Abstract
Purpose
This study aims to investigate the prognostic value of pretreatment C-reactive protein/albumin ratio (CAR) in human malignancies by an updated meta-analysis.
Methods
PubMed, Web of Science, Cochrane Library and Wanfang databases were searched. Pooled hazard ratios (HRs) and odds ratios (ORs) with their corresponding 95% confidence intervals (CIs) were used as effective values.
Results
A total of 25 studies with 12,097 patients were included in this meta-analysis. Pooled results showed that high pretreatment CAR was associated with poor overall survival (OS) (HR =1.99, 95% CI: 1.65–2.40, P=0.000) and poor disease-free survival (HR =1.55, 95% CI: 1.34–1.79, P=0.000). In addition, high pretreatment CAR was associated with increased 5-year mortality (OR =2.74, 95% CI: 2.11–3.55, P=0.000). Moreover, subgroup analysis demonstrated that high CAR was associated with poor OS despite variations in publication year, country, sample size, CAR cut-off value and treatment. However, high CAR was associated with poor OS in human malignancies except colorectal cancer (HR =1.64, 95% CI: 0.96–2.80, P=0.069).
Conclusion
High pretreatment CAR indicates poor prognosis in human malignancies except colorectal cancer. Thus, pretreatment CAR serves as a prognostic marker in human malignancies and could be used in the evaluation of prognosis in clinical work.
Introduction
Human malignancy remains a public health problem worldwide, and is reported to be the second leading cause of death in the US.Citation1 Based on GLOBOCAN estimates, ~14.1 million new cancer cases and 8.2 million deaths occurred in 2012 worldwide, and the burden is projected to grow worldwide due to the growth and aging of the population.Citation2 With the advance in early detection and treatment modality, the number of cancer survivors has increased steadily.Citation3 However, many challenges remain unsolved, such as identifying economical and practical markers for prognosis.
It is reported that the response of the body to cancer is not a unique mechanism but has many parallels with inflammation and wound healing, during which inflammatory cells and cytokines in tumors are more likely to contribute to tumor growth, progression and immunosuppression.Citation4 In clinical work, the commonly used inflammation markers include C-reactive protein (CRP), neutrophil, lymphocyte, platelet, D-dimer, fibrinogen, procalcitonin, and so on. Coincidentally, many inflammation-based scores, including Glasgow Prognostic Score, modified Glasgow Prognostic Score, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and lymphocyte-to-monocyte ratio, have been reported to be associated with the prognosis of human malignancies.Citation5–Citation7 Recently, numerous publications have tried to explore the correlation of pretreatment C-reactive protein/albumin ratio (CAR) with the prognosis of human malignancies, including one published meta-analysis based on only 10 available studies.Citation8
However, the prognostic role of pretreatment CAR in human malignancies remains inconclusive. Therefore, to clarify this issue, we performed this updated meta-analysis based on more available studies.
Methods
Search strategy
A comprehensive search of PubMed, Web of Science, Cochrane Library and Wanfang databases was performed up to March 13th 2017. The following keywords were used: (“C-reactive protein/Albumin ratio” or “C-reactive protein Albumin ratio” or “CRP/Alb ratio”) and (“Prognosis” or “Prognostic” or “recurrence” or “overall survival”). The detailed search strategy used in PubMed was as follows: “((((prognostic[Title/Abstract]) OR prognosis[Title/Abstract]) OR overall survival[Title/Abstract]) OR recurrence[Title/Abstract]) AND ((C-Reactive Protein[Title/Abstract]) AND albumin[Title/Abstract])”. During this process, we also screened the references list of retrieved articles manually, in order to gain more potential eligible studies.
Selection and exclusion criteria
Studies were included if they met all the following criteria: (1) investigating the prognostic role of CAR in human primary malignancies; (2) CAR was obtained before any treatment; (3) survival outcomes including overall survival (OS) or/and disease-free survival (DFS) were included; and (4) relative hazard ratios (HRs) with their corresponding 95% confidence intervals (CIs) were available or could be calculated according to the provided survival data or Kaplan–Meier curve.
Studies were excluded if they met any of the following criteria: (1) studies were reviews, letters, case reports and duplicates; (2) studies did not include any survival outcomes; (3) HRs with corresponding 95% CIs were not available or could not be calculated as mentioned. If multiple publications were based on the same origin of population, only the most informative or most recent one was enrolled.
Data extraction
Two investigators performed data extraction independently, during which discrepancies were solved by a consensus in this research team. The survival outcomes including OS, DFS and 5-year mortality were our main concerns. In addition, we extracted other relative basic information, including first author, publication year, country, cancer stage, cancer type, cut-off value for CAR, major treatment, data source and follow-up time. If results for both multivariate and univariate analyses were available, those for the former ones were extracted and used in the meta-analysis. When HRs with their 95% CIs were unavailable, the total number of events (including observed deaths, cancer-specific death and cancer recurrences) and the sample numbers of each group were extracted to calculate HRs.Citation9 In case neither crude HRs nor outcome data were available, Engauge Digitizer version 4.1 (http://sourceforge.net) was used to read the Kaplan–Meier curves, and then relative HRs with their 95% CIs were calculated using the methods described by Tierney et al.Citation9
Statistical analysis
In this meta-analysis, the pooled HRs and odds ratios (ORs) of included studies were calculated using STATA 10.0. Pooled HRs with corresponding 95% CIs were used to assess the effect of CAR on OS and DFS, and pooled ORs with corresponding 95% CIs were used to assess the effect of CAR on 5-year mortality. A combined HR or OR >1 indicated poor prognosis for patients with a high pretreatment CAR; otherwise, high pretreatment CAR served as a marker of good prognosis. Cochrane Q test and I2 statistic were used to test the potential heterogeneity across studies.Citation10 If heterogeneity was significant (I2.50% or/and P<0.1), we used a random-effect model; otherwise, a fixed-effect model was used. Both Begg’s test and Egger’s test were used to evaluate the potential publication bias.Citation11,Citation12 All P-values were two-sided, and statistical significance was defined as P<0.05.
Results
Literature search information
Initially, 1,021 studies were identified through systematic research in available databases. Next, 992 studies were excluded after reading title and abstract: including reviews, duplicates, letters and articles without survival outcomes. Finally, 25 studies (24 in English and 1 in Chinese)Citation13–Citation37 were included in this meta-analysis after excluding 4 studies (3 studies with survival outcomes unavailable, 1 study based on metastasizing tumor rather than primary cancer) ().
Characteristics of included studies
The characteristics of included studies are presented in . A total of 25 studies with 12,097 patients were included in this meta-analysis. The publication time ranged from 2014 to 2017, and all the studies were conducted in Asia (15 in China, 8 in Japan and 2 in Korea). The study sample sizes ranged from 40 to 2,685, with the median size of 386. The cut-off values for high pretreatment CAR ranged from 0.028 to 0.54, with the median cut-off value of 0.095. As shown in the table, this meta-analysis was based on multiple cancer sites (oral cavity in 1, larynx in 1, nasopharynx in 4, esophagus in 2, lung in 3, liver in 2, stomach in 2, pancreas in 4, colorectum in 4 and kidney in 2). The treatment methods were diverse, including surgical resection, chemotherapy, radiotherapy and multidisciplinary treatments. Among the 25 studies, all provided OS results, 8 provided DFS results and 7 provided 5-year mortality results.
Table 1 Characteristics of studies included in this meta-analysis
Meta-analysis results for OS
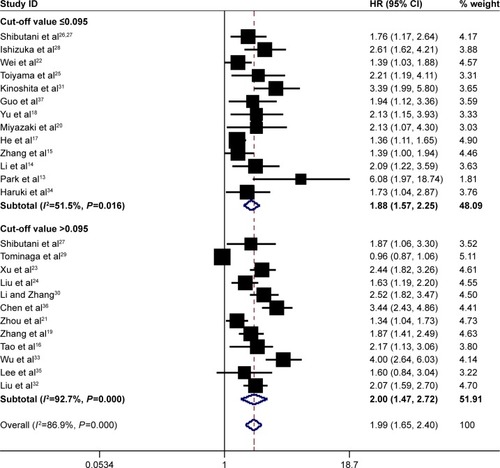
In total, 25 studies involving 12,097 patients investigated the prognostic role of pretreatment CAR in OS. Since heterogeneity was significant across studies (I2=86.9%, P=0.000), a random-effect model was used. Pooled results demonstrated that high pretreatment CAR was associated with poor OS (HR =1.99, 95% CI: 1.65–2.40, P=0.000), suggesting that patients with a high pretreatment CAR suffered from decreased OS rate ().
Figure 2 Forest plots for the prognostic impact of CAR on overall survival in human malignancies. Studies were grouped by cut-off value of CAR, and the median value was 0.095.
Abbreviations: CAR, C-reactive protein/albumin ratio; HR, hazard ratio; CI, confidence interval.
Meta-analysis results for DFS
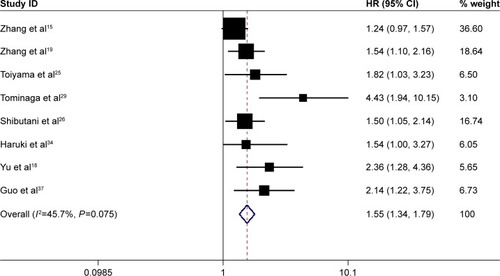
A total of 8 studies involving 4,226 patients reported the prognostic role of pretreatment CAR in DFS. The heterogeneity was not significant (I2=45.7%, P=0.075); therefore, a fixed-effect model was used. Combined results showed that high pretreatment CAR was associated with poor DFS (HR =1.55, 95% CI: 1.34–1.79, P=0.000), suggesting that patients with a high pretreatment CAR suffered from high tumor recurrence rate ().
Meta-analysis results for 5-year mortality rate
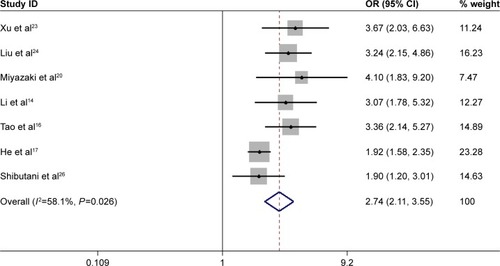
Totally, 5 studies with 5,551 patients assessed the prognostic effect of high pretreatment CAR on 5-year mortality. The pooled results showed that high pretreatment CAR was associated with high 5-year mortality (OR =2.74, 95% CI: 2.11–3.55, P=0.000, random-effect model), which also meant that patients with a high pretreatment CAR suffered from decreased survival rate ().
Subgroup analysis
Subgroup analysis showed that high pretreatment CAR was associated with poor OS despite variations in publication year, country, sample size, cut-off value for CAR and treatment (). However, when subgroup analysis was conducted according to cancer sites, the results showed that high pretreatment CAR was associated with poor OS in nasopharyngeal cancer (HR =1.56, 95% CI: 1.25–1.94, P=0.000), esophageal cancer (HR =1.84, 95% CI: 1.06–3.20, P=0.030), lung cancer (HR =1.63, 95% CI: 1.24–2.15, P=0.046), gastric cancer (HR =1.73, 95% CI: 1.31–2.28, P=0.000), hepatic carcinoma (HR =2.73, 95% CI: 2.07–3.60, P=0.000), pancreatic cancer (HR =2.25, 95% CI: 1.52–3.34, P=0.000), kidney cancer (HR =2.69, 95% CI: 1.54–4.69, P=0.000) and other cancers (including oral cancer and laryngeal cancer) (HR =3.22, 95% CI: 1.18–8.80, P=0.022), but not colorectal cancer (HR =1.64, 95% CI: 0.96–2.80, P=0.069) (). In addition, the HR for OS increased when the cut-off value of CAR increased, which could also be seen in . Therefore, high pretreatment CAR is significantly associated with poor OS despite potential confounding factors.
Figure 5 Forest plots for subgroup analysis based on the meta-analysis with overall survival according to different cancer types.
Abbreviations: HR, hazard ratio; CI, confidence interval.
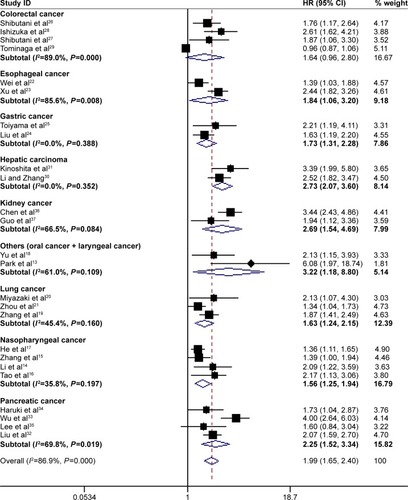
Table 2 Subgroup analysis for the prognostic impact of C-reactive protein/albumin ratio on overall survival in human malignancies
Publication bias
In this meta-analysis, both Begg’s test and Egger’s test were used to check the potential publication bias. No publication bias was found in the meta-analysis with OS (P=0.315) or 5-year mortality (P=0.764) when tested by Begg’s test. However, publication bias was found in the meta-analysis with OS (P=0.000) and 5-year mortality (P=0.024) when tested by Egger’s test, and DFS when tested by Begg’s test (P=0.035) as well as Egger’s test (P=0.006).
Sensitivity analysis
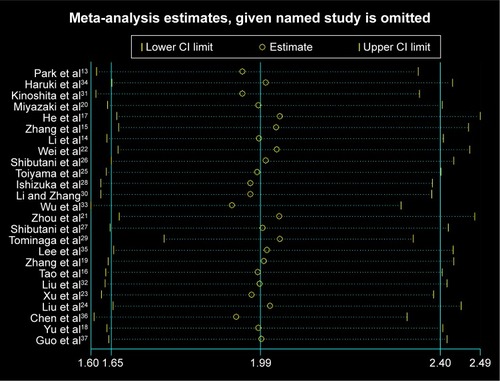
Since heterogeneity was found in the meta-analysis with OS, sensitivity analysis was performed for studies included in this meta-analysis. However, shows that the results of most of the included studies are close to the central line without obvious deviation.
Discussion
A meta-analysis is a quantitative method of analyzing and integrating available studies on the same topic, which has evolved as an alternative to the conventional narrative review.Citation38 A meta-analysis may help clinicians and researchers better understand the findings of clinical studies, draw conclusions about therapeutic effectiveness or plan new studies.Citation39 When compared with any single study, meta-analysis results are more convincing, but sometimes, they can also be limited by sampling bias, inadequate data and interpretation of bias outcomes.Citation40
Inflammation is a central aspect of the innate immune system response to tissue damage or infection. The relationship between inflammation and cancer has been recognized since 17th century.Citation4 Chronic inflammation can induce certain cancers and solid tumors (eg, hepatitis infection-related hepatocellular carcinoma, Helicobacter pylori infection-related gastric cancer and pancreatitis-related pancreatic carcinoma). Besides, inflammation is a critical component of tumor progression since tumor microenvironment could contribute to tumor invasion, migration and metastasis.Citation41 Therefore, the relationship between cancer and inflammation has become the forefront of clinical oncology.Citation42
CRP is a major component of any inflammatory reaction, which is generated from liver and belongs to pentraxin family.Citation43 A systematic review with 271 articles demonstrated that high CRP was associated with higher mortality in 90% of reports on people with primary solid tumors, especially gastrointestinal malignancies and kidney malignancies. In addition, high CRP was correlated with poor treatment response and increased tumor recurrence.Citation44
Human albumin (HA) is the most abundant plasma protein accounting for ~50% of the total protein content. HA is a small globular protein with a molecular weight of 66.5 kDa, consisting of a single chain of 585 amino acids.Citation45 As a major plasma protein, HA has been used for prognostic assessment in patients with different conditions, including hemodialysis patients, multiple myeloma patients and patients undergoing replacement albumin therapy.Citation46 In addition, pretreatment serum protein is used as a prognostic marker in many human malignancies, including urinary carcinoma,Citation47,Citation48 head and neck cancer,Citation49 lung cancer,Citation50 gynecological cancerCitation51,Citation52 and gastrointestinal cancers.Citation53,Citation54
As a combined product, CAR, especially its prognostic role in human malignancies, was investigated by many studies recently. On January 27th 2017, Li et al published a meta-analysis, including only 10 studies with 4,592 cancer patients, investigating the prognostic role of CAR in human cancer.Citation8 In their meta-analysis, the impact of pretreatment albumin–globulin ratio (AGR) on OS in human cancers was analyzed, but without DFS or 5-year mortality. As we all know, DFS and 5-year mortality rate are also considered as important indicators in the research on cancer prognosis. Moreover, the number of included studies and cancer patients was limited in this previously published paper. Therefore, we conducted this updated meta-analysis to investigate the prognostic value of pretreatment CAR in cancer patients. In this meta-analysis, we included 25 studies with 12,097 cases diagnosed with malignancy. The pooled results showed that high pretreatment CAR was associated with poor OS, poor DFS and high 5-year mortality, suggesting that pretreatment CAR might serve as a marker of poor survival rate and high tumor recurrence rate in human malignancies. Moreover, our subgroup meta-analysis showed that high pretreatment CAR was significantly associated with poor OS in patients with nasopharyngeal cancer, esophageal cancer, gastric cancer, liver cancer, pancreatic cancer, lung cancer, oral cancer and renal cancer, but not colorectal cancer. When compared with the previously published meta-analysis, our meta-analysis results are more reliable and convincing in addition to being based on more available studies.
However, this meta-analysis also has some limitations. Firstly, we did not perform methodological quality analysis for included studies, as no widely agreed quality criteria have been identified yet for assessing studies investigating prognosis.Citation55 Secondly, heterogeneity was found in the meta-analysis with OS and 5-year mortality. We performed subgroup meta-analysis based on potential confounding factors including publication time, country, sample size, cut-off value for CAR and treatment, but heterogeneity could not be solved. However, when conducting subgroup meta-analysis based on cancer sites, no heterogeneity was found in the meta-analysis with nasopharyngeal cancer, lung cancer, gastric cancer or liver cancer. The other reason is that the cut-off values for CAR were highly diverse, which may also account for the heterogeneity to some extent. Last but not least, publication bias was found in the meta-analysis, which might have been caused by the following reasons. Only articles in Chinese or English were included in this meta-analysis, though we did not set any language limitations during the searching process. In addition, some databases (eg, Embase database) were not available for our research group, and survival results were not provided or could not be calculated in some retracted articles.
At the same time, we also found other combined markers derived from albumin, such as albumin–bilirubin grade used for predicting prognosis in patients with hepatocellular carcinoma (HCC)Citation56,Citation57 and AGR used as a prognostic marker in patients with HCC,Citation58 urinary carcinoma,Citation59,Citation60 lung cancerCitation61 and endometrial cancer.Citation51 Besides, we also found one study investigating the impact of CAR on long-term outcomes following hepatic resection for colorectal liver metastases.Citation62 Thus, pretreatment CAR is a useful prognostic marker in cancer patients
In summary, high pretreatment CAR was associated with poor OS, poor DFS and high 5-year mortality in human malignancies. Pretreatment CAR might serve as a marker of poor survival rate and high tumor recurrence rate in human malignancies except colorectal cancers. Therefore, CAR could be used in the evaluation of prognosis of human malignancies in clinical work. More prospective studies with large sample size are needed to explore the prognostic role of pretreatment CAR in patients with colorectal cancer.
Acknowledgments
The authors are grateful to Lin An (Department of Dermatology, China-Japan Union Hospital of Jilin University, Changchun, Jilin, China) for her help in this research.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2017CA Cancer J Clin201767173028055103
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- MillerKDSiegelRLLinCCCancer treatment and survivorship statistics, 2016CA Cancer J Clin201666427128927253694
- BalkwillFMantovaniAInflammation and cancer: back to Virchow?Lancet2001357925553954511229684
- McMillanDCThe systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancerCancer Treat Rev201339553454022995477
- YodyingHMatsudaAMiyashitaMPrognostic significance of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: a systematic review and meta-analysisAnn Surg Oncol201623264665426416715
- NishijimaTFMussHBShacharSSTamuraKTakamatsuYPrognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: a systematic review and meta-analysisCancer Treat Rev2015411097197826481060
- LiNTianGWWangYZhangHWangZHLiGPrognostic role of the pretreatment C-reactive protein/albumin ratio in solid cancers: a meta-analysisSci Rep201774129828128229
- TierneyJFStewartLAGhersiDBurdettSSydesMRPractical methods for incorporating summary time-to-event data into meta-analysisTrials200781617555582
- HigginsJPThompsonSGQuantifying heterogeneity in a meta-analysisStat Med200221111539155812111919
- BeggCBMazumdarMOperating characteristics of a rank correlation test for publication biasBiometrics1994504108811017786990
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ199731571096296349310563
- ParkHCKimMYKimCHC-reactive protein/albumin ratio as prognostic score in oral squamous cell carcinomaJ Korean Assoc Oral Maxillofac Surg201642524325027847731
- LiJPChenSLLiuXMA novel inflammation-based stage (I stage) predicts overall survival of patients with nasopharyngeal carcinomaInt J Mol Sci20161711
- ZhangYZhouGQLiuXExploration and validation of C-reactive protein/albumin ratio as a novel inflammation-based prognostic marker in nasopharyngeal carcinomaJ Cancer20167111406141227471556
- TaoCJChenYYJiangFThe C-reactive protein/albumin ratio is an independent prognostic factor for overall survival in patients with nasopharyngeal carcinoma receiving intensity-modulated radiotherapyJ Cancer20167142005201127877215
- HeSWangYChenHC-reactive protein/albumin ratio (CAR) as a prognostic factor in patients with non-metastatic nasopharyngeal carcinomaJ Cancer20167152360236627994675
- YuSTZhouZCaiQPrognostic value of the C-reactive protein/albumin ratio in patients with laryngeal squamous cell carcinomaOnco Targets Ther20171087988428243123
- ZhangFYingLJinJThe C-reactive protein/albumin ratio predicts long-term outcomes of patients with operable non-small cell lung cancerOncotarget2017858835884227823974
- MiyazakiTYamasakiNTsuchiyaTRatio of C-reactive protein to albumin is a prognostic factor for operable non-small-cell lung cancer in elderly patientsSurg Today Epub20161116
- ZhouTZhanJHongSRatio of C-reactive protein/albumin is an inflammatory prognostic score for predicting overall survival of patients with small-cell lung cancerSci Rep201551048126084991
- WeiXLWangFHZhangDSA novel inflammation-based prognostic score in esophageal squamous cell carcinoma: the C-reactive protein/albumin ratioBMC Cancer20151535025934640
- XuXLYuHQHuWA novel inflammation-based prognostic score, the C-reactive protein/albumin ratio predicts the prognosis of patients with operable esophageal squamous cell carcinomaPLoS One2015109e013865726390126
- LiuXSunXLiuJPreoperative C-reactive protein/albumin ratio predicts prognosis of patients after curative resection for gastric cancerTransl Oncol20158433934526310380
- ToiyamaYShimuraTYasudaHClinical burden of C-reactive protein/albumin ratio before curative surgery for patients with gastric cancerAnticancer Res201636126491649827919972
- ShibutaniMMaedaKNagaharaHIsekiYHirakawaKOhiraMThe significance of the C-reactive protein to albumin ratio as a marker for predicting survival and monitoring chemotherapeutic effectiveness in patients with unresectable metastatic colorectal cancerSpringerplus201651179827812440
- ShibutaniMMaedaKNagaharaHIsekiYIkeyaTHirakawaKPrognostic significance of the preoperative ratio of C-reactive protein to albumin in patients with colorectal cancerAnticancer Res2016363995100126976989
- IshizukaMNagataHTakagiKIwasakiYShibuyaNKubotaKClinical significance of the C-reactive protein to albumin ratio for survival after surgery for colorectal cancerAnn Surg Oncol201623390090726530445
- TominagaTNonakaTSumidaYHidakaSSawaiTNagayasuTThe C-reactive protein to albumin ratio as a predictor of severe side effects of adjuvant chemotherapy in stage III colorectal cancer patientsPLoS One20161112e016796727930703
- LiMZhangPPredictive value of C-reactive protein/albumin ratio on prognosis of patients with primary hepatocellular carcinomaCancer Res Prev Treat2016437598601 Chinese
- KinoshitaAOnodaHImaiNThe C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinomaAnn Surg Oncol201522380381025190127
- LiuZJinKGuoMPrognostic value of the CRP/Alb ratio, a novel inflammation-based score in pancreatic cancerAnn Surg Oncol201724256156827650825
- WuMGuoJGuoLZuoQThe C-reactive protein/albumin ratio predicts overall survival of patients with advanced pancreatic cancerTumour Biol2016379125251253327344157
- HarukiKShibaHShiraiYThe C-reactive protein to albumin ratio predicts long-term outcomes in patients with pancreatic cancer after pancreatic resectionWorld J Surg20164092254226026956901
- LeeJMLeeHSHyunJJPrognostic value of inflammation-based markers in patients with pancreatic cancer administered gemcitabine and erlotinibWorld J Gastrointest Oncol20168755556227559435
- ChenZShaoYFanMPrognostic significance of preoperative C-reactive protein: albumin ratio in patients with clear cell renal cell carcinomaInt J Clin Exp Pathol2015811148931490026823819
- GuoSHeXChenQThe C-reactive protein/albumin ratio, a validated prognostic score, predicts outcome of surgical renal cell carcinoma patientsBMC Cancer201717117128264659
- KaterndahlDACohenPAQuantitatively reviewing the literature: the application of meta-analysisFam Pract Res J1987631231293331875
- L’AbbéKADetskyASO’RourkeKMeta-analysis in clinical researchAnn Intern Med198710722242333300460
- ThackerSBMeta-analysis. A quantitative approach to research integrationJAMA198825911168516893278147
- CoussensLMWerbZInflammation and cancerNature2002420691786086712490959
- MunnLLCancer and inflammationWiley Interdiscip Rev Syst Biol Med201792 Epub20161212
- AnsarWGhoshSC-reactive protein and the biology of diseaseImmunol Res201356113114223371836
- ShrotriyaSWalshDBennani-BaitiNThomasSLortonCC-reactive protein is an important biomarker for prognosis tumor recurrence and treatment response in adult solid tumors: a systematic reviewPLoS One20151012e014308026717416
- CaraceniPTufoniMBonavitaMEClinical use of albuminBlood Transfus201311Suppl 4s18s2524333308
- InfusinoIPanteghiniMSerum albumin: accuracy and clinical useClin Chim Acta2013419151823348571
- ChenZShaoYWangKPrognostic role of pretreatment serum albumin in renal cell carcinoma: a systematic review and meta-analysisOnco Targets Ther201696701671027822073
- KuJHKimMChoiWSKwakCKimHHPreoperative serum albumin as a prognostic factor in patients with upper urinary tract urothelial carcinomaInt Braz J Urol201440675376225615244
- DananDShonkaDCJrSelmanYChowZSmolkinMEJamesonMJPrognostic value of albumin in patients with head and neck cancerLaryngoscope201612671567157126864349
- EspinosaEFeliuJZamoraPSerum albumin and other prognostic factors related to response and survival in patients with advanced non-small cell lung cancerLung Cancer1995121–267767600032
- SeebacherVGrimmCReinthallerAThe value of serum albumin as a novel independent marker for prognosis in patients with endometrial cancerEur J Obstet Gynecol Reprod Biol2013171110110624011381
- AsherVLeeJBaliAPreoperative serum albumin is an independent prognostic predictor of survival in ovarian cancerMed Oncol20122932005200921735143
- LienYCHsiehCCWuYCPreoperative serum albumin level is a prognostic indicator for adenocarcinoma of the gastric cardiaJ Gastrointest Surg2004881041104815585392
- HeysSDWalkerLGDeehanDJEreminOESerum albumin: a prognostic indicator in patients with colorectal cancerJ R Coll Surg Edinb19984331631689654876
- AltmanDGSystematic reviews of evaluations of prognostic variablesBMJ2001323730622422811473921
- MaXLZhouJYGaoXHApplication of the albumin-bilirubin grade for predicting prognosis after curative resection of patients with early-stage hepatocellular carcinomaClin Chim Acta2016462152227520748
- LiMXZhaoHBiXYPrognostic value of the albumin-bilirubin grade in patients with hepatocellular carcinoma: validation in a Chinese cohortHepatol Res Epub2016825
- ZhangJLiuXYangZChenYLuoRThe pretreatment albumin to globulin ratio, a validated biomarker, predicts prognosis in hepatocellular carcinomaJ BUON201621492593427685915
- ZhangBYuWZhouLQPrognostic significance of preoperative albumin-globulin ratio in patients with upper tract urothelial carcinomaPLoS One20151012e014496126681341
- LiuJDaiYZhouFThe prognostic role of preoperative serum albumin/globulin ratio in patients with bladder urothelial carcinoma undergoing radical cystectomyUrol Oncol20163411484.e1484.e8
- ZhouTHeXFangWPretreatment albumin/globulin ratio predicts the prognosis for small-cell lung cancerMedicine (Baltimore)20169512e309727015181
- HarukiKShibaHHoriuchiTImpact of the C-reactive protein to albumin ratio on long-term outcomes after hepatic resection for colorectal liver metastasesAm J Surg Epub201726