Abstract
Long non-coding RNAs (lncRNAs) are a group of non-coding RNAs (ncRNAs) >200 nucleotides in length that govern diverse biological processes. Recent evidence suggests that lncRNAs are involved in cancer cell proliferation, apoptosis, invasion, migration, and metastasis. Dysregulation of lncRNAs has been observed in various tumors, and lncRNAs act as oncogenes or tumor suppressors in these malignancies. It has been revealed that lncRNA highly upregulated in liver cancer (HULC) is tightly correlated with a number of cancers such as hepatocellular carcinoma, gastric cancer, colorectal cancer, osteosarcoma, and diffuse large B-cell lymphoma. Depletion of HULC suppressed cancer cell proliferation, migration, and invasion and induced apoptosis. Additionally, HULC may function as a diagnostic biomarker and prognostic indicator for some tumors. In this review, we summarize the current knowledge of the role of HULC in cancer progression and the clinical management of human cancers.
Introduction
Long non-coding RNAs (lncRNAs) are transcripts of >200 nucleotides in length without protein-coding ability that have recently been found to be pervasively transcribed in the human genome.Citation1 Increasing numbers of studies have shown that lncRNAs exert important roles in cancer development and progression. The molecular functions executed by lncRNAs have been summarized as signals, decoys, guides, and scaffolds.Citation2 It has been elucidated that lncRNAs are involved in various biological processes including gene expression, RNA splicing, and ligand–receptor engagement, mediating the pathogenesis of numerous human diseases.Citation3–Citation5 Furthermore, recent investigations have demonstrated that lncRNAs are aberrantly expressed in many malignancies such as liver cancer, colorectal cancer (CRC), gastric cancer (GC), lung cancer, and breast cancer. Thus, lncRNAs play oncogenic and anti-tumor roles in tumor progression, and the levels of certain lncRNAs could be used as diagnostic markers and prognostic indicators.Citation6–Citation8
Highly upregulated in liver cancer (HULC) has been identified to closely associate with a number of tumors, including human hepatocellular carcinoma (HCC),Citation9 GC,Citation10 CRC,Citation11 osteosarcoma (OS),Citation12 and B-cell lymphoma.Citation13 Inhibition of HULC suppresses cancer cell proliferation and promotes apoptosis in some tumors. Emerging evidence suggests that HULC may act as a biomarker for tumor diagnosis and prognosis monitoring. In this review, we summarize the current knowledge of the role of HULC in the cancer progression and the clinical management of human cancers ().
Table 1 Functions and applications of upregulated HULC in various tumors
Characterization of HULC
HULC, located on chromosome 6p24.3 (data from UCSC website; http://genome-asia.ucsc.edu/cgi-bin/hgTracks?db=hg38&lastVirtModeType=default&lastVirtModeExtraState=&virtModeType=default&virtMode=0&nonVirtPosition=&position=chr6%3A8652209%2D8653846&hgsid=471875206_bqLwOkwNkIK5wmKztR6yq3iR2yzq), was first recognized as a novel messenger RNA (mRNA)-like lncRNA with two exons and dramatically increased expression in human HCC, using an HCC-specific complementary DNA (cDNA) microarray platform.Citation9 The full sequence of HULC can be obtained from the Entrez Gene database (https://www.ncbi.nlm.nih.gov/nuccore/NR_004855.2). Additionally, the prediction of HULC structure based on MFE and partition function can be obtained from ViennaRNA website (http://rna.tbi.univie.ac.at//cgi-bin/RNAWebSuite/RNAfold.cgi?PAGE=3&ID=0J41idlxsN). It has been widely reported that ~20% of all lncRNAs can bind to polycomb repressive complex 2 (PRC2), which consists of enhancer of zeste homolog 2 (EZH2), suppressor of zeste 12 (SUZ12), and embryonic ectoderm development (EED) proteins and mediates methyltransferase activities.Citation14–Citation16 HULC interaction with PRC2, which functions as a transcriptional silencer, could mediate CRC progression.Citation11
Function investigations found that depletion of HULC leads to genomic and morphological alterations in poorly differentiated SK-Hep-1 cells, implying its crucial roles in mesenchymal phenotype maintenance.Citation17 Furthermore, transfection of HCC cells with si-HULC significantly decreased cell proliferation, migration, and invasion and facilitated cell apoptosis.Citation17 HULC, identified as a microRNA (miRNA) sponge, can decrease the expression of a number of miRNAs.Citation18 Knockdown of HULC could impair translational repression of its target gene, which results in alterations in the patterns of the deacetylation and methylation of histones.Citation19 Furthermore, RNA immunoprecipitation (RIP) and RNA pull-down assays indicated that HULC can inhibit miR-9 expression through association with DNA methyltransferase mediating TNF-α-induced apoptosis.Citation20 Thus, the HULC-miR-9 pathway may be a potential target for atherosclerosis treatment. Recently, it has been recognized that patients with HCC with high HULC expression levels could benefit from metformin treatment, and the serum level of HULC may be a biomarker of treatment efficacy.Citation17
Potential clinical implications of HULC
HCC
HCC is the third leading cause of cancer-related death annually and the sixth most common malignancy worldwide.Citation21,Citation22 In China, HCC is one of the four leading causes of cancer-related death,Citation23 and this dismal prognosis is associated with frequent cancer metastasis, tumor recurrence, and a lack of curative treatment.Citation24 Large-scale studies have been undertaken to investigate the comprehensive treatment options for HCC treatment, including surgical resection, chemotherapy, orthotopic liver transplantation (OLT), transcatheter arterial chemoembolization (TACE), and targeted immunotherapy,Citation25,Citation26 and most of these therapies are only available at an early stage.Citation24 However, the patients are frequently diagnosed at an intermediate or advanced stage due to the lack of novel diagnostic biomarkers.Citation27 Therefore, identifications of effective novel molecular biomarkers and therapeutic targets in HCC are critical and will help to accelerate the research on HCC pathogenesis.
Panzitt et alCitation18 first demonstrated that lncRNA HULC was specifically expressed in hepatocytes and highly upregulated in HCC specimens (including 46 HCCs, four focal nodular hyperplasia [FNH], seven cirrhosis, and two non-neoplastic liver samples). Quantitative polymerase chain reaction (qPCR) was performed to investigate the lncRNA HULC expression at the mRNA levels and showed that HULC exhibited on average 33-fold (32.7±5.0, P=0.16) upregulation over the non-neoplastic liver pool in 76% (35/46) of HCCs. In addition, the aberrant expression of HULC in HCC tissues was related to tumor– node–metastasis (TNM) stage, intrahepatic metastases, HCC recurrence, and postoperative survival.Citation9 Recently, a preliminary analysis of the Gene Expression Omnibus (GEO) revealed that HULC was significantly associated with overall survival in patients with HCC (P=0.005).Citation28 Interestingly, Yang et alCitation28 verified that HULC had a significant association with vascular invasion, which should be a positive factor for HCC prognosis, and univariate and multivariate Cox regression analyses also confirmed that upregulation of HULC in tumor tissues contributed to better outcomes both for overall survival and in disease-free survival.
The detection of lncRNA HULC in blood from patients with HCC implies its potential use as a novel biomarker.Citation9 Li et alCitation29 demonstrated that lncRNA HULC and Linc00152 expression levels in plasma samples from patients with HCC were significantly upregulated and correlated with tumor growth and metastasis of HCC. Thus, HULC and Linc00152 may function as potential diagnostic biomarkers and therapeutic targets for patients with HCC.Citation29 Furthermore, HULC dysregulation can alter the expression of HCC-related genes to regulate gene expression. Du et alCitation30 reported that HBx-induced upregulation of HULC could promote HCC proliferation by silencing cyclin-dependent kinase inhibitor 2C (CDKN2C, P18) at the mRNA and protein levels. Moreover, Wan et alCitation31 illustrated that the expression of HULC and tumor suppressor miR-203 in HCC tissues or cell lines was inversely correlated and miR-203-induced HULC impairment dramatically diminished HCC cell proliferation and invasion and mediated cell apoptosis. Additionally, the abnormal activity of HULC could regulate human liver stem cell differentiation and tumor angiogenesis through miR-107/transcription factor E2F1/sphingosine kinase 1 (SPHK1) signaling to promote tumorigenesis in human HCC.Citation11,Citation32,Citation33
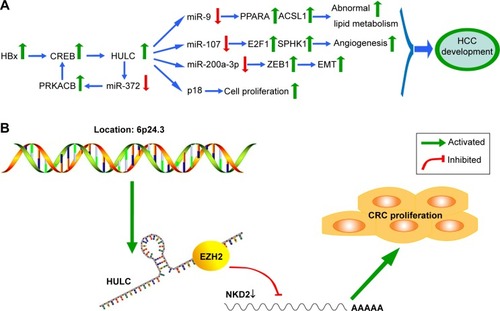
A functional and mechanistic study revealed that HULC could serve as a competing endogenous RNA (ceRNA) to sponge with miR-200a-3p and exert effects in HCC progression through zinc finger E-box-binding homeobox 1 (ZEB1)-induced epithelial–mesenchymal transition (EMT). Accordingly, Panzitt et alCitation18 discovered that downregulation of HULC can inhibit growth and metastasis in vitro and in vivo. The variant genotypes of rs7763881 in HULC discovered by Liu et alCitation34 have been investigated for their association with decreased HCC susceptibility in hepatitis B virus persistent carriers; a full investigation of the molecular mechanisms of HULC in the natural progression of hepatitis B virus infection to HCC development should be performed. Recently, Cui et alCitation35 concluded that retinoid X receptor alpha (RXRA) can activate the HULC promoter, and the corresponding feedback loop can upregulate the expression of HULC to facilitate malignant development via abnormal lipid metabolism in hepatoma cells through an miR-9-mediated RXRA signaling pathway. In addition, lncRNA HULC can also be induced by the transcription factor cAMP response element binding (CREB) protein through sequestration of miRNA-372.Citation19 The protumorigenic mechanisms of HULC in HCC are involved with different regulatory pathways (). These findings implied that HULC may be a crucial factor in HCC initiation and progression and confirm that lncRNA HULC could act as a novel prognostic marker and potential therapeutic target for HCC. However, larger samples and further mechanistic investigations are urgently needed to support these findings.
Figure 1 The protumorigenic mechanisms of HULC overexpression in HCC and CRC.
Abbreviations: HULC, highly upregulated in liver cancer; HCC, hepatocellular carcinoma; CRC, colorectal cancer; CREB, cAMP response element binding; SPHK1, sphingosine kinase 1; ZEB1, zinc finger E-box-binding homeobox 1; EMT, epithelial–mesenchymal transition; EZH2, enhancer of zeste homolog 2.
GC
GC is currently one of the leading causes of cancer-related death, especially in Asia,Citation36 and it has been defined as the second leading cause of cancer death in China with 679,000 newly reported cases and 498,000 GC-related deaths in 2015.Citation23 Owing to a lack of an effective molecule biomarker, most patients with GC are often at an advanced stage when diagnosed and the prognosis is poor even with surgery, chemotherapy, and radiotherapy due to relapse risk, distant metastasis, and chemoresistance.Citation37 Thus, a better understanding of the molecular mechanisms underlying the development of GC may help identify sensitive diagnostic and prognostic biomarkers and therapeutic targets.
Jin et alCitation10 investigated the serum HULC level of patients with GC using quantitative real-time polymerase chain reaction (qRT-PCR) and found high expression of circulating HULC in GC patients, implying its novel functions as a serum tumor marker for diagnosis and a monitoring indicator for prognosis. Moreover, they also showed that HULC overexpression in serum was associated with tumor size, lymph node metastasis, distant metastasis, TNM stage, and Helicobacter pylori infection. Another study elucidated significant HULC overexpression in GC cell lines and GC tissues compared with normal controls, and functional analyses revealed that depletion of HULC inhibited cell proliferation and invasion, induced apoptosis, and reversed EMT in GC.Citation38 Furthermore, elevated expression of HULC could mediate cancer cell proliferation and invasion and suppress apoptosis by activating autophagy.Citation38
In conclusion, silencing HULC expression could inhibit GC cell proliferation. Moreover, lncRNA HULC might serve as an oncogene in GC progression, suggesting its utilities as a candidate diagnostic biomarker and a novel therapeutic target. However, massive efforts are needed to explore the underlying regulatory mechanism.
CRC
CRC is the third most common malignancy worldwide with 1,200,000 newly diagnosed cases annuallyCitation39,Citation40 and is characterized by a poor prognosis with ~600,000 deaths each year.Citation41 The diagnostic biomarkers frequently used in the clinic at present have been shown to lack sensitivity and specificity, which leads to difficulties of early diagnosis and challenges for CRC therapy.Citation42,Citation43 Therefore, identifications of novel diagnostic and therapeutic biomarkers would be of great clinical significance.
Matouk et alCitation44 reported that HULC was expressed neither in primary CRC samples nor in their normal counterparts. Moreover, upregulation of HULC was found in patients with CRC who had distant metastasis of liver cancer, highlighting its specificity to malignant cells located in the liver.Citation44 However, the earlier investigation has been reported and updated in recent years. Yang et alCitation11 demonstrated that HULC was upregulated in CRC tissues and cells and associated with poor prognosis and shorter survival for CRC patients. Moreover, depletion of HULC suppressed tumorigenicity of CRC cells in vivo and impaired CRC cells proliferation, migration, and invasion and induced cell apoptosis in vitro.Citation11 Importantly, the oncogenic function mediated by HULC is partially induced by epigenetically silencing NKD2 expression via direct interaction with EZH2 (a part of PRC2) ().Citation11 These observations implied that HULC would be a crucial biomarker and useful therapeutic target in CRC.
Pancreatic cancer (PC)
PC is a malignant neoplasmCitation45 and ranks as the fourth leading cause of cancer-related death in Western society.Citation39 Although comprehensive treatments for PC have been rapidly developed recently, the therapeutic efficacy of PC is still far from satisfactory as the early detection and diagnosis of PC are difficult.Citation46,Citation47 Therefore, the major drawback with PC is late diagnosis.
The expressions of HULC in 304 patients with PC were investigated by qRT-PCR, and the findings showed that HULC expression in tumor tissues was significantly higher than that in adjacent normal tissues.Citation48 Moreover, they also showed that higher expression of HULC was significantly correlated with larger tumor size, advanced lymph node metastasis, and vascular invasion. Further studies revealed that reducing HULC expression significantly impaired PC cell proliferation by blocking the G1/S-phase transition in vitro.Citation48
Taucher et alCitation49 reported that HULC expression levels could help to distinguish cancer tissues from healthy tissues, and the certain HULC levels had a significant relationship with dismal overall survival and recurrence of PC in patients. Additionally, multivariate analyses confirmed that HULC could be an independent predictor for overall survival (P=0.032),Citation48 and Kaplan–Meier analysis demonstrated significant differences between patients with high and low HULC expression. Thus, HULC may serve as a highly sensitive screening biomarker and a potential therapeutic target in patients with PC.Citation48
OS
OS, the most common malignant bone tumor that often presents with structural chromosomal alterations, occurs predominantly in adolescents and young adults and accounts for 5% of childhood cancers.Citation50,Citation51 Despite advances in clinical treatments involving surgical resection combined with chemotherapy and/or radiotherapy,Citation52,Citation53 the invasive nature of the disease continues to limit the 5-year survival with OS to merely 20%. Therefore, new, non-conventional treatments using therapeutic targets may help to improve clinical efficacy.
Sun et alCitation54 showed that HULC was significantly upregulated in OS tissues and cell lines compared with normal controls. And the tumors in patients with dramatically expressed HULC often show more aggressive behavior and/or metastatic potential.Citation54 Sun et alCitation54 revealed that the proliferation, migration, and invasion capacity impairment in OS cell lines resulted in part from inhibition of HULC using small interfering RNA (siRNA). Moreover, they also reported that HULC level was correlated with clinical stage, distant metastasis, prognosis, and survival rates in patients with OS, in terms of both overall and event-free survival.Citation54 To further study the relationship between HULC expression and clinicopathological characteristics of patients with OS, a larger sample size is needed.Citation12
It has been verified that HULC could be a potential prognostic biomarker in OS.Citation12 Sun et alCitation54 further confirmed that HULC may be implicated in early prognosis and could act as a therapeutic target in patients with OS. These results highlight the critical and novel role of HULC in OS.
Other human cancers
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of lymphoma.Citation55 Peng et alCitation13 demonstrated that HULC was remarkably overexpressed in both DLBCL tissues and cell lines, and HULC expression was closely associated with DLBCL characteristics, such as Ann Arbor stages, B symptoms, CHOP-like (cyclophosphamide, doxorubicin, vincristine, and prednisone) treatment, rituximab treatment, and international prognostic index (IPI). Furthermore, a large number of samples from the long-term follow-ups indicated that HULC can be considered as an independent diagnostic and prognostic marker in patients with DLBCL.Citation13 Additionally, HULC depletion could significantly inhibit cell proliferation and promote apoptosis by repressing cyclin D1 and Bcl-2 in DLBCL cells, and targeting HULC may be a potential gene therapy for patients with DLBCL.Citation13
Glioma accounts for ~80% of primary malignant brain tumors and exhibits exceptionally high morbidity and mortality.Citation56 It has been documented that HULC, vascular endothelial growth factor (VEGF), and endothelial cell-specific molecule 1 (ESM-1) expression, as well as microvessel density, are positively correlated with grade dependency in tissues from glioma patients. They further discovered that HULC could act as an oncogene in gliomas, and its depletion impairs glioma cell proliferation, adhesion, migration, invasion, and angiogenesis induced by ESM-1 through cell cycle alteration, which leads to angiogenesis suppression.Citation57 Moreover, ESM-1 involvement of the phosphatidylinositol 3 kinase/protein kinase B/mechanistic target of rapamycin signaling pathway can induce anoikis and blocked the cell cycle progression at G1/S phase in glioma, altering the expressions of tumor-related genes.Citation57 These findings implied that HULC could promote pro-angiogenesis activity and serve as a potential therapeutic target in glioma treatment.Citation57
In summary, aberrant expression of HULC plays important roles in the progression of DLBCL and glioma, and there is an urgent need to develop novel therapeutic modalities that specifically and sensitively target the pathogenesis of these lethal malignancies.
Concluding remarks and future perspectives
Dysregulation of lncRNAs is correlated with various cancers. Diagnostic biomarkers and therapeutic targets are essential for early diagnosis and treatment. The rapid development of next-generation sequencing and high-resolution microarray techniques can help to identify the expression and role of lncRNAs in malignancies. HULC involvement has been identified in many cancers, mediating cancer cell proliferation, apoptosis, invasion, and metastasis. Importantly, HULC possesses tremendous diagnostic and therapeutic potential in many cancers.
Although HULC has been characterized, the underlying mechanisms contributing to cancer progression remain unclear. Further investigations of HULC can bring novel insights into the diagnosis and treatment of cancer, and more efforts should be devoted to the development of HULC-based treatment. However, its clinical implications in cancer prevention and treatment still have a long way to go.
Author contributions
Zhonghua Ma and Hesuyuan Huang contributed equally to this work. Yetao Xu and Xuezhi He contributed to drafting and editing of the manuscript. Jirong Wang and Bingqing Hui participated in the conception of the idea. Hao Ji and Jing Zhou contributed to literature search. Keming Wang participated in the conception and coordination. All authors contributed toward data analysis, drafting and revising the paper and agreed to be accountable for all aspects of the work.
Acknowledgments
This work was supported by the Medical Science and Technology Development Foundation, Jiangsu Province Department of Health (H201407), the Six Talents Peak Project of Jiangsu Province (WSN-050), the Natural Science Foundation of Jiangsu Province of China (BK20151578), the Natural Science Fund Project of Jiangsu Province (15KJB320006), and the Medical Science and Technology Development Foundation of Nanjing (YKK13178).
Disclosure
The authors report no conflicts of interest in this work.
References
- JiangYMaloufGGZhangJLong non-coding RNA profiling links subgroup classification of endometrioid endometrial carcinomas with trithorax and polycomb complex aberrationsOncotarget2015637398653987626431491
- LiXWuZFuXHanWlncRNAs: insights into their function and mechanics in underlying disordersMutat Res Rev Mutat Res201476212125485593
- HeYMengXMHuangCLong noncoding RNAs: novel insights into hepatocellular carcinomaCancer Lett20143441202724183851
- WangKCChangHYMolecular mechanisms of long noncoding RNAsMol Cell201143690491421925379
- TsaiMCManorOWanYLong noncoding RNA as modular scaffold of histone modification complexesScience2010329599268969320616235
- WangFYuanJHWangSBOncofetal long noncoding RNA PVT1 promotes proliferation and stem cell-like property of hepatocellular carcinoma cells by stabilizing NOP2Hepatology20146041278129025043274
- YangFZhangLHuoXSLong noncoding RNA high expression in hepatocellular carcinoma facilitates tumor growth through enhancer of zeste homolog 2 in humansHepatology20115451679168921769904
- YuanSXYangFYangYLong noncoding RNA associated with microvascular invasion in hepatocellular carcinoma promotes angiogenesis and serves as a predictor for hepatocellular carcinoma patients’ poor recurrence-free survival after hepatectomyHepatology20125662231224122706893
- LiSPXuHXYuYLncRNA HULC enhances epithelial-mesenchymal transition to promote tumorigenesis and metastasis of hepatocellular carcinoma via the miR-200a-3p/ZEB1 signaling pathwayOncotarget2016727424314244627285757
- JinCShiWWangFLong non-coding RNA HULC as a novel serum biomarker for diagnosis and prognosis prediction of gastric cancerOncotarget2016732517635177227322075
- YangXJHuangCQPengCWHouJXLiuJYLong noncoding RNA HULC promotes colorectal carcinoma progression through epigenetically repressing NKD2 expressionGene2016592117217827496341
- UzanVRLengertABoldriniEHigh expression of HULC is associated with poor prognosis in osteosarcoma patientsPLoS One2016116e156774
- PengWWuJFengJLong noncoding RNA HULC predicts poor clinical outcome and represents pro-oncogenic activity in diffuse large B-cell lymphomaBiomed Pharmacother20167918819327044827
- SanulliSJustinNTeissandierAJarid2 methylation via the PRC2 complex regulates H3K27me3 deposition during cell differentiationMol Cell201557576978325620564
- LiuCLiSDaiXPRC2 regulates RNA polymerase III transcribed non-translated RNA gene transcription through EZH2 and SUZ12 interaction with TFIIIC complexNucleic Acids Res201543136270628426038315
- XuBKonzeKDJinJWangGGTargeting EZH2 and PRC2 dependence as novel anticancer therapyExp Hematol201543869871226027790
- GandhySUImaniradPJinUHSpecificity protein (Sp) transcription factors and metformin regulate expression of the long non-coding RNA HULCOncotarget2015628263592637226317792
- PanzittKTschernatschMMGuellyCCharacterization of HULC, a novel gene with striking up-regulation in hepatocellular carcinoma, as noncoding RNAGastroenterology2007132133034217241883
- WangJLiuXWuHCREB up-regulates long non-coding RNA, HULC expression through interaction with microRNA-372 in liver cancerNucleic Acids Res201038165366538320423907
- MaYHuangDYangFLong noncoding RNA highly upregulated in liver cancer regulates the tumor necrosis factor-alpha-induced apoptosis in human vascular endothelial cellsDNA Cell Biol201635629630026981838
- JemalASiegelRWardECancer statistics, 2008CA Cancer J Clin2008582719618287387
- GohGBChangPETanCKChanging epidemiology of hepatocellular carcinoma in AsiaBest Pract Res Clin Gastroenterol201529691992826651253
- ChenWZhengRBaadePDCancer statistics in China, 2015CA Cancer J Clin201666211513226808342
- GeorgeJPatelTNoncoding RNA as therapeutic targets for hepatocellular carcinomaSemin Liver Dis2015351637425632936
- CervelloMMcCubreyJACusimanoALampiasiNAzzolinaAMontaltoGTargeted therapy for hepatocellular carcinoma: novel agents on the horizonOncotarget20123323626022470194
- ZhuQLiNZengXHepatocellular carcinoma in a large medical center of China over a 10-year period: evolving therapeutic option and improving survivalOncotarget2015664440445025686836
- MaluccioMCoveyARecent progress in understanding, diagnosing, and treating hepatocellular carcinomaCA Cancer J Clin201262639439923070690
- YangZLuYXuQTangBParkCKChenXHULC and H19 played different roles in overall and disease-free survival from hepatocellular carcinoma after curative hepatectomy: a preliminary analysis from gene expression omnibusDis Markers2015201519102926136615
- LiJWangXTangJHULC and Linc00152 act as novel biomarkers in predicting diagnosis of hepatocellular carcinomaCell Physiol Biochem201537268769626356260
- DuYKongGYouXElevation of highly up-regulated in liver cancer (HULC) by hepatitis B virus X protein promotes hepatoma cell proliferation via down-regulating p18J Biol Chem201228731263022631122685290
- WanDShenSFuSmiR-203 suppresses the proliferation and metastasis of hepatocellular carcinoma by targeting oncogene ADAM9 and oncogenic long non-coding RNA HULCAnticancer Agents Med Chem201616441442326179263
- GuiXLiHLiTPuHLuDLong noncoding RNA CUDR regulates HULC and beta-catenin to govern human liver stem cell malignant differentiationMol Ther201523121843185326347501
- WangRSunQWangPNotch and Wnt/beta-catenin signaling pathway play important roles in activating liver cancer stem cellsOncotarget2016755754576826735577
- LiuYPanSLiuLA genetic variant in long non-coding RNA HULC contributes to risk of HBV-related hepatocellular carcinoma in a Chinese populationPLoS One201274e3514522493738
- CuiMXiaoZWangYLong noncoding RNA HULC modulates abnormal lipid metabolism in hepatoma cells through an miR-9-mediated RXRA signaling pathwayCancer Res201575584685725592151
- TanPYeohKGGenetics and molecular pathogenesis of gastric adenocarcinomaGastroenterology201514951153116226073375
- SakaMMoritaSFukagawaTKataiHPresent and future status of gastric cancer surgeryJpn J Clin Oncol201141330731321242182
- ZhaoYGuoQChenJHuJWangSSunYRole of long non-coding RNA HULC in cell proliferation, apoptosis and tumor metastasis of gastric cancer: a clinical and in vitro investigationOncol Rep201431135836424247585
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- YuXLiZYuJChanMTWuWKMicroRNAs predict and modulate responses to chemotherapy in colorectal cancerCell Prolif201548550351026202377
- YangMHYuJChenNElevated microRNA-31 expression regulates colorectal cancer progression by repressing its target gene SATB2PLoS One2013812e8535324386467
- WangRFSongBRPengJJThe prognostic value of preoperative serum CEA and CA19-9 values in stage I–III colorectal cancerHepatogastroenterology20146113299499926158155
- FungKYNiceEPriebeIColorectal cancer biomarkers: to be or not to be? Cautionary tales from a road well travelledWorld J Gastroenterol201420488889824574763
- MatoukIJAbbasiIHochbergAGalunEDweikHAkkawiMHighly upregulated in liver cancer noncoding RNA is overexpressed in hepatic colorectal metastasisEur J Gastroenterol Hepatol200921668869219445043
- MalvezziMBertuccioPLeviFLa VecchiaCNegriEEuropean cancer mortality predictions for the year 2014Ann Oncol20142581650165624759568
- MayorSImmunotherapy improves overall survival in pancreatic cancerLancet Oncol2015162e58
- BergmannLMauteLHeilGA prospective randomised phase-II trial with gemcitabine versus gemcitabine plus sunitinib in advanced pancreatic cancer: a study of the CESAR Central European Society for Anticancer Drug Research-EWIVEur J Cancer2015511273625459392
- PengWGaoWFengJLong noncoding RNA HULC is a novel biomarker of poor prognosis in patients with pancreatic cancerMed Oncol2014311234625412939
- TaucherVManggeHHaybaeckJNon-coding RNAs in pancreatic cancer: challenges and opportunities for clinical applicationCell Oncol (Dordr)201639429531827060060
- HeHNiJHuangJMolecular mechanisms of chemoresistance in osteosarcoma (Review)Oncol Lett2014751352136224765137
- LiuJJLiuSWangJGTelangiectatic osteosarcoma: a review of literatureOnco Targets Ther2013659360223745051
- GuoJReddickWEGlassJODynamic contrast-enhanced magnetic resonance imaging as a prognostic factor in predicting event-free and overall survival in pediatric patients with osteosarcomaCancer2012118153776378522180392
- HanGWangYBiWZMagnetic resonance imaging is appropriate for determining the osteotomy plane for appendicular osteosarcoma after neoadjuvant chemotherapyMed Oncol20122921347135321350876
- SunXHYangLBGengXLWangRZhangZCIncreased expression of lncRNA HULC indicates a poor prognosis and promotes cell metastasis in osteosarcomaInt J Clin Exp Pathol2015832994300026045809
- IqbalJNaushadHBiCGenomic signatures in B-cell lymphoma: how can these improve precision in diagnosis and inform prognosis?Blood Rev2016302738826432520
- OmuroADeAngelisLMGlioblastoma and other malignant gliomas: a clinical reviewJAMA2013310171842185024193082
- ZhuYZhangXQiLHULC long noncoding RNA silencing suppresses angiogenesis by regulating ESM-1 via the PI3K/Akt/mTOR signaling pathway in human gliomasOncotarget2016712144291444026894862
