic study will lead to a better understanding of the role of desmosomes in malignancy and have implications for cancer treatment.
Disclosure
The authors report no conflicts of interest in this work.
References
- HolthoferBWindofferRTroyanovskySLeubeREStructure and function of desmosomesInt Rev Cytol20072646516317964922
- DusekRLAttardiLDDesmosomes: new perpetrators in tumour suppressionNat Rev Cancer201111531732321508970
- GetsiosSHuenACGreenKJWorking out the strength and flexibility of desmosomesNat Rev Mol Cell Biol20045427128115071552
- KowalczykAPGreenKJStructure, function, and regulation of desmosomesProg Mol Biol Transl Sci20131169511823481192
- BrookeMANitoiuDKelsellDPCell-cell connectivity: desmosomes and diseaseJ Pathol2012226215817121989576
- GarrodDChidgeyMDesmosome structure, composition and functionBiochim Biophys Acta20081778357258717854763
- JohnsonJLKoetsierJLSiricoAThe desmosomal protein desmoglein 1 aids recovery of epidermal differentiation after acute UV light exposureJ Invest Dermatol201413482154216224594668
- CollinsJELeganPKKennyTPMacGarvieJHoltonJLGarrodDRCloning and sequence analysis of desmosomal glycoproteins 2 and 3 (desmocollins): cadherin-like desmosomal adhesion molecules with heterogeneous cytoplasmic domainsJ Cell Biol199111323813912010468
- ParkerAEWheelerGNArnemannJDesmosomal glycoproteins II and III. Cadherin-like junctional molecules generated by alternative splicingJ Biol Chem19912661610438104452037591
- CuiTChenYYangLDSC3 expression is regulated by p53, and methylation of DSC3 DNA is a prognostic marker in human colorectal cancerBr J Cancer201110461013101921364582
- HatzfeldMThe p120 family of cell adhesion moleculesEur J Cell Biol2005842–320521415819401
- PeiferMMcCreaPDGreenKJWieschausEGumbinerBMThe vertebrate adhesive junction proteins beta-catenin and plakoglobin and the Drosophila segment polarity gene armadillo form a multigene family with similar propertiesJ Cell Biol199211836816911639851
- ChoiHJWeisWIStructure of the armadillo repeat domain of plakophilin 1J Mol Biol2005346136737615663951
- MertensCKuhnCFrankeWWPlakophilins 2a and 2b: constitutive proteins of dual location in the karyoplasm and the desmosomal plaqueJ Cell Biol19961354100910258922383
- SchmidtALangbeinLRodeMPratzelSZimbelmannRFrankeWWPlakophilins 1a and 1b: widespread nuclear proteins recruited in specific epithelial cells as desmosomal plaque componentsCell Tissue Res199729034814999369526
- SmithEAFuchsEDefining the interactions between intermediate filaments and desmosomesJ Cell Biol19981415122912419606214
- GreenKJParryDASteinertPMStructure of the human desmoplakins. Implications for function in the desmosomal plaqueJ Biol Chem19902651911406114072391353
- Al-JassarCBikkerHOverduinMChidgeyMMechanistic basis of desmosome-targeted diseasesJ Mol Biol2013425214006402223911551
- McGrathJAMcMillanJRShemankoCSMutations in the plakophilin 1 gene result in ectodermal dysplasia/skin fragility syndromeNat Genet19971722402449326952
- BroussardJAGetsiosSGreenKJDesmosome regulation and signaling in diseaseCell Tissue Res2015360350151225693896
- TselepisCChidgeyMNorthAGarrodDDesmosomal adhesion inhibits invasive behaviorProc Natl Acad Sci U S A19989514806480699653140
- ChidgeyMDawsonCDesmosomes: a role in cancer?Br J Cancer200796121783178717519903
- KurzenHMunzingIHartschuhWExpression of desmosomal proteins in squamous cell carcinomas of the skinJ Cutan Pathol2003301062163014744087
- CaiFZhuQMiaoYShenSSuXShiYDesmoglein-2 is over-expressed in non-small cell lung cancer tissues and its knockdown suppresses NSCLC growth by regulation of p27 and CDK2J Cancer Res Clin Oncol20171431596927629878
- ChenYJChangJTLeeLDSG3 is overexpressed in head neck cancer and is a potential molecular target for inhibition of oncogenesisOncogene200726346747616878157
- FurukawaCDaigoYIshikawaNPlakophilin 3 oncogene as prognostic marker and therapeutic target for lung cancerCancer Res200565167102711016103059
- BrownLWaseemACruzINDesmoglein 3 promotes cancer cell migration and invasion by regulating activator protein 1 and protein kinase C-dependent-Ezrin activationOncogene201433182363237423752190
- KolegraffKNavaPHelmsMNParkosCANusratALoss of desmocollin-2 confers a tumorigenic phenotype to colonic epithelial cells through activation of Akt/beta-catenin signalingMol Biol Cell20112281121113421325624
- ChenJO’SheaCFitzpatrickJEKosterMIKochPJLoss of desmocollin 3 in skin tumor development and progressionMol Carcinog201251753554521681825
- CuiTChenYYangLThe p53 target gene desmocollin 3 acts as a novel tumor suppressor through inhibiting EGFR/ERK pathway in human lung cancerCarcinogenesis201233122326233322941060
- OshiroMMKimCJWozniakRJEpigenetic silencing of DSC3 is a common event in human breast cancerBreast Cancer Res200575R669R68016168112
- CuiTChenYYangLDiagnostic and prognostic impact of desmocollins in human lung cancerJ Clin Pathol201265121100110623002285
- LiTWTingJHYokoyamaNNBernsteinAvan de WeteringMWatermanMLWnt activation and alternative promoter repression of LEF1 in colon cancerMol Cell Biol200626145284529916809766
- MacDonaldBTTamaiKHeXWnt/beta-catenin signaling: components, mechanisms, and diseasesDev Cell200917192619619488
- MiravetSPiedraJMiroFItarteEGarcia de HerrerosADunachMThe transcriptional factor Tcf-4 contains different binding sites for beta-catenin and plakoglobinJ Biol Chem200227731884189111711551
- AlaeeMDaneshGPasdarMPlakoglobin reduces the in vitro growth, migration and invasion of ovarian cancer cells expressing N-cadherin and mutant p53PLoS One2016115e015432327144941
- SechlerMBorowiczSVan ScoykMNovel role for gamma-catenin in the regulation of cancer cell migration via the induction of hepatocyte growth factor activator inhibitor type 1 (HAI-1)J Biol Chem201529025156101562025925948
- FangWKLiaoLDLiLYDown-regulated desmocollin-2 promotes cell aggressiveness through redistributing adherens junctions and activating beta-catenin signalling in oesophageal squamous cell carcinomaJ Pathol2013231225727023836524
- Bass-ZubekAEHobbsRPAmargoEVPlakophilin 2: a critical scaffold for PKC alpha that regulates intercellular junction assemblyJ Cell Biol2008181460561318474624
- Schmitt-GraeffAKoeningerAOlschewskiMThe Ki67+ proliferation index correlates with increased cellular retinol-binding protein-1 and the coordinated loss of plakophilin-1 and desmoplakin during progression of cervical squamous lesionsHistopathology2007511879717593084
- SchwarzJAyimASchmidtADifferential expression of desmosomal plakophilins in various types of carcinomas: correlation with cell type and differentiationHum Pathol200637561362216647960
- Sobolik-DelmaireTReddyRPashajARobertsBJWahlJK3rdPlakophilin-1 localizes to the nucleus and interacts with single-stranded DNAJ Invest Dermatol2010130112638264620613778
- AignerKDescovichLMikulaMThe transcription factor ZEB1 (deltaEF1) represses Plakophilin 3 during human cancer progressionFEBS Lett200758181617162417391671
- VillaretDBWangTDillonDIdentification of genes overexpressed in head and neck squamous cell carcinoma using a combination of complementary DNA subtraction and microarray analysisLaryn-goscope20001103 pt 1374381
- CheungIYFengYDanisKNovel markers of subclinical disease for Ewing family tumors from gene expression profilingClin Cancer Res200713236978698318056173
- AngstBDNillesLAGreenKJDesmoplakin II expression is not restricted to stratified epitheliaJ Cell Sci199097pt 22472572277092
- DelvaETuckerDKKowalczykAPThe desmosomeCold Spring Harb Perspect Biol200912a00254320066089
- ChunMGHanahanDGenetic deletion of the desmosomal component desmoplakin promotes tumor microinvasion in a mouse model of pancreatic neuroendocrine carcinogenesisPLoS Genet201069e100112020862307
- WanHSouthAPHartIRIncreased keratinocyte proliferation initiated through downregulation of desmoplakin by RNA interferenceExp Cell Res2007313112336234417475244
- YangLChenYCuiTDesmoplakin acts as a tumor suppressor by inhibition of the Wnt/beta-catenin signaling pathway in human lung cancerCarcinogenesis201233101863187022791817