Abstract
Compared with various malignant tumors, lung cancer has high incidence and the highest mortality worldwide. Non-small-cell lung cancer (NSCLC), the most common kind of lung cancer, is still a great threat to the world, including China. Surgery, platinum-based chemotherapy, and radiotherapy are still the primary treatments for NSCLC patients in the clinic, whereas immunotherapy and targeted therapy are gradually playing more important roles. A next-generation tyrosine kinase inhibitor (TKI), afatinib, was developed as a targeted reagent for epidermal growth factor receptor (EGFR). This targeted drug was effective in a series of trials. The US Food and Drug Administration then approved afatinib as a new first-line treatment for EGFR L858R and exon 19 deletion mutant patients in 2013. This review focused on current clinical studies of afatinib. Although this TKI was not widely available in China until recently, we aim to provide a reference for its future use in China.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Non-small-cell lung cancer (NSCLC) is the most common type of lung cancer (>80%),Citation1 which is still a leading cause of death among various cancers worldwide.Citation2 Malignant lung epithelial cells seem to be responsible for the two leading pathologies of NSCLC, namely adenocarcinoma and squamous cell carcinoma.Citation3 In China, over 7,333,000 new lung cancer patients were diagnosed, and nearly 6,102,000 patients die of lung cancer each year.Citation4 A suitable treatment is important to benefit a large number of Chinese NSCLC patients and relieve their suffering.
Surgery to remove the solid tumor and the nearby lymph nodes in early-stage NSCLC patients is an optimal choice.Citation5 However, one challenge is that most NSCLC patients are diagnosed at an advanced stage where surgery is not suitable. It is well known that many patients benefited from chemotherapy.Citation6 Recently, the immunotherapy and targeted therapy also showed unexpected potency in NSCLC patients with some special characteristics.Citation7,Citation8 For example, epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) was proven to be much more effective in patients with mutant EGFR than traditional chemotherapy.Citation9,Citation10 In addition, trials for a second-generation TKI, afatinib, which was recently approved in China, show inspiring results. This review focuses on current studies of afatinib and discusses its efficacy and safety in Chinese NSCLC patients to facilitate its further use in China.
TKI and EGFR/HER2 in NSCLC
EGFR and human epidermal growth factor receptor 2 (HER2) are the most two important members of the ERBB receptor tyrosine kinase family (ERBB1–4).Citation11,Citation12 EGFR tyrosine kinase can be activated and can cause a series of effects when it is combined with its special ligands such as epidermal growth factor (EGF), epigen (EPG), and transforming growth factor-α (TGF-α).Citation13 It has been reported that EGFR is overexpressed in some human carcinoma cell lines such as MDA and A431.Citation14 Subsequent experiments showed that a high level of EGFR and HER2 expression may be a sign for cancers including NSCLC.Citation15–Citation17 HER2 is a half-functional receptor without the ability to bind other ligands; however, HER2 maintains the tyrosine kinase activity when heterodimerized with other receptors such as EGFR.Citation11 Some studies have indicated that patients with HER2 mutations demonstrated phenotypes similar to those with EGFR mutations.Citation18,Citation19 There is evidence that both EGFR and HER2 can facilitate cancer cell survival during anticancer therapy.Citation20–Citation22 Many signal pathways such as the mitogen-activated protein kinase (MAPK) pathway have been involved in this intricate process.Citation23,Citation24 The MAPK pathway was found to play a critical role in cell growthCitation25 and was demonstrated to be an important downstream pathway of EGFR.Citation26 Another significant pathway regulated by EGFR/HER2, which helps in the progression of cancer, is the phosphatidylinositol 3 kinase–serine/threonine kinase (PI3K-AKT) pathway,Citation27 a well-known pathway that has an important relationship with the occurrence of various cancers.Citation28 In recent years, research has shown that autophagy may be affected when the expression level of EGFR is abnormal.Citation29 Some evidence indicates that the EGFR may interfere in cancer processes through the regulation of expression levels of cancer-related proteins.Citation30 These evidences indicate that EGFR and HER2 play an important role in NSCLC through various pathways. The pathways associated with EGFR and HER2 are summarized in .
Figure 1 Signaling pathways of EGFR and HER2.
Abbreviations: AKT, serine/threonine kinase; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; HER2, human epidermal growth factor receptor 2; PI3K, phosphatidylinositol 3 kinase; TGF-α, transforming growth factor-α.
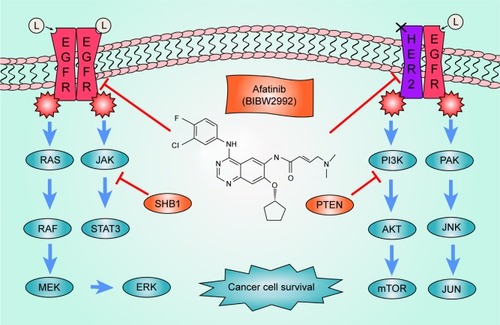
To selectively target mutant EGFR in cancer patients, the first-generation TKI therapies, gefitinib and erlotinib, were developed.Citation31,Citation32 It was reported that first-generation TKI therapies showed a notable curative effect in patients with deletion mutations in exon 19 or L858R mutations in exon 21.Citation9 However, secondary mutations such as T790M can severely hamper the efficacy of gefitinib and erlotinib in the TKI therapy process.Citation33 Researchers also wondered if the efficacy of the first-generation TKI could be improved by changing its reversible targeted characteristics.Citation34 These concerns prompted pharmacologists to develop a new-generation TKI, which may be more effective than the first generation.
Afatinib (BIBW2992) is an irreversible next-generation EGFR inhibitor that targets both EGFR and HER2Citation35 and is effective in NSCLC patients with first-generation TKI resistance.Citation36 The irreversible antagonism facilitates its anticancer activity compared with the first-generation TKI, although some studies revealed that T790M mutation can reduce the efficacy of irreversible TKI.Citation37 It is noted that the third-generation TKI therapies, osimertinib (AZD9291), rociletinib (CO1686), and olmutinib (HM61713), have been reported to be powerful for T790M-mutant EGFR patients.Citation38–Citation40 Once these new-generation TKIs are approved by China Food and Drug Administration (CFDA), they will be a good alternative to afatinib in Chinese patients with T790M-mutant EGFR.Citation41 Afatinib can inhibit EGFR and HER2 through modifying the ATB-binding site cys797 (EGFR), cys805 (HER2), and cys803 (ERBB4) in covalent binding.Citation35 In vitro experiments showed that afatinib is effective in reducing the phosphorylation levels of EGFR and suppressing the survival of cancer cell lines such as H1666 and HCC827.Citation42 Xenograft models and clinical trials showed that afatinib has a strong antitumor effect against NSCLC with a high expression of EGFR levels and was efficacious in EGFR L858R/T790M-mutant patients.Citation43,Citation44 These evidences highlight the advantage of afatinib compared with that of first-generation TKI drugs and demonstrate the effectiveness of afatinib as a therapy for mutant EGFR NSCLC patients.
EGFR/HER2 mutation in Chinese NSCLC patients
As clinical trials have previously indicated, afatinib is effective in EGFR-mutated patients and suggests that the frequency of mutant EGFR in NSCLC patients is a key factor in the utilization of afatinib. It was reported that activation can be found only in tumor tissues and not in normal tissues of the same patient,Citation45 and these mutations were more familiar in non-smoking women.Citation46,Citation47 The frequency of EGFR mutations among different regions also varies, particularly between those from Asia and non-Asian regions.Citation48 It was reported that EGFR deletion in exon 19 or L858R in exon 21 accounted for over 85% of all EGFR mutant individuals,Citation49 indicating that afatinib can benefit this large segment of NSCLC patients. The frequency of mutant EGFR in Chinese patients was over 30%,Citation50 whereas the frequency of mutant EGFR in White and Caucasian patients was ~10%.Citation51 This difference indicates that afatinib can benefit about one-third of the Chinese NSCLC patients with mutant EGFR. The mutation frequency of EGFR in NSCLC patients from different regions is listed in .
Table 1 EGFR mutation status in cancer patients all over the world
It is worth noting that HER2 is also reported to be a therapeutic target of afatinib.Citation62,Citation63 There are three kinds of abnormal HER2s, including overexpression of HER2 protein, amplification of the HER2 gene, and mutation in the HER2 gene.Citation64 HER2 mutation was reported to be the driving mutation of NSCLC,Citation65 and afatinib is a good choice for patients with HER2 mutations according to the National Comprehensive Cancer Network (NCCN) guideline.Citation66 However, unlike the EGFR mutation status, the mutation rate of HER2 in NSCLC is much lower (2%) and is similar between Chinese and non-Chinese patients.Citation67–Citation69 The rate of HER2 amplification or overexpression in NSCLC is higher than the mutation rate,Citation64 but there are no clinical trials on the use of afatinib in HER2 amplification or overexpression in NSCLC patients to guide the use of afatinib for this segment of the population.
Efficacy and safety of afatinib in Chinese NSCLC patients
To investigate the efficacy and safety of afatinib in NSCLC patients, a series of clinical trials were carried out comparing its effectiveness with traditional chemotherapy and first-generation TKI therapy. Information about some important trials studying NSCLC and afatinib are listed in .Citation70–Citation77 In this review, trials for primarily Chinese populations are discussed in detail.
Table 2 Clinical trials about afatinib
Efficacy
There have been many clinical trials of afatinib to evaluate its efficacy compared with that of traditional chemotherapy. The largest scale clinical trial of afatinib in a Chinese population (327 Chinese patients) was NCT1121393.Citation74 The 364 NSCLC patients were enrolled from 36 centers in China, Thailand, and South Korea. Control group was treated with gemcitabine and cisplatin (122 patients), whereas others (242 patients) were treated with afatinib. In this trial, the progression-free survival (PFS) period and Response Evaluation Criteria in Solid Tumors (RECIST) assessment results were collected as the criteria to evaluate the efficacy of afatinib versus traditional chemotherapy. The clinical characteristics of these two groups were similar except for their Eastern Cooperative Oncology Group (ECOG) performance status (p=0.004, χ2 test), which was worse in the afatinib group. All patients had mutant EGFR (Leu858Arg, exon 19 deletions, or others) in their tumor tissues.Citation74 The outcome of the therapeutic efficacy evaluation indicated that afatinib is more desirable for EGFR mutant patients, especially for patients mutated with Leu858Arg or exon 19 deletions. The median PFS period of the afatinib group was 13.7 months (95% confidence interval [CI], 11.5–13.9), while it was 5.6 months (95% CI, 0.19–0.36) in the gemcitabine and cisplatin group according to the investigators. Similar results were obtained for the RECIST assessment: 66.9% complete response (CR) + partial response (PR) patients in afatinib group and 23.0% in chemotherapy group according to the investigators.Citation74 Compared with clinical trials of afatinib and traditional chemotherapy all over the world, similar conclusions were drawn regarding the efficacy of afatinib not only as a first-line therapy but also as a second-line regimen.Citation71,Citation73 The median PFS periods of the afatinib group were 11.1 (first-line therapy) and 5.6 (second-line therapy) months, which were significantly higher than those of the chemotherapy group (6.8 months for first-line therapy and 2.9 months for second-line therapy). The RECIST assessment in the two global trials (NCT949650 and NCT1085136) also demonstrated the superior performance of afatinib (). These results suggest that afatinib is more effective than platinum-based chemotherapies for EGFR-mutated patients including Chinese patients as both a first-line and second-line cancer therapy.
To compare the efficacy of afatinib with the first-generation TKI, more trials were selected for discussion in this review. NCT2625168 is a clinical trial under a compassionate use program that compares the efficacy between afatinib and erlotinib groups in Chinese NSCLC patients who failed in pre-therapy with first-generation TKI and chemotherapy.Citation77 Twenty-five patients were treated with afatinib, whereas 28 patients were treated with erlotinib. All patients had documented EGFR mutations including exon 18 and exon 19 mutations. More patients in the afatinib group were evaluated as having worse ECOG2 stages, whereas the other clinical characteristics were similar between the two groups. RECIST assessment and survival time were recorded in this trial to evaluate the efficacy of afatinib compared with that of erlotinib in Chinese NSCLC patients who received a less than ideal first-line TKI therapy. Although the object response rate (ORR; CR + PR, 20% in afatinib group vs 7.1% in erlotinib group, p=0.17), the median PFS period (4.1 months in afatinib group vs 3.3 months in erlotinib group, p=0.97), and the median overall survival (OS; 10.3 months in afatinib group vs 10.8 months in erlotinib group, p=0.51) duration were similar between two groups, the disease control rate of the afatinib-treated group (CR + PR + stable disease =68.0%) was significantly superior to that of the erlotinib group (39.3%) in all patients. In addition, the PFS period of the afatinib-treated group was significantly superior to that of the erlotinib group in patients aged 70 years or younger or for whom the time to progression for the first-generation TKI therapy was ≥18 months. The author also compared the efficacy of afatinib in this study with that in the LUX-Lung 1 clinical trial;Citation78 a similar outcome was obtained, indicating that the results were solid even though the sample size was limited in this trial. Two additional global trials (NCT1466660 and NCT1523587) also indicated that afatinib was more ideal for EGFR mutant patients in first-line and second-line antitumor therapies (). However, similar to the results of NCT2625168, the median PFS period of the afatinib-treated group was similar to the group treated with gefitinib in NCT1466660 (11.0 months in the afatinib group vs 10.9 months in the gefitinib group).Citation75 As a second-line regimen, the median PFS period of the afatinib-treated group was 2.4 months, whereas the median PFS period of the erlotinib-treated group was 1.9 months.Citation76
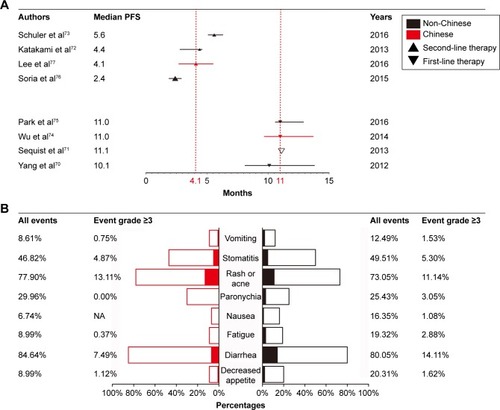
In addition to clinical trials conducted worldwide, a retrospective study conducted in Linkou Chang-Gung Memorial Hospital, Taiwan, indicated that compared with the first-generation TKI, afatinib is better for Chinese NSCLC patients as well. In this study, 448 patients who had EGFR-activating mutations were treated with three different kinds of TKI (gefitinib, 304 patients; erlotinib, 63 patients; and afatinib, 81 patients) as a first-line therapy in one hospital enrolled from February 16, 2011 to October 30, 2015.Citation79 The aim of this study was to compare the efficacy among these three different TKI therapies. Similar to the NCT1466660 trial, this study showed that Chinese EGFR-mutated NSCLC patients can also benefit more from afatinib than gefitinib (p<0.001) when TKI was chosen as the first-line therapy regimen.Citation75 Consistent results were obtained in multivariable analysis (hazard ratio [HR], 0.51; 95% CI, 0.34–0.78). Although many limitations existed in this study, such as side effects that were not reported, it was obvious from this study that compared with gefitinib, afatinib is an effective first-line treatment for Chinese NSCLC patients with EGFR mutations. Another research study indicated that afatinib showed strong efficacy for adenocarcinoma patients with both EGFR and HER2 mutations in kinase domains; this is a potential advantage of afatinib over the first-generation TKI therapies.Citation80 We compared the efficacy between Chinese and non-Chinese patients; this result is shown in . A non-significant difference was observed.
Figure 2 The difference of efficacy and safety between Chinese and non-Chinese patients.
Abbreviations: CI, confidence interval; NA, not applicable; NSCLC, non-small-cell lung cancer; PFS, progression-free survival.
Safety
Although the efficacy of afatinib is much better than that of traditional chemotherapy in EGFR mutant patients, the safety of afatinib is undoubtedly as important as its efficacy. Significantly higher frequencies of some adverse events including dyspnea, diarrhea, rashes, and acne were observed in afatinib group. On the other hand, afatinib may be safer due to its lower incidence of other adverse events. In NCT1121393, patients in the gemcitabine and cisplatin group showed a higher frequency of some adverse events, including vomiting, nausea, neutropenia, and leucopenia than patients in the afatinib group (). In addition, there were 21 patients (11.5%) who discontinued their afatinib therapy, whereas 45 (39.8%) patients discontinued their chemotherapy for intolerable adverse events in this study.Citation74 Furthermore, 23 afatinib patients (10.0%) and 17 chemotherapy patients (15.3%) discontinued their therapy for intolerable adverse events in NCT949650.Citation71 In NCT1085136, afatinib and paclitaxel were provided as a combined regimen. As a result, a larger proportion of patients (18.9%) discontinued their therapy for intolerable adverse events, whereas in the control group 6.8% of patients discontinued their chemotherapy.Citation73 Although these adverse reactions may be a shortcoming of afatinib, it is obvious that these trials successfully demonstrated the safety of afatinib compared with that of traditional platinum-based chemotherapy in Chinese NSCLC patients.
The efficacy of afatinib is superior to erlotinib (), but compared with erlotinib, afatinib resulted in more diarrhea events but less acneiform rash. Similar treatment interruption rates (28% in the afatinib group vs 28.6% in the erlotinib group, p=0.96) and the rate of patients with dose reductions (24.0% in the afatinib group vs 17.9% in the erlotinib group, p=0.58) were obtained in the two groups. All patients in both groups continued the therapy despite treatment-related toxicity.Citation77 In NCT1466660 and NCT1523587, similar treatment interruption rates were found: 11.2% in the afatinib group versus 10.6% in the gefitinib group in NCT1466660 and 17.3% in the afatinib group versus 13.1% in the erlotinib group in NCT1523587. In consideration of the limited sample size, we compared the safety between Chinese and non-Chinese patients; these results are presented in . We found that there was no significant difference between Chinese NSCLC patients and non-Chinese NSCLC patients, indicating the experience of dealing with adverse side effects of afatinib can be learned from other countries also. Overall, these trials indicate that afatinib is a powerful therapy regimen for NSCLC patients with mutant EGFR.
Companion diagnosis
It is obvious that Chinese patients carry a higher EGFR-mutated frequency than European and American patients (). A suitable EGFR mutant detection procedure would benefit this part of patients who could potentially receive afatinib therapy. It is reported that mutant EGFR can be potentially detected in biopsy and blood.Citation81–Citation84 Biopsy has been widely used for EGFR mutant detection for years.Citation85,Citation86 There are many DNA extraction kits designed for fresh or formalin-fixed paraffin-embedded biopsy samples to conveniently ensure high-quality tumor DNA.Citation87 However, transthoracic biopsies are invasive, and the heterogeneity of tumors may lead to inaccurate results.Citation88,Citation89 In this case, cell-free tumor DNA (Cft-DNA) has arisen as a new possibility for tumor somatic mutation detection in recent years, and it is reported that EGFR mutations could be successfully detected in Cft-DNA from serum.Citation90 This approach indicates that blood sample may replace biopsy samples for Chinese NSCLC patients to detect EGFR mutations with qualified techniques. Many techniques such as high-resolution melting analysis, immunohistochemistry, polymerase chain reaction-restriction fragment length polymorphism, amplification refractory mutation system (ARMS), and direct sequencing have been reported as effective in detecting mutated EGFR in biopsy and Cft-DNA.Citation86,Citation91–Citation93 Meanwhile, increasingly emerged technology such as the new generation of sequencing may benefit patients too.Citation94,Citation95 For most patients who need to detect mutant EGFR results quickly for their therapy in clinic, ARMS is preferred for its economical and reliable characteristics.Citation96,Citation97 For drug resistance population, direct sequencing could obtain more information in genomic scale so that more potential therapeutic choices would be provided.Citation98,Citation99 Thus, the development of companion diagnosis-related technology will benefit more NSCLC patients.
Conclusion and challenge
As discussed, it is obvious that the efficacy and safety of afatinib in Chinese EGFR mutant NSCLC patients are similar to those in patients with the same mutation worldwide (). This clue indicates that afatinib can be utilized in Chinese populations as indicated in the NCCN guideline.Citation66 In addition, the mutation frequency of EGFR in Chinese NSCLC patients is higher than that in European and American patients, indicating that a higher proportion of Chinese NSCLC patients could benefit from afatinib. Moreover, as variation detection technology has recently rapidly developed in China, more NSCLC patients can be conveniently detected if their EGFR mutation is suitable for afatinib therapy. We have reached the conclusion that afatinib could be utilized as a powerful antitumor drug in the near future in Chinese NSCLC patients.
However, there are still some challenges we must face in afatinib therapy in Chinese patients. First, over 30% of EGFR-mutated patients are not susceptible to afatinib for unknown reasons. This drug resistance phenomenon attenuated the efficacy of afatinib. Some evidence indicates that some afatinib-resistant patients may carry other mutated genes such as L792F and C797S in EGFR.Citation100 Some abnormal pathways were also reported to be the reason for this resistance to afatinib.Citation101,Citation102 In these cases, genomic scale sequencing is suitable to reveal the underlying mechanism in drug-resistant patients from information in their mutation profile. Second, it is important to develop a suitable technique for patients to fully and accurately obtain their genomic codes for personalized therapy. Although next-generation sequencing (NGS) is popular due to its high-throughput characteristics, the error occurred in PCR process, and the short length of reads limited its ability. The third-generation sequencing is superior in these aspects compared with NGS, but it is still immature, so revolutions are required in the future. Finally, the number and sample size of afatinib clinical trials in Chinese NSCLC patients are limited. More trials should be conducted in the future to draw more solid conclusions about the efficacy and safety of afatinib in the treatment of NSCLC in Chinese patients.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81773823 and 81573463), Hunan Provincial Natural Science Foundation of China Grant (2015JJ1024), and National Science and Technology Major Project (2017ZX09304014).
Disclosure
The authors report no conflicts of interest in this work.
References
- EttingerDSWoodDEAisnerDLNon-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncologyJ Natl Compr Canc Netw201715450453528404761
- SiegelRLMillerKDJemalACancer statistics, 2017CA Cancer J Clin201767173028055103
- TravisWDBrambillaENicholsonAGWHO PanelThe 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classificationJ Thorac Oncol20151091243126026291008
- ChenWZhengRBaadePDCancer statistics in China, 2015CA Cancer J Clin201666211513226808342
- DattaDLahiriBPreoperative evaluation of patients undergoing lung resection surgeryChest200312362096210312796194
- SchillerJHHarringtonDBelaniCPEastern Cooperative Oncology GroupComparison of four chemotherapy regimens for advanced non-small-cell lung cancerN Engl J Med20023462929811784875
- ThomasALiuSVSubramaniamDSGiacconeGRefining the treatment of NSCLC according to histological and molecular subtypesNat Rev Clin Oncol201512951152625963091
- AnagnostouVKBrahmerJRCancer immunotherapy: a future paradigm shift in the treatment of non-small cell lung cancerClin Cancer Res201521597698425733707
- KazandjianDBlumenthalGMYuanWHeKKeeganPPazdurRFDA approval of gefitinib for the treatment of patients with metastatic EGFR mutation-positive non-small cell lung cancerClin Cancer Res20162261307131226980062
- GeLShiRProgress of EGFR-TKI and ALK/ROS1 inhibitors in advanced non-small cell lung cancerInt J Clin Exp Med201587103301033926379824
- DaviesRLGrosseVAKucherlapatiRBothwellMGenetic analysis of epidermal growth factor action: assignment of human epidermal growth factor receptor gene to chromosome 7Proc Natl Acad Sci U S A1980777418841926254014
- KlapperLNGlatheSVaismanNThe ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factorsProc Natl Acad Sci U S A19999694995500010220407
- SandersJMWampoleMEThakurMLWickstromEMolecular determinants of epidermal growth factor binding: a molecular dynamics studyPLoS One201381e5413623382875
- BowersGReardonDHewittTThe relative role of ErbB1–4 receptor tyrosine kinases in radiation signal transduction responses of human carcinoma cellsOncogene200120111388139711313882
- GustersonBCowleyGMcIlhinneyJOzanneBFisherCReevesBEvidence for increased epidermal growth factor receptors in human sarcomasInt J Cancer19853666896932999006
- ChernevaRGeorgievODimovaIRukovaBPetrovDTonchevaDEGFR and hTERT expression as a diagnostic approach for non-small cell lung cancer in high risk groupsBiomark Cancer20102576324179385
- RicciardiGRRussoAFranchinaTNSCLC and HER2: between lights and shadowsJ Thorac Oncol20149121750176225247338
- ShigematsuHTakahashiTNomuraMSomatic mutations of the HER2 kinase domain in lung adenocarcinomasCancer Res20056551642164615753357
- StephensPHunterCBignellGLung cancer: intragenic ERBB2 kinase mutations in tumoursNature20044317008525526
- GatelyKFordeLCuffeSHigh coexpression of both EGFR and IGF1R correlates with poor patient prognosis in resected non-small-cell lung cancerClin Lung Cancer2014151586624210543
- DouillardJYPirkerRO’ByrneKJRelationship between EGFR expression, EGFR mutation status, and the efficacy of chemotherapy plus cetuximab in FLEX study patients with advanced non-small-cell lung cancerJ Thorac Oncol20149571772424662454
- LuYWangYZhangMHER2-siRNA delivered by EGFR-specific single chain antibody inhibits NSCLC cell proliferation and tumor growthOncotarget2016717235942360726988752
- KitanoHChungJYYlayaKProfiling of phospho-AKT, phospho-mTOR, phospho-MAPK and EGFR in non-small cell lung cancerJ Histochem Cytochem201462533534624487999
- HynesNEMacDonaldGErbB receptors and signaling pathways in cancerCurr Opin Cell Biol200921217718419208461
- GomezNCohenPDissection of the protein kinase cascade by which nerve growth factor activates MAP kinasesNature199135363401701731716348
- BreindelJLHaskinsJWCowellEPZhaoMNguyenDXSternDFEGF receptor activates MET through MAPK to enhance non-small cell lung carcinoma invasion and brain metastasisCancer Res201373165053506523794705
- NishimuraYTakiguchiSItoSItohKEGFstimulated AKT activation is mediated by EGFR recycling via an early endocytic pathway in a gefitinibresistant human lung cancer cell lineInt J Oncol20154641721172925653196
- FrumanDARommelCPI3K and cancer: lessons, challenges and opportunitiesNat Rev Drug Discov201413214015624481312
- HensonEChenYGibsonSEGFR family members’ regulation of autophagy is at a crossroads of cell survival and death in cancerCancers (Basel)201794E2728338617
- JonesRBGordusAKrallJAMacBeathGA quantitative protein interaction network for the ErbB receptors using protein microarraysNature2006439707316817416273093
- LynchTJBellDWSordellaRActivating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinibN Engl J Med2004350212129213915118073
- ShepherdFARodrigues PereiraJCiuleanuTNational Cancer Institute of Canada Clinical Trials GroupErlotinib in previously treated non-small-cell lung cancerN Engl J Med2005353212313216014882
- YunCHMengwasserKETomsAVThe T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATPProc Natl Acad Sci U S A200810562070207518227510
- YoshidaTZhangGSmithMATyrosine phosphoproteomics identifies both codrivers and cotargeting strategies for T790M-related EGFR-TKI resistance in non-small cell lung cancerClin Cancer Res201420154059407424919575
- SolcaFDahlGZoephelATarget binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blockerJ Pharmacol Exp Ther2012343234235022888144
- HeigenerDFSchumannCSebastianMAfatinib Compassionate Use Consortium (ACUC)Afatinib in non-small cell lung cancer harboring uncommon EGFR mutations pretreated with reversible EGFR inhibitorsOncologist201520101167117426354527
- SosMLRodeHBHeynckSChemogenomic profiling provides insights into the limited activity of irreversible EGFR Inhibitors in tumor cells expressing the T790M EGFR resistance mutationCancer Res201070386887420103621
- OxnardGRThressKSAldenRSAssociation between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancerJ Clin Oncol201634283375338227354477
- KimESOlmutinib: first global approvalDrugs201676111153115727357069
- SequistLVSoriaJCGoldmanJWRociletinib in EGFR-mutated non-small-cell lung cancerN Engl J Med2015372181700170925923550
- TranPNKlempnerSJProfile of rociletinib and its potential in the treatment of non-small-cell lung cancerLung Cancer (Auckl)20167919728210165
- LiDAmbrogioLShimamuraTBIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer modelsOncogene200827344702471118408761
- WongCHMaBBHuiCWTaoQChanATPreclinical evaluation of afatinib (BIBW2992) in esophageal squamous cell carcinoma (ESCC)Am J Cancer Res20155123588359926885448
- JanjigianYYSmitEFGroenHJDual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutationsCancer Discov2014491036104525074459
- ShigematsuHLinLTakahashiTClinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancersJ Natl Cancer Inst200597533934615741570
- LeeSYKimMJJinGSomatic mutations in epidermal growth factor receptor signaling pathway genes in non-small cell lung cancersJ Thorac Oncol20105111734174020881644
- ShigematsuHGazdarAFSomatic mutations of epidermal growth factor receptor signaling pathway in lung cancersInt J Cancer2006118225726216231326
- GirardNSimaCSJackmanDMNomogram to predict the presence of EGFR activating mutation in lung adenocarcinomaEur Respir J201239236637221778168
- SharmaSVBellDWSettlemanJHaberDAEpidermal growth factor receptor mutations in lung cancerNat Rev Cancer20077316918117318210
- LuRLHuCPYangHPLiYYGuQHWuLBiological characteristics and epidermal growth factor receptor tyrosine kinase inhibitors efficacy of EGFR mutation and its subtypes in lung adenocarcinomaPathol Oncol Res201420244545124297623
- RosellRMoranTQueraltCSpanish Lung Cancer GroupScreening for epidermal growth factor receptor mutations in lung cancerN Engl J Med20093611095896719692684
- ShiYAuJSThongprasertSA prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER)J Thorac Oncol20149215416224419411
- HongSFangWHuZA large-scale cross-sectional study of ALK rearrangements and EGFR mutations in non-small-cell lung cancer in Chinese Han populationSci Rep20144726825434695
- XieGXieFWuPThe mutation rates of EGFR in non-small cell lung cancer and KRAS in colorectal cancer of Chinese patients as detected by pyrosequencing using a novel dispensation orderJ Exp Clin Cancer Res2015346326081767
- LiamCKLeowHRHowSHEpidermal growth factor receptor mutations in non-small cell lung cancers in a multiethnic Malaysian patient populationAsian Pac J Cancer Prev201415132132624528049
- TomitaMAyabeTChosaEKawagoeKNakamuraKEpidermal growth factor receptor mutations in Japanese men with lung adenocarcinomasAsian Pac J Cancer Prev20141524106271063025605150
- ArrietaOCardonaAFFederico BramugliaGCLICaPGenotyping non-small cell lung cancer (NSCLC) in Latin AmericaJ Thorac Oncol20116111955195922005474
- ShiauCJBabwahJPda Cunha SantosGSample features associated with success rates in population-based EGFR mutation testingJ Thorac Oncol20149794795624922009
- ZaricBStojsicVKovacevicTClinical characteristics, tumor, node, metastasis status, and mutation rate in domain of epidermal growth factor receptor gene in Serbian patients with lung adenocarcinomaJ Thorac Oncol2014991406141025122436
- Maki-NevalaSRontyMMorelMEpidermal growth factor receptor mutations in 510 Finnish non-small-cell lung cancer patientsJ Thorac Oncol20149688689124828666
- RamlauRCuferTBerzinecPINSIGHT study TeamEpidermal growth factor receptor mutation-positive non-small-cell lung cancer in the real-world setting in central Europe: the INSIGHT studyJ Thorac Oncol20151091370137426291014
- SuzawaKToyookaSSakaguchiMAntitumor effect of afatinib, as a human epidermal growth factor receptor 2-targeted therapy, in lung cancers harboring HER2 oncogene alterationsCancer Sci20161071455226545934
- MazieresJBarlesiFFilleronTLung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: results from the European EUHER2 cohortAnn Oncol201627228128626598547
- MarNVredenburghJJWasserJSTargeting HER2 in the treatment of non-small cell lung cancerLung Cancer201587322022525601485
- ZhangYSunYPanYFrequency of driver mutations in lung adenocarcinoma from female never-smokers varies with histologic subtypes and age at diagnosisClin Cancer Res20121871947195322317764
- National Comprehensive Cancer NetworkNon-Small Cell Lung Cancer (Version 9, 2017) Available from: https://www.nccn.org/professionals/physician_gls/default.aspxAccessed January 3, 2018
- WangRZhangYPanYComprehensive investigation of oncogenic driver mutations in Chinese non-small cell lung cancer patientsOncotarget2015633343003430826486077
- SongZYuXShiZZhaoJZhangYHER2 mutations in Chinese patients with non-small cell lung cancerOncotarget2016747781527815827825109
- MazieresJPetersSLepageBLung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectivesJ Clin Oncol201331161997200323610105
- YangJCShihJYSuWCAfatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): a phase 2 trialLancet Oncol201213553954822452895
- SequistLVYangJCYamamotoNPhase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutationsJ Clin Oncol201331273327333423816960
- KatakamiNAtagiSGotoKLUX-Lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or bothJ Clin Oncol201331273335334123816963
- SchulerMYangJCParkKLUX-Lung 5 InvestigatorsAfatinib beyond progression in patients with non-small-cell lung cancer following chemotherapy, erlotinib/gefitinib and afatinib: phase III randomized LUX-lung 5 trialAnn Oncol201627341742326646759
- WuYLZhouCHuCPAfatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trialLancet Oncol201415221322224439929
- ParkKTanEHO’ByrneKAfatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trialLancet Oncol201617557758927083334
- SoriaJCFelipECoboMLUX-Lung 8 InvestigatorsAfatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trialLancet Oncol201516889790726156651
- LeeVHLeungDKChoyTSEfficacy and safety of afatinib in Chinese patients with EGFR-mutated metastatic non-small-cell lung cancer (NSCLC) previously responsive to first-generation tyrosine-kinase inhibitors (TKI) and chemotherapy: comparison with historical cohort using erlotinibBMC Cancer20161614726911310
- HirshVCadranelJCongXJSymptom and quality of life benefit of afatinib in advanced non-small-cell lung cancer patients previously treated with erlotinib or gefitinib: results of a randomized phase IIb/III trial (LUX-Lung 1)J Thorac Oncol20138222923723328549
- KuanFCLiSHWangCLLinMHTsaiYHYangCTAnalysis of progression-free survival of first-line tyrosine kinase inhibitors in patients with non-small cell lung cancer harboring leu858Arg or exon 19 deletionsOncotarget2017811343135327935868
- De GreveJTeugelsEGeersCClinical activity of afatinib (BIBW 2992) in patients with lung adenocarcinoma with mutations in the kinase domain of HER2/neuLung Cancer201276112312722325357
- ZhangLJCaiLLiZRelationship between epidermal growth factor receptor gene mutation and copy number in Chinese patients with non-small cell lung cancerChin J Cancer2012311049149922572014
- JiangBLiuFYangLSerum detection of epidermal growth factor receptor gene mutations using mutant-enriched sequencing in Chinese patients with advanced non-small cell lung cancerJ Int Med Res20113941392140121986139
- WeiFLinCCJoonANoninvasive saliva-based EGFR gene mutation detection in patients with lung cancerAm J Respir Crit Care Med2014190101117112625317990
- ChenSZhaoJCuiLLiuYUrinary circulating DNA detection for dynamic tracking of EGFR mutations for NSCLC patients treated with EGFR-TKIsClin Transl Oncol201719333234027468867
- TakanoTOheYTsutaKEpidermal growth factor receptor mutation detection using high-resolution melting analysis predicts outcomes in patients with advanced non small cell lung cancer treated with gefitinibClin Cancer Res20071318 pt 15385539017875767
- JannePABorrasAMKuangYA rapid and sensitive enzymatic method for epidermal growth factor receptor mutation screeningClin Cancer Res2006123 pt 175175816467085
- HuYCZhangQHuangYHLiuYFChenHLComparison of two methods to extract DNA from formalin-fixed, paraffin-embedded tissues and their impact on EGFR mutation detection in non-small cell lung carcinomaAsian Pac J Cancer Prev20141562733273724761893
- VanderlaanPAYamaguchiNFolchESuccess and failure rates of tumor genotyping techniques in routine pathological samples with non-small-cell lung cancerLung Cancer2014841394424513263
- VogelsteinBPapadopoulosNVelculescuVEZhouSDiazLAJrKinzlerKWCancer genome landscapesScience201333961271546155823539594
- SacherAGPaweletzCDahlbergSEProspective validation of rapid plasma genotyping for the detection of EGFR and KRAS mutations in advanced lung cancerJAMA Oncol2016281014102227055085
- SeoANParkTIJinYNovel EGFR mutation-specific antibodies for lung adenocarcinoma: highly specific but not sensitive detection of an E746_A750 deletion in exon 19 and an L858R mutation in exon 21 by immunohistochemistryLung Cancer201483331632324412618
- AsanoHToyookaSTokumoMDetection of EGFR gene mutation in lung cancer by mutant-enriched polymerase chain reaction assayClin Cancer Res2006121434816397022
- MaMShiCQianJTengJZhongHHanBComparison of plasma and tissue samples in epidermal growth factor receptor mutation by ARMS in advanced non-small cell lung cancerGene20165911586427370697
- van DijkELAugerHJaszczyszynYThermesCTen years of next-generation sequencing technologyTrends Genet201430941842625108476
- GoodwinSMcPhersonJDMcCombieWRComing of age: ten years of next-generation sequencing technologiesNat Rev Genet201617633335127184599
- EllisonGDonaldEMcWalterGA comparison of ARMS and DNA sequencing for mutation analysis in clinical biopsy samplesJ Exp Clin Cancer Res20102913220925915
- KozuYTsutaKKohnoTThe usefulness of mutation-specific antibodies in detecting epidermal growth factor receptor mutations and in predicting response to tyrosine kinase inhibitor therapy in lung adenocarcinomaLung Cancer2011731455021129809
- WonJKKeamBKohJConcomitant ALK translocation and EGFR mutation in lung cancer: a comparison of direct sequencing and sensitive assays and the impact on responsiveness to tyrosine kinase inhibitorAnn Oncol201526234835425403583
- ThompsonJCYeeSSTroxelABDetection of therapeutically targetable driver and resistance mutations in lung cancer patients by next-generation sequencing of cell-free circulating tumor DNAClin Cancer Res201622235772578227601595
- KobayashiYAzumaKNagaiHCharacterization of EGFR T790M, L792F, and C797S mutations as mechanisms of acquired resistance to afatinib in lung cancerMol Cancer Ther201716235736427913578
- HashidaSYamamotoHShienKAcquisition of cancer stem cell-like properties in non-small cell lung cancer with acquired resistance to afatinibCancer Sci2015106101377138426202045
- LeeYWangYJamesMJeongJHYouMInhibition of IGF1R sig-naling abrogates resistance to afatinib (BIBW2992) in EGFR T790M mutant lung cancer cellsMol Carcinog2016555991100126052929
