Abstract
Purpose
Refractory pediatric leukemia remains one of the leading causes of death in children. Intensification of current chemotherapy regimens to improve the outcome in these children is often limited by the effects of drug resistance and cumulative toxicity. Hence, the search for newer agents and novel therapeutic approaches are urgently needed to formulate the next-generation early-phase clinical trials for these patients.
Materials and methods
A comprehensive library of antimicrobials, including eight HIV protease inhibitors (nelfinavir [NFV], saquinavir, indinavir, ritonavir, amprenavir, atazanavir, lopinavir, and darunavir), was tested against a panel of pediatric leukemia cells by in vitro growth inhibition studies. Detailed target modulation studies were carried out by Western blot analyses. In addition, drug synergy experiments with conventional and novel antitumor agents were completed to identify effective treatment regimens for future clinical trials.
Results
Several of the HIV protease inhibitors showed cytotoxicity at physiologically relevant concentrations (half-maximal inhibitory concentration values ranging from 1–24 µM). In particular, NFV was found to exhibit the most potent antileukemic properties across all cell lines tested. Mechanistic studies show that NFV leads to the induction of autophagy and apoptosis possibly through the induction of endoplasmic reticulum stress. Furthermore, interference with cell signaling pathways, including Akt and mTOR, was also noted. Finally, drug combination studies have identified agents with potential for synergy with NFV in its antileukemic activity. These include JQ1 (BET inhibitor), AT101 (Bcl-2 family inhibitor), and sunitinib (TK inhibitor).
Conclusion
Here, we show data demonstrating the potential of a previously unexplored group of drugs to address an unmet therapeutic need in pediatric oncology. The data presented provide preclinical supportive evidence and rationale for future studies of these agents for refractory leukemia in children.
Introduction
Despite the recent advances achieved in the treatment of leukemia in children, the prognosis of relapsed and refractory disease remains dismal with more than 50% mortality rate.Citation1–Citation3 Acute and long-term toxicities of current chemotherapy agents often limit their further intensification, thus highlighting an urgent need for novel therapeutic approaches to develop newer, safer treatment protocols. One approach to circumvent such toxicity concerns is to consider activity in compounds with previously established clinical data and use, such as that of antimicrobials.
Employing agents from the field of infectious diseases in the fight against cancer has historical precedence, as exampled by the chemotherapeutic fludarabine, which is a fluorinated nucleotide analog of the antiviral agent vidarabine;Citation4 or the tetracycline, doxycycline which has shown activity against breast cancer.Citation5 Here, we bring focus to a class of antivirals – the HIV protease inhibitors (PIs) – which have activity against pediatric leukemia.
The HIV PIs were first developed in the early 1990s, beginning with saquinavir that was rationally designed to fit the HIV aspartyl protease – an enzyme for which there are no human analogs.Citation6 First approved for use by 1995, the PIs have had over two decades of clinical use in the treatment regimens of HIV patients, including use in children.Citation6 In HIV patients on PI-based therapy, it was noted that there was a dramatic drop in the rates of HIV-associated malignancies, unrelated to the viral loads or CD4 counts.Citation7,Citation8 This had led to studies that evaluated the effects of PIs in cancer therapy.Citation9–Citation11 Some of the earlier studies included murine xenograft models of Kaposi’s sarcoma, whereby Sgadari and colleagues demonstrated several PIs had effects on tumor growth, invasion, angiogenesis, and survival.Citation10,Citation11 Ikezoe et al pursued the PIs’ application to multiple myeloma, demonstrating effects on cell cycle arrest and STAT3 and ERK signaling.Citation12 By the early 2000s, PIs have been shown to have activity in vitro against a number of adult solid tumors, the results of which are reported in a landmark paper by Gills et al.Citation13 Here, a 60-cell line panel of malignancies, including breast, colon, lung, renal, leukemia, and melanoma, were treated with nelfinavir (NFV) to reveal growth inhibition at ranges between 10−5 and 10−6 M.Citation13 Additionally, they confirm cytotoxicity via apoptosis and provide evidence for mechanisms of endoplasmic reticulum (ER) stress and autophagy.Citation13
Supported by the findings from these studies, a number of clinical trials have been conducted, with promising early results ().Citation14–Citation18 For example, in treatment-resistant multiple myeloma, a phase I clinical trial of NFV with bortezimib showed partial response in one-third of patients, as well as safety and tolerability of NFV at doses used.Citation17 Similarly, in a phase I trial of advanced stage non-small-cell lung cancer, NFV given in combination with radiation resulted in complete response in five of nine patients and partial response in the remaining four patients.Citation18 Again, for a phase I trial in pancreatic cancer, NFV with radiation showed partial computed tomography (CT) responses in five of ten patients with a substantial reduction in tumor size atypical for radiation treatment alone.Citation16 Finally, a phase I a clinical trial of NFV against various adult solid tumors also showed partial responses in up to 3 of 11 of evaluable patients with drug safety and tolerability.Citation15 Additionally, there is some preclinical work on PI-derivatives showing promising results, including in leukemia.Citation19–Citation22
Despite the early promising data obtained from adult cancer studies, however, specific investigations directed against pediatric malignancies so far have not been reported. The present study describes the identification NFV-mediated cytotoxicity in pediatric leukemia cells and evaluation of mechanisms involved in this process. We also provide information on agents with potential for effective drug combination for evaluation in future treatment protocols.
Material and methods
Cell lines and cell culture
Cell lines used were SEM, C1, Molt3, TIB202, Molm13, and MV4-11, purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and are previously described.Citation23 Additionally, the cell line Poetic2.2 was also employed – a line developed in our laboratory derived from a 13-year old pre-B acute lymphoblastic leukemia patient with a p16 deletion (Conjoint Health Research Ethics Board [CHREB] number: HREBA.CC-16-0286). Cells were maintained in Opti-MEM media (Gibco, Invitrogen Corporation, Burlington, ON, Canada) supplemented with 10% heat-inactivated fetal bovine serum, 100 units/mL of penicillin, and 100 µg/mL of streptomycin (Gibco) and incubated in a humidified 5% CO2 incubator. Cells were passaged approximately every 3–5 days, by centrifuging the suspension and resuspending the pellet in fresh media. Primary leukemic cells were obtained after ethics board approval (CHREB number: HREBA.CC-16-0286) and written informed consent from the parent or guardian. Normal lymphocytes were obtained from healthy volunteers after receipt of their verbal consent. Lymphocytes were isolated by density gradient centrifugation using Ficoll-Paque Plus (GE Healthcare Life Sciences, Mississauga, ON, Canada).
Materials and reagents
The antimicrobial library, consisting of 112 compounds (68 antibacterials; 39 antivirals; and five other) was purchased from Selleck Chemicals (Burlington, ON, Canada) and contained the PIs, NFV, indinavir, amprenavir, and atazanavir. Additional PIs – lopinavir, ritonavir, darunavir (DRV), and saquinavir were purchased from Sigma-Aldrich (St Louis, MO, USA). Compounds were dissolved in dimethylsulfoxide (DMSO) to a stock concentration of 10 mM. Subsequent dilutions were made using cell culture media, as needed, to gain concentrations of 40 to 10−6 µM.
In vitro cytotoxicity assays
For the antimicrobial library screening, three cell lines were used in the testing, C1, Molt3, and Poetic2.2. Cells were passaged during exponential growth and seeded in 96-well plates (Grenier BioOne, Monroe, NC, USA) at 5×103 cells per well. Using four antimicrobial drug dilutions (0.01, 0.1, 1, and 10 µM) and the corresponding DMSO vehicle control, the drugs were added to the plates and incubated. After 4 days, cell viability was quantified using Alamar blue assay (Invitrogen, Burlington, ON, Canada) and cytotoxicity curves created. For the more detailed cytotoxicity curves, a larger dilution series was employed, and calculation of the half- and quarter-maximal inhibitory concentrations (IC50 and IC25, respectively) were determined for each drug based on independent cytotoxicity plots.
Drug combination studies
Pediatric leukemia cells were cultured as described above. SEM and Molm13 cell lines were selected as representative cell lines for the set and underwent the initial synergism screen against 35 chemotherapeutic agents, both conventional and novel. For the drug combination studies, the chemotherapy agents were first plated at 1 and 0.1 µM concentrations in triplicate wells of 96-well plates. NFV was added to each according to predetermined IC25 values. Cells were then plated at 5×103 cells/well. After 4 days of incubation, cell survival was quantified as above. Agents with the highest activity (ie, lowest IC50) in combination with NFV were selected for more detailed analyses against all seven cell lines to confirm the consistency of the findings across the broader panel. Various concentrations, ranging from 10 to 10−6 µM, were added to the wells alone or in combination with the corresponding IC25 of NFV for that cell line. Cell numbers and viability were determined after 4 days. The combination indices (CIs) were determined as previously described,Citation24 where a CI <1 indicates synergy between the agents, CI =1 indicates that the agents are working additively, and CI >1 indicates that the agents are antagonistic.
Target modulation analyses
Target modulation by PIs was determined by Western blot analyses. Briefly, cells were incubated with the PI or appropriate control for 6, 12, or 24 h, as indicated, then lysed using radioimmunoprecipitation assay-based lysis buffer containing phosphatase and PIs. Lysates were resolved and transferred electrophoretically, and then probed using specific antibodies (Cell Signaling, Danvers, MA, USA; EMD Millipore, Billerica, MA, USA; and AbCam, Cambridge, UK).
Results
PIs show potential as antileukemics
Testing of the antimicrobial library against a panel of leukemia cell lines revealed several compounds with cytotoxic effects (data not shown), including several drugs from the antiviral class, the HIV PIs. As a group, these agents showed a class-effect cytotoxicity at the upper 10 µM concentration of the screen, reaching IC20–25 for NFV, ritonavir, and lopinavir. This provided the initial suggestion to further investigate this class of agents.
PIs as a class are cytotoxic to leukemia
Extending the findings from the initial screen, eight different US Food and Drug Administration-approved PIs were tested for cytotoxicity against the seven pediatric leukemia cell lines. Their IC50 values are reported in and range from 1 to >40 µM. As a comparator for clinically relevant plasma levels, the table also includes the typical drug levels obtained from HIV dosing regimens for each of the corresponding PIs. Boxes have been color-coded according to presumed feasibility of achieving that level in the plasma based on current HIV dosing regimens.Citation25,Citation26 Overall, the PIs with the most consistent activity across cell lines was NFV. Lopinavir, ritonavir, and saquinavir also showed activity against many of the cell lines tested.
Table 1 Summary of IC50 values (µM) for eight FDA-approved HIV-protease inhibitors tested against seven different pediatric leukemia cell lines
NFV shows selective targeting of leukemic cells
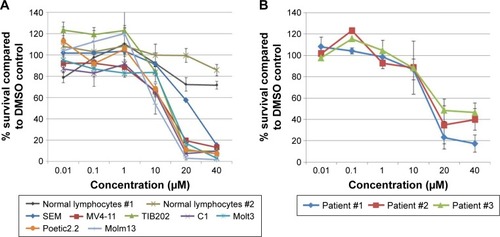
Given the findings from the class analyses, NFV was selected for more detailed cytotoxicity assays. Assessment of activity against seven cell lines gave IC50s ranging from 1 to 24 µM (). To determine the degree of selectivity of NFV toward leukemia cells, two populations of normal lymphocytes from healthy volunteers were also screened and showed minimal cytotoxicity at the upper concentrations tested (). Efficacy against primary leukemic cells was also demonstrated, showing analogous cytotoxicity curves with IC50 ranging from 16–20 µM ().
Figure 1 (A) Cytotoxicity assays of NFV against seven pediatric leukemia cell lines. Normal lymphocyte cytotoxicity is included for control. For the cell lines, plots are representative of at least triplicate independent experiments. (B) Cytotoxicity of NFV against primary leukemia cells.
NFV induces ER stress, leading to autophagy and apoptosis
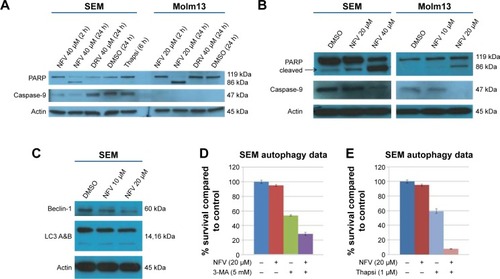
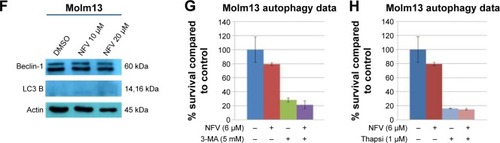
To delineate the underlying mechanisms of NFV on leukemia, we selected two of the above cell lines, SEM (an ALL cell line) and Molm13 (an AML cell line). Here, we show that treatment of these cells with NFV induces ER stress in the cell, as shown by elevated levels of the phosphorylated ER stress marker, eIF2α – a protein subunit that controls the initiation of mRNA translation. In its phosphorylated form, P-eIF2α prevents the further translation of proteins, reducing the ER workload in an attempt to allow the cell to recover.Citation27 When cells are treated with NFV, a concentration- and time-dependent increase in this marker was observed (). Also, there is simultaneous elevation of a downstream marker, CHOP, and an additional stress signaling marker, ATF3. This added cellular stress may subsequently induce the processes of autophagy and apoptosis (). Immunoblots of autophagy markers show relatively stable levels of Beclin-1 and LC3B), in the treated and untreated cells (), consistent with the complexity and specificity of measuring autophagy in such systems.Citation28 We thus show additional support of the cells’ reliance on this pathway through the use of autophagy inhibitors, 3-methyladenine and thapsigargin, which result in significant increase in cell death when the cell cannot rely on this pathway (). Analysis of apoptosis markers shows increasing cleavage of both PARP and caspase-9 (via loss of the whole caspase-9) in both a time- and concentration-dependent manner, supporting cytotoxicity through apoptosis (). As a negative control, we included darunavir – a PI with no cytotoxic effects at the doses tested, and it is noted that DRV does not induce elevation of any of the stress or apoptosis markers.
Figure 2 ER stress induced in NFV-treated and untreated cells.
Abbreviations: Conc, concentration; DMSO, dimethylsulfoxide; DRV, darunavir; ER, endoplasmic reticulum; NFV, nelfinavir.
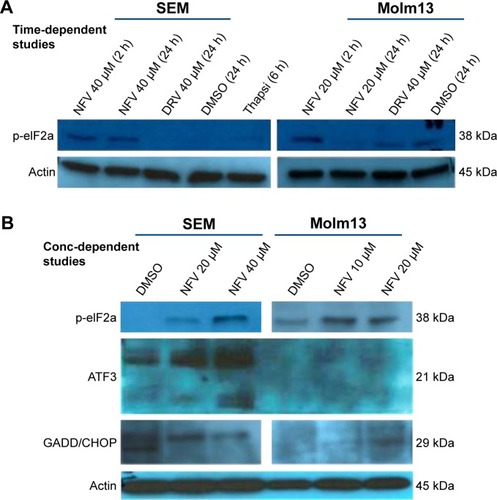
Figure 3 Autophagy and apoptosis in NFV-treated and untreated cells.
Abbreviations: 3-MA, 3-methyladenine; DMSO, dimethylsulfoxide; DRV, darunavir; NFV, nelfinavir; Thapsi, thapsigargin.
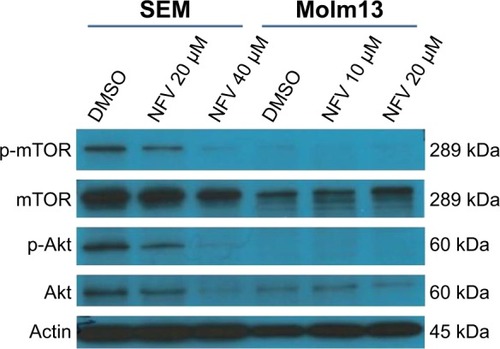
NFV inhibits Akt and mTOR signaling
Akt and mTOR signaling pathways are central to cellular proliferation and regulation of apoptosis in cells and are often upregulated in many types of cancers.Citation29 To determine the effects of NFV on these pathways, immunoblots were performed and show decreased phosphorylation of both Akt and mTOR with increasing concentrations of NFV ().
NFV activity shows synergy with specific antileukemic agents
To determine which drugs NFV could be combined with to provide enhanced antileukemic activity, the SEM and Molm13 cell lines were screened against a panel of 35 different chemotherapy agents, both novel and conventional, with diverse mechanisms of action. The drug panel was screened alone and in combination with NFV at two different concentrations, 1 µM and 0.1 µM ( and S1). Based on these results, compounds that showed more than an additive change in cytotoxicity when combined were selected for more detailed studies to assess synergy and determine CIs. Agents selected were JQ1 (BET inhibitor), AT101 (Bcl-2 family inhibitor), and Sunitinib (TK inhibitor). CIs when tested across the panel of leukemia cell lines ranged from 0.07 to 0.95 for JQ1; 0.05–1.8 for AT101; and 0.013–0.6 for Sunitinib, implying synergistic effects across most cell lines (; ).
Figure 5 Combination studies with known chemotherapy regimens in SEM.
Abbreviations: DMSO, dimethylsulfoxide; IC25, quarter-maximal inhibitory concentration; NFV, nelfinavir.
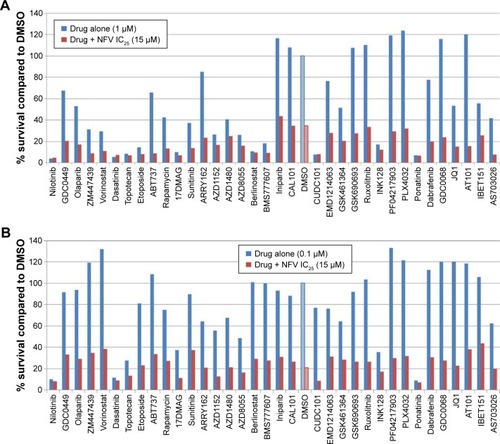
Figure 6 Combination studies of NFV with JQ1, Sunitinib, and AT101.
Abbreviations: DMSO, dimethylsulfoxide; IC25, quarter-maximal inhibitory concentration; NFV, nelfinavir.
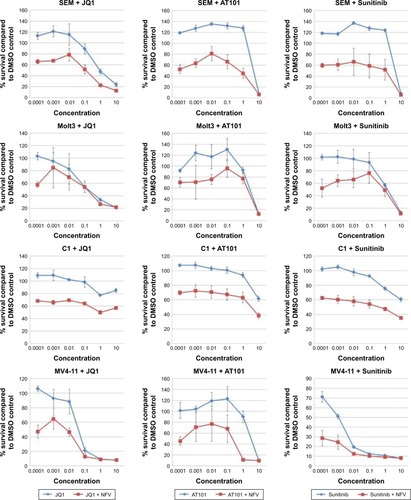
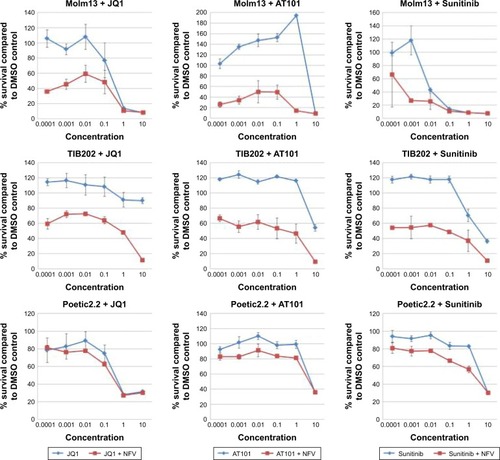
Table 2 Combination indices of nelfinavir in combination with JQ1, AT101, and Sunitinib
Discussion
The acute and long-term toxicities of chemotherapy regimens used to treat refractory and relapsed pediatric leukemia have prompted the search for newer, safer agents. Here, through a search of antimicrobials, we identify the HIV PIs, with their decades of use and availability of pharmacokinetic and toxicity data, as potential candidates for the safe combination and incorporation into chemotherapy regimens.
The PIs have only just begun to be noticed for their anticancer properties with preliminary associations made in HIV patients with malignancies.Citation7,Citation8 NFV, in particular, has received the most attention with preliminary studies emerging in numerous adult malignancies, including castration-resistant prostate cancer,Citation30,Citation31 rectal cancer,Citation32,Citation33 pancreatic cancer,Citation34 breast cancer,Citation35 and lung cancerCitation14,Citation18,Citation36 (). Though never before reported in pediatric cancers, our current work on pediatric leukemias suggest that NFV would also have significant antileukemic properties, with IC50s ranging from 1 to 15 µM – concentrations that are physiologically obtainable based on current oral HIV dosing regimens.Citation25,Citation26
The mechanism by which NFV exerts its effects appears to involve the induction of ER stress (), which may drive the cell toward autophagy and ultimately, apoptosis (). A similar mechanism has been shown in some adult cancers in vitro where NFV has been shown to induce ER stress in head and neck, breast, and prostate cancer cells.Citation37–Citation39 The autophagy induction is further characterized by the application of autophagy inhibitors, 3-methyladenine and thapsigargin, which greatly reduce cell survival when combined with NFV (). This is consistent with previous findings where, when similar inhibitors resulted in lethal or near-lethal cellular toxicity.Citation40 When the cancer cells’ homeostatic mechanisms are overwhelmed, cells undergo apoptosis. Here, we also show elevated levels of apoptosis markers, including cleaved PARP and caspase-9, in response to NFV treatment, supporting this mechanism of cell death ().
NFV also acts by targeting crucial cell-signaling pathways, such as Akt and mTOR.Citation40 Here, we show that these mechanisms are present in its effect in pediatric leukemia cells, showing both time- and concentration-dependent effects on cell signaling (). Again, such signaling pathways are highlighted as a key target and one of the hallmarks in cancer.Citation41 Interestingly, it is this off-target effect on mTOR that has also been shown to play a role in the treatment of HIV infection.Citation42 Additional pathways implicated in the literature with NFV include oxidative stress mechanisms,Citation26,Citation43 decreased angiogenesis,Citation14,Citation44 and cell cycle arrest.Citation14,Citation26,Citation45
In future clinical trials, the inclusion of NFV in combination, rather than as a single agent, would provide additional benefits with enhanced tumor killing, reduction of chemotherapy-specific toxicities, and, ideally, avoidance of therapy resistance. NFV has already been considered in combination with other chemotherapy agents in clinical trials, including with doxorubicin for breast cancerCitation35 and with bortezomib for myeloma.Citation17 For both cases, NFV was able to act synergistically with the standard therapeutics to overcome drug resistance. In the former case, NFV was able to enhance the effects of doxorubicin against a multidrug-resistant breast cancer cell line (MCF-7/Dox), considered to be multifactorial and involving suppression of P-gp-mediated drug efflux, inhibition of Akt activation, and induction ER stress, leading to autophagy.Citation35 Similarly, in the case of myeloma, NFV was able to enhance bortezomib to overcome both bortezomib and carfilzomib resistance, again, attributed to ER stress leading to increased unfolded protein response (UPR) pathway-induced apoptosis.Citation46 Additionally, owing to its influence on cellular stress, NFV has also been considered in combination with radiation therapy.Citation33,Citation39,Citation47,Citation48 To consider NFV’s application in the treatment of pediatric leukemia, we examined a panel of mechanistically diverse chemotherapeutic drugs in combination with NFV. This screen revealed synergy with the BET inhibitor, JQ1, with CIs ranging from 0.07 to 0.97. Based on the evidence of NFV’s influence on cellular stress and signaling targets, synergy with agents that alter epigenetic processes may be postulated. Indeed, there is evidence of synergy in literature of epigenetic drugs with both ER stress inducers and mTOR inhibitors.Citation49–Citation51 Similarly, for other pathways revealed, the Bcl-2 family inhibitor, AT101, and the TK inhibitor, Sunitinib, synergy with drugs that induce ER stress have been shown previously.Citation36,Citation52 For these combinations, in our studies, CIs ranged from 0.05 to 1.8 and 0.013 to 0.6, respectively, providing evidence for their use together.
Conclusion
Pediatric leukemia remains a critical area for ongoing research as our current therapies remain toxic to healthy cells and relapses of chemoresistant-disease continue to occur at unacceptable rates. Here we show that HIV PIs, namely NFV, that has been employed for decades against the HIV virus in both adults and children, has selective anticancer effects with a therapeutic window compared to normal lymphocytes. We have also identified the potential mechanisms behind this observation. Finally, we show that the drug is able to act synergistically with certain currently approved antileukemic agents providing the framework to design of a future in vivo studies and subsequently early-phase clinical trials for refractory leukemia in children.
Acknowledgments
This study was supported in part by the POETIC Foundation, Alberta Children’s Hospital Foundation, and the Kids Cancer Care Foundation of Alberta (KCCFA).
Supplementary materials
Figure S1 Combination studies with known chemotherapy regimens in Molm13.
Notes: Drugs were plated in 96-well plates alone and in combination with the IC50 of NFV, determined from the corresponding cell line cytotoxicity screens. (A) Plated drugs at 1 µM final concentration; (B) drugs at 0.1 µM. The cells were then added to each (5,000 cells/well) and incubated for four days before being read by Alamar blue assay. The control (DMSO) and NFV alone are shown with patterned bars.
Abbreviations: DMSO, dimetthylsulfoxide; IC50, half-maximal inhibitory concentration; NFV, nelfinavir.
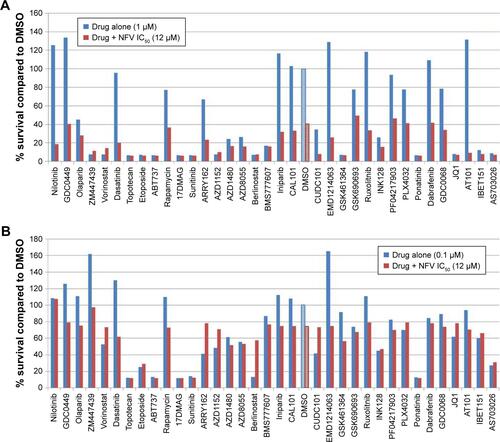
Table S1 Summary of clinical trials involving NFV to date (as per the NCI Clinical Trials Database)
Disclosure
The authors report no conflicts of interest in this work.
References
- AdamsonPCImproving the outcome for children with cancer: development of targeted new agentsCA: Cancer J Clin201565321222025754421
- GuestEMStamRWUpdates in the biology and therapy for infant acute lymphoblastic leukemiaCurr Opin Pediatr2017291202627841777
- TagaTTomizawaDTakahashiHAdachiSAcute myeloid leukemia in children: Current status and future directionsPediatr Int2016582718026645706
- JacksonGHUse of fludarabine in the treatment of acute myeloid leukemiaHematol J20045Suppl 1S62S6715079154
- DuivenvoordenWCHirteHWSinghGUse of tetracycline as an inhibitor of matrix metalloproteinase activity secreted by human bone-metastasizing cancer cellsInvasion Metastasis19971763123229949290
- FlexnerCHIV drug development: the next 25 yearsNat Rev Drug Discov200761295996617932493
- MoniniPSgadariCToschiEBarillariGEnsoliBAntitumour effects of antiretroviral therapyNat Rev Cancer200441186187515516959
- ChowWAJiangCGuanMAnti-HIV drugs for cancer therapeutics: back to the future?Lancet Oncol2009101617119111246
- SchmidtkeGHolzhütterHGBogyoMHow an inhibitor of the HIV-I protease modulates proteasome activityJ Biol Chem199927450357343574010585454
- SgadariCBarillariGToschiEHIV protease inhibitors are potent anti-angiogenic molecules and promote regression of Kaposi sarcomaNat Med20028322523211875492
- SgadariCMoniniPBarillariGEnsoliBUse of HIV protease inhibitors to block Kaposi’s sarcoma and tumour growthLancet Oncol20034953754712965274
- IkezoeTSaitoTBandobashiKYangYKoefflerHPTaguchiHHIV-1 protease inhibitor induces growth arrest and apoptosis of human multiple myeloma cells via inactivation of signal transducer and activator of transcription 3 and extracellular signal-regulated kinase 1/2Mol Cancer Ther20043447347915078991
- GillsJJLopiccoloJTsurutaniJNelfinavir, A lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis in vitro and in vivoClin Cancer Res200713175183519417785575
- KoltaiTNelfinavir and other protease inhibitors in cancer: mechanisms involved in anticancer activityF1000Research20154926097685
- BlumenthalGMGillsJJBallasMSA phase I trial of the HIV protease inhibitor nelfinavir in adults with solid tumorsOncotarget20145188161817225327558
- BrunnerTBGeigerMGrabenbauerGGPhase I trial of the human immunodeficiency virus protease inhibitor nelfinavir and chemo-radiation for locally advanced pancreatic cancerJ Clin Oncol200826162699270618509182
- DriessenCKrausMJoergerMTreatment with the HIV protease inhibitor nelfinavir triggers the unfolded protein response and may overcome proteasome inhibitor resistance of multiple myeloma in combination with bortezomib: a phase I trial (SAKK 65/08)Haematologica2016101334635526659919
- RenganRMickRPrymaDA phase I trial of the HIV protease inhibitor nelfinavir with concurrent chemoradiotherapy for unresectable stage IIIA/IIIB non-small cell lung cancer: a report of toxicities and clinical responseJ Thorac Oncol20127470971522425919
- Maksimovic-IvanicDFagonePMcCubreyJBendtzenKMijatovicSNicolettiFHIV-protease inhibitors for the treatment of cancer: Repositioning HIV protease inhibitors while developing more potent NO-hybridized derivatives?Int J Cancer201714081713172627870005
- SinghRKesharwaniPMehraNKSinghSBanerjeeSJainNKDevelopment and characterization of folate anchored Saquinavir entrapped PLGA nanoparticles for anti-tumor activityDrug Dev Ind Pharm201541111888190125738812
- YouJHeZChenLCH05-10, a novel indinavir analog, is a broad-spectrum antitumor agent that induces cell cycle arrest, apoptosis, endoplasmic reticulum stress and autophagyCancer Sci2010101122644265120946116
- Maksimovic-IvanicDMojicMBulatovicMThe NO-modified HIV protease inhibitor as a valuable drug for hematological malignancies: Role of p70S6KLeuk Res201539101088109526220866
- JayanthanARuanYTruongTHNarendranAAurora Kinases as Druggable Targets in Pediatric Leukemia: Heterogeneity in Target Modulation Activities and Cytotoxicity by Diverse Novel Therapeutic AgentsPLoS ONE201497e10274125048812
- ChouTCDrug combination studies and their synergy quantification using the Chou-Talalay methodCancer Res201070244044620068163
- AcostaEPKakudaTNBrundageRCAndersonPLFletcherCVPharmacodynamics of human immunodeficiency virus type 1 protease inhibitorsClin Infect Dis200030Suppl 2S151S15910860900
- XiangTDuLPhamPZhuBJiangSNelfinavir, an HIV protease inhibitor, induces apoptosis and cell cycle arrest in human cervical cancer cells via the ROS-dependent mitochondrial pathwayCancer Lett20153641798825937300
- OslowskiCMUranoFMeasuring ER stress and the unfolded protein response using mammalian tissue culture systemMethods Enzymol2011490719221266244
- BarthSGlickDMacleodKFAutophagy: assays and artifactsJ Pathol2010221211712420225337
- AltomareDATestaJRPerturbations of the AKT signaling pathway in human cancerOncogene200524507455746416288292
- GuanMFousekKChowWANelfinavir inhibits regulated intramembrane proteolysis of sterol regulatory element binding protein-1 and activating transcription factor 6 in castration-resistant prostate cancerFEBS J2012279132399241122540830
- GuanMSuLYuanYCLiHChowWANelfinavir and nelfinavir analogs block site-2 protease cleavage to inhibit castration-resistant prostate cancerSci Rep20155969825880275
- HillEJRobertsCFranklinJMClinical trial of oral nelfinavir before and during radiation therapy for advanced rectal cancerClin Cancer Res20162281922193126861457
- MeynREKrishnanSSkinnerHDEverything old is new again: using nelfinavir to radiosensitize rectal cancerClin Cancer Res20162281834183626920893
- WilsonJMFokasEDuttonSJARCII: A phase II trial of the HIV protease inhibitor Nelfinavir in combination with chemoradiation for locally advanced inoperable pancreatic cancerRadiother Oncol2016119230631127117177
- ChakravartyGMathurAMalladePNelfinavir targets multiple drug resistance mechanisms to increase the efficacy of doxorubicin in MCF-7/Dox breast cancer cellsBiochimie2016124536426844637
- KawabataSGillsJJMercado-MatosJRSynergistic effects of nelfinavir and bortezomib on proteotoxic death of NSCLC and multiple myeloma cellsCell Death Dis20123e35322825471
- ChoHYThomasSGoldenEBEnhanced killing of chemo-resistant breast cancer cells via controlled aggravation of ER stressCancer Lett20092821879719345476
- MathurAAbd ElmageedZYLiuXSubverting ER-stress towards apoptosis by nelfinavir and curcumin coexposure augments docetaxel efficacy in castration resistant prostate cancer cellsPloS One201498e10310925121735
- LiuRZhangLYangJHIV protease Inhibitors sensitize human head and neck squamous carcinoma cells to radiation by activating endoplasmic reticulum stressPloS One2015105e012592825933118
- JohnsonCEHuntDKWiltshireMEndoplasmic reticulum stress and cell death in mTORC1-overactive cells is induced by nelfinavir and enhanced by chloroquineMol Oncol20159367568825498902
- HanahanDWeinbergRAHallmarks of cancer: the next generationCell201114464667421376230
- DoniaMMcCubreyJABendtzenKNicolettiFPotential use of rapamycin in HIV infectionBr J Clin Pharmacol201070678479321175433
- Ben-RomanoRRudichAEtzionSNelfinavir induces adipocyte insulin resistance through the induction of oxidative stress: differential protective effect of antioxidant agentsAntivir Ther20061181051106017302375
- PoreNGuptaAKCernigliaGJMaityAHIV protease inhibitors decrease VEGF/HIF-1alpha expression and angiogenesis in glioblastoma cellsNeoplasia200681188989517132220
- JiangWMikochikPJRaJHHIV protease inhibitor nelfinavir inhibits growth of human melanoma cells by induction of cell cycle arrestCancer Res20076731221122717283158
- KrausMBaderJOverkleeftHDriessenCNelfinavir augments proteasome inhibition by bortezomib in myeloma cells and overcomes bortezomib and carfilzomib resistanceBlood Cancer J20133e10323454896
- GodaJSPachporTBasuTChopraSGotaVTargeting the AKT pathway: Repositioning HIV protease inhibitors as radiosensitizersIndian J Med Res2016143214515927121513
- LinAMaityAMolecular pathways: a novel approach to targeting hypoxia and improving radiotherapy efficacy via reduction in oxygen demandClin Cancer Res20152191995200025934887
- LeeDHQiJBradnerJESynergistic effect of JQ1 and rapamycin for treatment of human osteosarcomaInt J Cancer201513692055206425307878
- ParkMAZhangGMartinAPVorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activationCancer Biol Ther20087101648166218787411
- CottiniFAndersonKNovel therapeutic targets in multiple myelomaClin Advan Hematol Oncol201513423624826352582
- ClarkeRCookKLHuREndoplasmic reticulum stress, the unfolded protein response, autophagy, and the integrated regulation of breast cancer cell fateCancer Res20127261321133122422988