Abstract
Objective
To evaluate the effect of postoperative hepatic artery-infusion chemotherapy (HAIC) on survival probability in patients with hepatocellular carcinoma (HCC) after radical hepatectomy.
Patients and methods
This retrospective study included 85 patients with HCC who received radical hepatectomy from May 2005 to May 2010. Among these patients, 42 underwent two sessions of HAIC (5-fluoruracil [1,000 mg/m2], oxaliplatin [85 mg/m2], and mitomycin-C [6 mg/m2]) after radical hepatectomy (HAIC group), and 43 underwent radical hepatectomy only (the control group). HAIC-related side effects and long-term survival were retrospectively analyzed.
Results
The HAIC group showed a significantly higher 5-year intrahepatic recurrence-free survival probability and lower risk of intrahepatic recurrence (HR 0.5615, 95% CI 0.3234–0.9749 [log-rank test]; P=0.0332). The HAIC group also had significantly higher 5-year disease-free survival probability (HR 0.591, 95% CI 0.3613–0.9666 [log-rank test]; P=0.0298) and overall survival probability than the control group (HR 0.5768, 95% CI 0.3469–0.9589 [log-rank test]; P=0.0278). No HAIC-related deaths in the HAIC group were reported. All toxicities and complications were controlled, and no patients quit the treatment.
Conclusion
HAIC can effectively and safely reduce intrahepatic recurrence and improve the long-term survival of patients with HCC after radical hepatectomy.
Introduction
Hepatocellular carcinoma (HCC) leads to 690,000 deaths annually worldwide. More than half of these deaths occur in China, and thus the disease is considered the second-most fatal cancer in the country.Citation1 Various treatment options are currently available, such as surgical resection, radiofrequency ablation, percutaneous ethanol injection, transcatheter arterial chemoembolization, radiotherapy, and hepatic artery-infusion chemotherapy (HAIC).Citation2
Radical resection is the optimal therapy for patients with HCC with early disease stage and good liver-function reserve.Citation3,Citation4 However, more than 80% of patients with HCC develop recurrence within 5 years after radical hepatectomy, which significantly harms long-term survival.Citation5–Citation7 Postoperative recurrence of HCC is related to multicenter growth of tumor and microscopic tumor thrombosis.Citation8,Citation9 Both univariate and multivariate analyses of the prognostic factors indicate that intrahepatic recurrence significantly affects survival.Citation10,Citation11 Macroscopic tumor foci are resected by surgery; however, undetected residual microscopic foci will then lead to relapse.Citation8 Therefore, elimination of the residual microscopic foci plays an important role in improving the long-term survival of HCC after surgery. The present study aims to determine the safety and efficacy of HAIC in patients with HCC after radical hepatectomy.
Patients and methods
Patients
The medical records of 85 patients with HCC who had received radical hepatectomy in our hospital from May 2005 to May 2010 were retrospectively analyzed. Baseline characteristics – age, sex, serum AFP level, operating time, blood loss during surgery, etiology, degree of cirrhosis, resection modalities, microvascular invasion (MVI), Child–Pugh stage, tumor size, number of tumors, and tumor differentiation – were collected and compared. The presence of MVI was determined by pathological findings from surgical specimens.
Among the patients, 42 underwent two courses of HAIC after radical hepatectomy (HAIC group) and 43 underwent radical hepatectomy without postoperative HAIC (control group). Entry criteria were 1) age 18–75 years, 2) Karnofsky performance score ≥70, 3) no allergy to the chemotherapy agent, 4) no previous anticancer therapy, 5) Child–Pugh class A–B, 6) no portal vein thrombosis or systemic metastasis, 7) no refractory ascites or organ dysfunction, 8) no recurrence or remnant tumor detected within 1 month after radical hepatectomy, and 9) full follow-up data.
The protocol was approved by the ethics committee of the First People’s Hospital, affiliated to Huzhou Normal College, and followed the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. Signed informed consent was obtained from all patients.
HAIC administration
HAIC was administered within 3 weeks after surgery for two sessions, with an interval of 4 weeks. The procedure included oxaliplatin (Oxa; 85 mg/m2) as a 2-hour infusion, followed by fluorouracil (5FU; 1,000 mg/m2) as a 5-hour infusion and mitomycin C (6 mg/m2) as a >1-hour infusion. HAIC was administered via a catheter, with one tip inserted into the common hepatic artery or proper hepatic artery and the other end connected to the micropump.Citation12 The catheter was removed after HAIC. Patients were carefully monitored, and symptomatic treatments were administered. Treatment would be terminated in cases of life-threatening side effects or upon the request of the patient.
Assessment and follow-up
All complications and toxicities assessed according to the Common Terminology Criteria for Adverse Events (version 4.0) were recorded. Patients were followed up every 4 weeks in the first year after surgery and every 3 months thereafter. The follow-up assessment included physical examination, biological testing, AFP level, chest radiography, abdominal ultrasonography and computed tomography, or magnetic resonance imaging. Recurrence was diagnosed by imaging and if necessary biopsy. Once recurrence or metastasis was confirmed, transcatheter arterial chemoembolization, radiofrequency ablation, or surgery was performed.
Statistical analysis
Data are expressed as means ± SD. Statistical analysis was conducted using the two-sample t-test and adjusted χ2 test between the two groups. Fisher’s exact test was also employed if individual cell size was fewer than five counts. Survival probabilities were calculated using the Kaplan–Meier method, and survival curves were analyzed using the log-rank test. P<0.05 was considered statistically significant.
Results
Baseline characteristics
Baseline characteristics are listed in . No significant differences in age, sex, serum AFP level, operating time, blood loss during surgery, etiology, degree of cirrhosis, resection modalities, MVI, Child–Pugh stage, tumor size, number of tumors, or tumor differentiation were indicated.
Table 1 Baseline characteristics
Intrahepatic recurrence-free survival
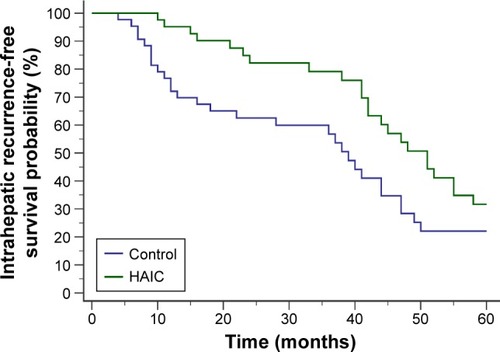
In the first 5 postoperative years, 23 of 42 patients in the HAIC group and 29 of 43 patients in the control group developed intrahepatic recurrence. The HAIC group obtained significantly higher intrahepatic recurrence-free survival probability (HR 0.5615, 95% CI 0.3234–0.9749 [log-rank test]; P=0.0332) ().
Figure 1 Intrahepatic recurrence-free survival curves after radical hepatectomy.
Abbreviation: HAIC, hepatic artery-infusion chemotherapy.
Disease-free survival
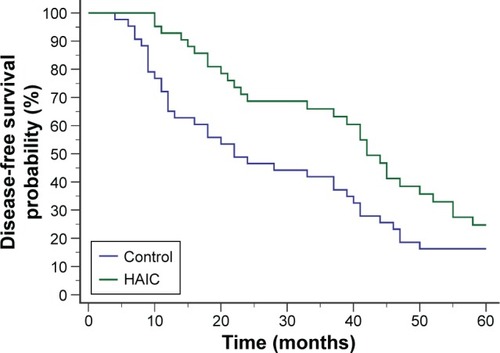
In the first 5 postoperative years, 29 patients in the HAIC group and 36 patients in the control group developed recurrence. The 5-year disease-free survival probability was significantly higher in the HAIC group than in the control group (HR 0.591, 95% CI 0.3613–0.9666 [log-rank test]; P=0.0298) ().
Figure 2 Disease-free survival curves after radical hepatectomy.
Abbreviation: HAIC, hepatic artery-infusion chemotherapy.
Overall survival
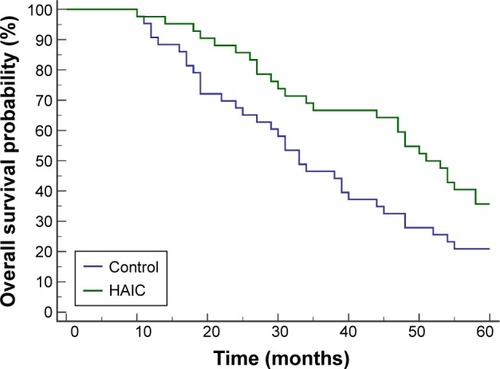
In the first 5 postoperative years, 27 patients in the HAIC group and 34 patients in the control group had died. The HAIC group obtained significantly higher overall survival probability than the control group (HR 0.5768, 95% CI 0.3469–0.9589 [log-rank test]; P=0.0278) ().
Figure 3 Overall survival curves after radical hepatectomy.
Abbreviation: HAIC, hepatic artery-infusion chemotherapy.
Toxicity and complications
No HAIC-induced deaths in the HAIC group were reported. Patients in the HAIC group mainly experienced paresthesia (14 of 42), neutropenia (15 of 42), thrombocytopenia (16 of 42), anemia (10 of 42), nausea or vomiting (30 of 42), diarrhea (8 of 42), and hepatic toxicity (25 of 42). All toxicities and complications were controlled, and no patients quit the treatment.
Discussion
HCC is a global public health problem, and causes millions of death annually worldwide. It is considered one of the most common cancers in China.Citation13 Due to the difficulty of early diagnosis and the high frequency of metastasis and recurrence, the prognosis of HCC remains poor.
Liver tumors have been demonstrated to receive abundant blood supply from the hepatic artery.Citation14–Citation16 HAIC is a hepatically directed treatment that takes advantage of relatively selective hepatic arterial tumor vascularization. Several meta-analyses and clinical studies have concluded that postoperative HAIC can decrease recurrence after surgery and improve long-term survival.Citation17–Citation19 HAIC draws interest as a potential treatment option, because compared with intravenous infusion, extraction of chemotherapeutic drugs from the hepatic arterial circulation via the first-pass effect can produce higher local concentrations and fewer systemic side effects. Accordingly, we started the protocol of arterial infusion of Oxa (85 mg/m2 on day 1) followed by 5FU (1,000 mg/m2 on day 1) and mitomycin C (6 mg/m2). The feasibility and safety of HAIC with Oxa, mitomycin C, and 5FU for HCC treatment have been reported previously.Citation20
In the current study, fewer patients in the HAIC group than in the control group developed intrahepatic recurrence during the first 5 years after surgery. The HAIC group obtained significantly higher 5-year intrahepatic recurrence-free survival probability and lower risk of intrahepatic recurrence (HR 0.5615, 95% CI 0.3234–0.9749; P=0.0332). Consequently, the HAIC group also obtained significantly higher 5-year disease-free survival (HR 0.591, 95% CI 0.3613–0.9666; P=0.0298) and overall survival probability compared with the control group (HR 0.5768, 95% CI 0.3469–0.9589 [log-rank test]; P=0.0278). No HAIC-related deaths in the HAIC group were reported. No patients quit the treatment because of uncontrollable toxicities or complications.
In conclusion, HAIC is an effective and safe procedure for preventing postoperative intrahepatic recurrence and improving the long-term survival of patients with HCC after curative resection. The current protocol can be safely employed in a prospective randomized study.
Author contributions
MF, CWT, and JBS designed the study and wrote the manuscript. MF and CWT contributed equally to this study. WMF, YB, and YYZ conducted patient management and monitoring. YB and WMF collected and analyzed the data. All authors contributed toward data analysis, drafting, and revising the paper, and agree to be accountable for all aspects of the work.
Acknowledgments
This study was supported by the Foundation of Huzhou Bureau of Science and Technology (grant 2016YZB02).
Disclosure
The authors report no conflicts of interest in this work.
References
- JemalABrayFCenterMMFerlayJWardEFormanDGlobal cancer statisticsCA Cancer J Clin2011612699021296855
- MiyakiDAikataHHondaYHepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma according to Child-Pugh classificationJ Gastroenterol Hepatol201227121850185723020312
- UkaKAikataHTakakiSPretreatment predictor of response, time to progression, and survival to intraarterial 5-fluorouracil/interferon combination therapy in patients with advanced hepatocellular carcinomaJ Gastroenterol2007421084585317940838
- TangCWZhuMFengWMBaoYZhengYYChinese herbal medicine, jianpi ligan decoction, improves prognosis of unresectable hepatocellular carcinoma after transarterial chemoembolization: a retrospective studyDrug Des Devel Ther20161024612466
- ImamuraHMatsuyamaYTanakaERisk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomyJ Hepatol200338220020712547409
- PoonRTFanSTNgIOLoCMLiuCLWongJDifferent risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinomaCancer200089350050710931448
- SitzmannJVAbramsRImproved survival for hepatocellular cancer with combination surgery and multimodality treatmentAnn Surg199321721491548382468
- LiQWangJSunYPostoperative transhepatic arterial chemoembolization and portal vein chemotherapy for patients with hepatocellular carcinoma: a randomized study with 131 casesDig Surg200623423524016943671
- ChengHYWangXChenDXuAMJiaYCThe value and limitation of transcatheter arterial chemoembolization in preventing recurrence of resected hepatocellular carcinomaWorld J Gastroenterol200511233644364615962394
- KonishiMRyuMKinoshitaTInoueKSurgical treatment of hepatocellular carcinoma with direct removal of the tumor thrombus in the main portal veinHepatogastroenterology200148411421142411677978
- TangCShenJFengWCombination therapy of radiofrequency ablation and transarterial chemoembolization for unresectable hepatocellular carcinoma: a retrospective studyMedicine (Baltimore)20169520e375427196501
- KasugaiHKojimaJTatsutaMTreatment of hepatocellular carcinoma by transcatheter arterial embolization combined with intraarterial infusion of a mixture of cisplatin and ethiodized oilGastroenterology19899749659712550311
- BoschFXRibesJDiazMCleriesRPrimary liver cancer: worldwide incidence and trendsGastroenterology20041275 Suppl 1S5S1615508102
- AckermanNBLienWMKondiESSilvermanNAThe blood supply of experimental liver metastases – I: the distribution of hepatic artery and portal vein blood to “small” and “large” tumorsSurgery1969666106710725402533
- ConwayJGPoppJAJiSThurmanRGEffect of size on portal circulation of hepatic nodules from carcinogen-treated ratsCancer Res1983437337433786850642
- ArcherSGGrayBNVascularization of small liver metastasesBr J Surg19897665455482474356
- MathurinPRaynardBDharancySMeta-analysis: evaluation of adjuvant therapy after curative liver resection for hepatocellular carcinomaAliment Pharmacol Ther200317101247126112755838
- TanakaKShimadaHTogoSUse of transcatheter arterial infusion of anticancer agents with Lipiodol to prevent recurrence of hepatocellular carcinoma after hepatic resectionHepatogastroenterology199946261083108810370671
- FukudaSOkudaKImamuraMImamuraIEriguchiNAoyagiSSurgical resection combined with chemotherapy for advanced hepatocellular carcinoma with tumor thrombus: report of 19 casesSurgery2002131330031011894035
- GuthoffILotspeichEFesterCHepatic artery infusion using oxaliplatin in combination with 5-fluorouracil, folinic acid and mitomycin C: oxaliplatin pharmacokinetics and feasibilityAnticancer Res2003236D5203520814981990
