Abstract
Introduction
Endometrial cancer is the one of the most common cancers of the genital organ. HE4 and MMP2 are both proteins whose serum levels increase in endometrial cancer.
Aim
To explore the diagnostic potential of the serum levels of HE4 and MMP2 in patients with endometrial cancer and benign endometrial diseases. To assess the relationship between the serum levels of HE4 and MMP2 and the typical prognostic factors in patients with endometrial cancer.
Materials and methods
Included in the study was a group of 112 patients presenting with bleeding abnormalities at the Pomeranian Medical University in years 2012–2016. Serum HE4 concentrations were measured using the Elecsys Electrochemiluminescence Immunoassay (ECLIA). MMP2 concentrations were quantified in the serum using multiplex immunoassays.
Results
We observed statistically significant differences in mean serum levels of HE4 and MMP2 between the group of endometrial cancer patients and the group of patients with no changes in the endometrium (P=0.002/0.003). The diagnostic potential of HE4 and MMP2 in differentiation of high (International Federation of Gynecology and Obstetrics [FIGO] III and IV) vs low (FIGO I and II) clinical stage of tumor and prediction of cellular differentiation grade (G1 vs G3) on the basis of the analysis of the area under the curve is, respectively, 0.86 and 0.82 for HE4 and 0.82 and 0.74 for MMP2. The HE4 marker was significantly more specific than MMP2 in every study group and amounted to 93% vs 86% in all patients included in the analysis, 94% vs 84% in pre-menopausal patients and 84% vs 79% in post-menopausal patients.
Conclusion
HE4 and MMP2 are characterized by high specificity and may be useful as biomarkers in the diagnostics of endometrial cancer. When determined preoperatively, HE4 is correlated with the prognostic factors of endometrial cancer and may be helpful in the planning of individual treatment of endometrial cancer patients.
Keywords:
Introduction
Endometrial cancer is the second most common cancer of the genital organ. It is discovered quite early, most commonly in patients reporting at the physician’s office due to abnormal perimenopausal bleeding. Type I endometrial cancer is diagnosed in 80% of patients. It is associated with the well-known risk factors such as obesity, hypertension or type II diabetes. Type II endometrial cancer is more often diagnosed in elderly patients. Its histopathological types are associated with poorer prognosis. As in other cancer diseases, the 5-year survival and the likelihood of recurrence depend on the clinical stage and the histopathological type of the tumor.Citation1 The vast majority of endometrial cancer patients are characterized by significant obesity and background internal diseases. Radical surgical treatment including iliac and paraaortic lymphadenectomy may be a significant burden to these patients. Therefore, markers that could be helpful in identification of patients with poorer prognosis are being sought. This is all the more important as the sensitivity of detection by means of imaging methods is in the range of 70%–82% for lymph node metastasesCitation2 and 40%–74% for the thickness of myometrial infiltration.Citation3 Today, overexpression of HE4 in endometrial cancer is being increasingly raised. HE4 is a well-known protein used in the diagnostics of ovarian cancer.Citation4 In endometrial cancer, significant overexpression was also observed for metalloproteinase 2 that is strongly associated with invasiveness and proliferation of this disease.Citation5
Aim
To explore the behavior and diagnostic potential of the serum levels of HE4 and MMP2 in patients with endometrial cancer and benign endometrial diseases.
To assess the relationship between the serum levels of HE4 and MMP2 and the typical risk factors in endometrial cancer patients.
Materials and methods
Included in the study was a group of 112 patients presenting with bleeding abnormalities at the Pomeranian Medical University in years 2012–2016. Excluded from the study were patients with high creatinine levels, chronic renal diseases, respiratory diseases or circulatory failure, in whom HE4 levels might be elevated in a non-specific manner. Patients were classified as pre-menopausal and post-menopausal according to their hormonal status.
Following the surgical treatment and histopathological examinations, patients were divided into 2 groups, including:
patients with endometrial cancer, n=62;
patients with normal endometrium, n=50.
The detailed distribution of patients with endometrial cancer divided into subgroups is given in .
Table 1 Patients with endometrial cancer divided into subgroups
Methods
Five milliliters of blood was collected from each patient for determination of HE4 and MMP2. Serum HE4 concentrations were measured using the Elecsys Electrochemiluminescence Immunoassay (ECLIA) from Roche running on the cobas e 601 analyzer. The normal range was <70 pmol/L for pre-menopausal women and <140 pmol/L for post-menopausal women.
MMP2 assay analysis was performed as follows: 25 µL of each standard, control and samples were added to the plate together with multiplex antibody capture bead solution, and the plate was incubated with agitation for 2 hours at room temperature. Subsequently, the well was washed with 200 µL wash buffer 2 times by using hand-held magnet. A total of 25 µL of detection antibody cocktail was pipetted to each well, and the plate was sealed and incubated at room temperature for 1 hour on a plate shaker. After this step, 25 µL of streptavidin–phycoerythrin mixture was added to the plate and incubated with agitation for 30 minutes in dark. Finally, after washing, the microspheres in each well were resuspended in 100 µL sheath fluid and shaken at room temperature for 5 minutes. The plate was then read and analyzed on the Luminex analyzer, and analyte concentrations were determined from 5 different standard curves showing median fluorescence intensity (MFI) vs protein concentration.
The statistical analysis was performed using STASTICA 10.0 PL program. The descriptive characteristic of the examined population of patients was determined using minimum, maximum, mean and median values. Since the distributions of studied traits are not normal distributions, positional parameters, medians and non-parametric tests were used to compare median values (Kruskal–Wallis and post hoc Dunn test to compare 3 groups and Mann–Whitney U test to compare 2 groups). For the selected groups, the receiver operating characteristic (ROC) curves were obtained and the area under the curve (AUC) was calculated with 95% confidence intervals according to the non-parametric method of DeLong. A P-value of <0.05 was considered as statistically significant.
Ethical approval
Resolution number: KB-0012/77/12 of the Bioethics Committee of the Pomeranian University of Medicine in Szczecin of 13 October 2012.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was provided by the patients and the physician. All the patients have signed informed consent for the study, which has been signed and initialed by the doctor on each side. Patients read carefully the information and were able to ask questions.
Results
Comparative analysis of the study groups
presents the patients’ characteristics and the mean levels and comparisons of the tested proteins. We observed statistically significant differences in mean serum levels of HE4 and MMP2 between the group of endometrial cancer patients and the group of patients with bleeding abnormalities and no changes within the endometrium, both as regards the entire study population as in the individual hormonal status subgroups (pre- vs post-menopausal).
Table 2 Comparative analysis of the study groups pre- vs post-menopausal
Comparative analysis according to the prognostic factors
and present the values and comparisons of HE4 and MMP2 serum levels depending on various prognostic factors. In our analysis of all the currently established adverse risk factors of endometrial cancer, we found out that there are no statistically significant differences in HE4 and MMP2 levels between both types of cancer (I vs II) and between G1 and G2 tumors. However, the differences in HE4 levels were closer to the statistical significance threshold, with median levels amounting to 89.6 pmol/L in endometrioid-type tumors and 101.1 pmol/L in non-endometrioid-type tumors, P=0.053.
Table 3 Comparative analysis according to the prognostic factors for HE4
Table 4 Comparative analysis according to the prognostic factors for MMP2
No differences in serum MMP2 levels were also observed for different blood vessel invasion status and different endometrial infiltration depths, while significantly higher MMP2 levels were observed in poorly differentiated tumors compared to well-differentiated tumors (P=0.02), high-stage tumors compared to low-stage tumors (P=0.001) and tumors with positive status of lymph vessel invasion (P=0.02) and lymph node metastases (P=0.003).
The serum levels of HE4 were significantly higher in poorly differentiated tumors (P=0.007), high-stage tumors (P=0.002) and tumors with blood vessel involvement (P=0.001), lymph vessel invasion (P=0.004), lymph node metastases (P=0.005) and larger myometrial infiltration depths (P=0.0002).
Analysis of ROC curves, assay sensitivity and specificity
In order to evaluate the diagnostic values of HE4 and MMP2, ROC curves were plotted and the AUCs were calculated. For HE4, the AUC values were 0.92 for all study patients, 0.92 for pre-menopausal patients and 0.86 for post-menopausal patients. The AUC values for MMP2 were 0.79, 0.98 and 0.71, respectively (–). The diagnostic potential of HE4 and MMP 2 in differentiation of high (International Federation of Gynecology and Obstetrics [FIGO] III and IV) vs low (FIGO I and II) clinical stage of tumor and prediction of cellular differentiation grade (G1 vs G3) on the basis of the analysis of the AUC is, respectively, 0.86 and 0.82 for HE4 and 0.82 and 0.74 for MMP2. The curves are presented in and . presents the sensitivity and specificity values for HE4 and MMP2 according to the hormonal status of patients. Higher sensitivity was observed for MMP2 in all patients included in the analysis (68%) and in post-menopausal patients (67%) compared to HE4 (67% and 64%, respectively). In pre-menopausal patients, the sensitivity was higher for HE4 (78%) than for MMP2 (73%). On the other hand, the HE4 marker was significantly more specific than MMP2 in every study group and amounted to 93% vs 86% in all patients included in the analysis, 94% vs 84% in pre-menopausal patients and 84% vs 79% in post-menopausal patients.
Figure 1 The ROC curve for MMP2 protein in patients without division due to the hormonal status (AUC =0.79).
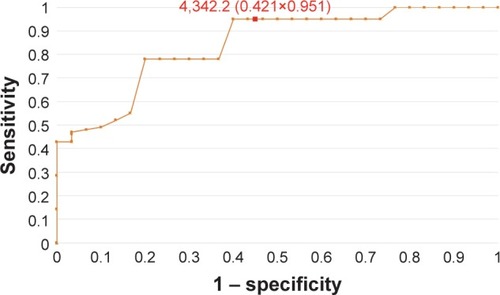
Figure 2 The ROC curve for HE4 protein in patients without division due to the hormonal status (AUC =0.92).
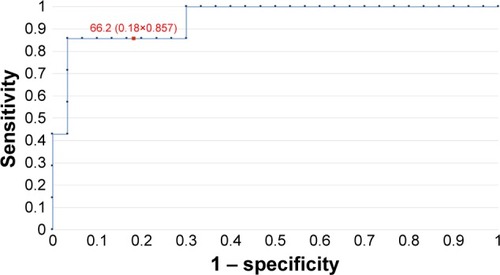
Figure 3 The ROC curves for HE4 and MMP2 proteins in patients before menopause (AUC =0.92/0.84).
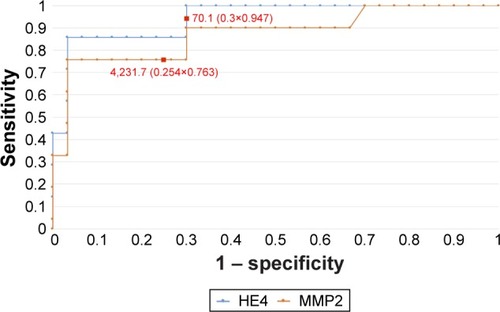
Figure 4 The ROC curves for HE4 and MMP2 proteins in post-menopausal women (AUC =0.86/0.71).
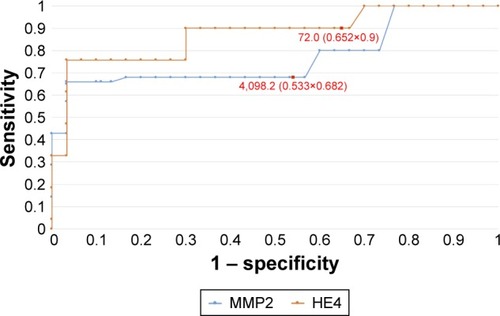
Figure 5 The ROC curves for HE4 and MMP2 proteins depending on staging (AUC =0.86/0.82).
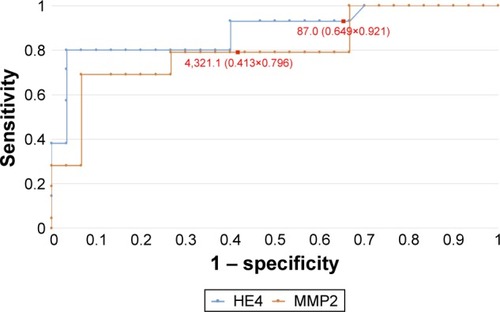
Figure 6 The ROC curves for HE4 and MMP2 proteins in G1 and G3 grading (AUC =0.82/0.74).
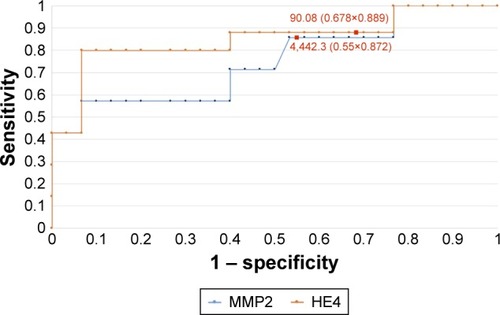
Table 5 Sensitivity and specificity values for HE4 and MMP2
Discussion
Since 2011, when Food and Drug Administration (FDA) approved HE4 as a marker for use in the diagnostics of ovarian cancer, its blood levels are studied and the possibilities are being explored for its use in diagnostics and monitoring of other tumors, including endometrial cancer. In our studies, the serum levels of HE4 were significantly higher in endometrial cancer patients in both pre- and post-menopausal women. This was in line with the results obtained by Angioli et al.Citation6 Similar data were reported by Liu et al,Citation7 who, while comparing the possibilities for early detection of endometrial cancer by means of Ca125 and HE4, demonstrated that HE4 is more sensitive and specific regardless of the age and the hormonal status of patients. Gasiorowska et alCitation8 reported that the threshold HE4 level indicating the cutoff between benign endometrial lesions and endometrial cancer was 58.08 pmol/L. In our case, this value was slightly higher and amounted to 66.2 pmol/L.
Available in the literature are two meta-analyses that assess the possibilities for using HE4 in early detection of endometrial cancer. The sensitivity and specificity of the detection of endometrial cancer on the basis of single HE4 measurements were 56% and 89%Citation9 and 59% and 92%, respectively.Citation10 Bie and ZhangCitation10 and Hu et alCitation9 highlighted that HE4 is more sensitive than Ca125; however, the results were unsatisfactory. In our studies, we were able to achieve the sensitivity at the level of 67% with the specificity at the level of 93%.
Also compared was the high expression of HE4 in endometrial cancer tissues compared to benign endometrial pathologies, amounting to 85.7% and 16.7%, respectively.Citation11 In addition, tissue expression was observed to be higher in endometrial cancer compared to atypical endometrial hyperplasia, amounting to 84.6% and 66.6%, respectively.Citation4
The most recent studies by Chen et alCitation12 showed that MACC1 plays an important role in endometrial cancer, as its high expression confirmed in vivo in endometrial cancer cell lines was correlated with high expression of MMP2. MACC1 is currently considered a key regulator in the development of numerous cancers. Also numerous other authors pointed to the high expression of MMP2 in patients with endometrial cancer.Citation5,Citation13,Citation14 Guo et alCitation15 demonstrated that active MMP2 constitutes the main form of the protein in endometrial cancer, as confirmed by means of electrophoretic assay.
To date, no reports are available on the serum levels of MMP2, comparing the levels of the protein in the serum of patients with endometrial cancer to that in patients with normal endometrium. In our studies, we observed that the levels of MMP2 in endometrial cancer patients were statistically significantly higher than those in patients with normal endometrium. The sensitivity and specificity of the protein in the detection of endometrial cancer were 68% and 86%, respectively. The values are comparable to those obtained for HE4. The respective AUC values for MMP2 compared to HE4 were 0.77 and 0.83. Angioli et alCitation6 highlighted that ultrasound scans and serum HE4 measurements allow for better preoperative assessment of the clinical stage of tumor than NMR (with the sensitivity values of 96.3% and 91%, respectively), facilitating significant reduction in the costs of diagnostic examinations.
Angioli et alCitation6 demonstrated statistically significant differences in HE4 levels in patients with different types of endometrial cancer.Citation6 In our studies, serum HE4 levels were higher in patients with type II endometrial cancer, albeit no statistical significance threshold was achieved, in line with the results obtained by other authors.Citation16–Citation18
Similarly to Abdalla et alCitation18 and Dobrzycka et al,Citation19 we observed no differences in HE4 levels between the subgroups of patients with well- and moderately differentiated endometrial tumors while observing statistically significant differences between the subgroups of patients with well- and poorly differentiated endometrial tumors.
Other studies demonstrated that patients with well-differentiated G1 endometrial tumors of low clinical staging had their serum HE4 levels significantly lower than patients with G3 tumors of FIGO stages III and IV. The authors suggested that this observation could be used in the future to identify patients requiring systemic lymphadenectomy.Citation10 Capriglione et alCitation20 observed that the serum HE4 levels were statistically significantly higher for all negative prognostic factors (low histopathological differentiation, high stage, blood vessel and lymph vessel involvement and lymph node metastases).Citation20 These reports are in line with our results including the HE4 levels being higher in patients with the involvement of lymphatic and blood vessels and in patients with tumor metastases into the lymph nodes. Yilmaz et al and Kemik et al confirmed that in endometrial cancer patients, HE4 marker may be used for individual treatment planning.Citation21,Citation22
The differences in the expression of MMP2 for different histopathological types of tumors were not determined. In our studies, MMP2 levels were found to be higher in non-endometrioid carcinomas than in endometrioid adenocarcinomas. Karahan et alCitation23 and Chen et alCitation12 demonstrated that MMP2 expression was much higher in patients with higher clinical stage of the disease. Higher expression of MMP2 in higher tumor stage patients was also demonstrated in immunohistochemical studies by Graesslin et alCitation24 and Li et al.Citation25 We observed that the serum MMP2 levels were markedly higher in patients with higher clinical stage of the disease (FIGO III and IV). However, we are aware that the reports on high tissue expression are not completely comparable to those on high serum levels.
We were also able to observe statistically significant differences in MMP2 levels between the well- and poorly differentiated endometrial cancer. Talvensaari-Mattila et al, who conducted their studies only in type I endometrial cancer patients, observed that the overexpression of MMP2 in the tissues was correlated with tumor differentiation and was thus associated with poorer prognosis. MMP2 was overexpressed in 100% of poorly differentiated tumors compared to only 70% in well-differentiated tumors.Citation13 Similar reports were presented by Li et al,Citation25 Graesslin et al,Citation24 Guo et al,Citation15 and Karahan et al,Citation23 who considered MMP2 to be a marker facilitating identification of patients with higher likelihood of both local and distant metastases. In our study, higher serum MMP2 levels were observed in patients with lymph node metastases and lymphatic vessel involvement but not in patients with deep myometrial infiltration or blood vessel involvement. Guo et al demonstrated a markedly higher expression of MMP2 for deeper myometrial infiltration.
Conclusion
Both human epididymal epithelial cell protein subfraction 4 and metalloproteinase 2 are characterized by high specificity and may be useful as biomarkers in the diagnostics of endometrial cancer. When determined preoperatively, HE4 is correlated with the prognostic factors of endometrial cancer and may be helpful in the planning of individual treatment of endometrial cancer patients.
Disclosure
The authors report no conflicts of interest in this work.
References
- FelixASYangHPBellDWShermanMEEpidemiology of endometrial carcinoma: etiologic importance of hormonal and metabolic influencesAdv Exp Med Biol201794334627910063
- AlcazarJLPinedaLMartinez-Astorquiza CorralTTransvaginal/transrectal ultrasound for assessing myometrial invasion in endometrial cancer: a comparison of six different approachesJ Gynecol Oncol201526320120726197857
- SanjuanACoboTPahisaJPreoperative and intraoperative assessment of myometrial invasion and histologic grade in endometrial cancer: role of magnetic resonance imaging and frozen sectionInt J Gynecol Cancer200616138539016445663
- LiXGaoYTanMExpression of HE4 in endometrial cancer and its clinical significanceBiomed Res Int2015201543746826539494
- WangDWangDWangNLongZRenXLong non-coding RNA BANCR promotes endometrial cancer cell proliferation and invasion by regulating MMP2 and MMP1 via ERK/MAPK signaling pathwayCell Physiol Biochem2016403–464465627898420
- AngioliRCapriglioneSScalettaGThe role of HE4 in endometrial cancer recurrence: how to choose the optimal follow-up programTumour Biol20163744973497826531723
- LiuXZhaoFHuLSunYValue of detection of serum human epididymis secretory protein 4 and carbohydrate antigen 125 in diagnosis of early endometrial cancer of different pathological subtypesOnco Targets Ther201581239124326060409
- GasiorowskaEMagnowskaMIzyckaNWarcholWNowak-MarkwitzEThe role of HE4 in differentiating benign and malignant endometrial pathologyGinekol Pol201687426026427321096
- HuLDuSGuoWChenDLiYComparison of serum human epididymis protein 4 and carbohydrate antigen 125 as markers in endometrial cancer: a meta-analysisInt J Gynecol Cancer201626233134026807564
- BieYZhangZDiagnostic value of serum HE4 in endometrial cancer: a meta-analysisWorld J Surg Oncol20141216924885319
- DengLGaoYLiXExpression and clinical significance of annexin A2 and human epididymis protein 4 in endometrial carcinomaJ Exp Clin Cancer Res20153419626362938
- ChenSZongZHWuDDSunKXLiuBLZhaoYThe role of metastasis-associated in colon cancer 1 (MACC1) in endometrial carcinoma tumorigenesis and progressionMol Carcinog201656413611371
- Talvensaari-MattilaASantalaMSoiniYTurpeenniemi-HujanenTPrognostic value of matrix metalloproteinase-2 (MMP-2) expression in endometrial endometrioid adenocarcinomaAnticancer Res2005256B4101410516309203
- Honkavuori-ToivolaMSantalaMSoiniYTurpeenniemi-HujanenTTalvensaari-MattilaACombination of strong MMP-2 and weak TIMP-2 immunostainings is a significant prognostic factor in endometrial carcinomaDis Markers201335426126624344400
- GuoWChenGZhuCWangHExpression of matrix metalloproteinase-2, 9 and it’s tissue inhibitor-1, 2 in endometrial carcinomaZhonghua Fu Chan Ke Za Zhi2002371060460712487935
- BignottiERagnoliMZanottiLDiagnostic and prognostic impact of serum HE4 detection in endometrial carcinoma patientsBr J Cancer201110491418142521468050
- BrennanDJHackethalAMetcalfAMANECS GroupSerum HE4 as a prognostic marker in endometrial cancer – a population based studyGynecol Oncol2014132115916524211402
- AbdallaNPiorkowskiRStanirowskiPSlomkaACendrowskiKSawickiWAssessment of levels of the tumor markers HE4 and CA125 considering staging, grading and histological types of endometrial cancerPrz Menopauzalny201615313313727980523
- DobrzyckaBMackowiak-MatejczykBTerlikowskaKMKinalskiMTerlikowskiSJUtility of HE4 to identify patients with endometrioid endometrial cancer who may require lymphadenectomyAdv Med Sci2016611232726344910
- CapriglioneSPlottiFMirandaAFurther insight into prognostic factors in endometrial cancer: the new serum biomarker HE4Expert Rev Anticancer Ther201717191827892774
- KemikPSaatliBYildirimNDiagnostic and prognostic values of preoperative serum levels of YKL-40, HE-4 and DKK-3 in endometrial cancerGynecol Oncol20161401646926607777
- YilmazSAAltinkayaSOKerimogluOSThe role of human epididymis secretory protein E4 in patients with endometrial cancer and premalignant endometrial lesionsJ Obstet Gynaecol2017371586328006994
- KarahanNGuneyMBaspinarSOralBKapucuogluNMunganTExpression of gelatinase (MMP-2 and MMP-9) and cyclooxygenase-2 (COX-2) in endometrial carcinomaEur J Gynaecol Oncol200728318418817624083
- GraesslinOCortezAFauvetRLorenzatoMBirembautPDaraiEMetalloproteinase-2, -7 and -9 and tissue inhibitor of metalloproteinase-1 and -2 expression in normal, hyperplastic and neoplastic endometrium: a clinical-pathological correlation studyAnn Oncol200617463764516407419
- LiSShenXYangZClinical significance of MMP2 overexpression in endometrial adenocarcinomaNan Fang Yi Ke Da Xue Xue Bao201434342342524670463
