Abstract
Endometriosis is an inflammatory pathology associated with a negative effect on life quality. Recently, this pathology was connected to ovarian cancer, in particular with endometrioid ovarian cancer. microRNAs (miRNAs) are a class of RNA transcripts ~19–22 nucleotides in length, the altered miRNA pattern being connected to pathological status. miRNAs are highly stable transcripts, and these can be assessed from formalin-fixed paraffin-embedded (FFPE) samples leading to the identification of miRNAs that could be developed as diagnostic and prognostic biomarkers, in particular those involved in malignant transformation. The aim of our study was to evaluate miRNA expression pattern in FFPE samples from endometriosis and ovarian cancer patients using PCR-array technology and also to compare the differential expression pattern in ovarian cancer versus endometriosis. For the PCR-array study, we have used nine macrodissected FFPE samples from endometriosis tissue, eight samples of ovarian cancers and five normal ovarian tissues. Quantitative real-time PCR (qRT-PCR) was used for data validation in a new patient cohort of 17 normal samples, 33 endometriosis samples and 28 ovarian cancer macrodissected FFPE samples. Considering 1.5-fold expression difference as a cut-off level and a P-value <0.05, we have identified four miRNAs being overexpressed in endometrial tissue, while in ovarian cancer 15 were differentially expressed (nine overexpressed and six downregulated). The expression level was confirmed by qRT-PCR for miR-93, miR-141, miR-155, miR-429, miR-200c, miR-205 and miR-492. Using the interpretative program Ingenuity Pathway Analysis revealed several deregulated pathways due to abnormal miRNA expression in endometriosis and ovarian cancer, which in turn is responsible for pathogenesis; this differential expression of miRNAs can be exploited as a therapeutic target. A higher number of altered miRNAs were detected in endometriosis versus ovarian cancer tissue, most of them being linked with epithelial-to-mesenchymal transition.
Introduction
Endometriosis is presented as a benign gynecological condition that affect ~5%–10% of premenopausal women and is characterized by the presence of ectopic endometrial implants in pelvic area; however, these implants can also be extended to the abdomen and even to the central nervous system. In some cases, these implants can undergo malignant transformation and can lead to a condition called endometriosis-associated ovarian cancer (EAOC).Citation1 This progression is now supported by epidemiological studies and some molecular data; the development of cancer from endometriosis is a slowly progressing process that is difficult to be revealed by clinical evidences.Citation1,Citation2 A recent study affirms that ~2.5% of women diagnosed with ovarian endometriosis undergo malignant conversion, but this percentage can be seriously underestimated due to unspecific molecular processes that intervene in the transit from benign to malignant status.Citation3 It is consequently anticipated to be a multifaceted process, based on the characteristic features of the disease, where coding and noncoding transcripts play an important role.Citation4
The molecular characteristics of this carcinogenic event are still poorly understood, although there are a number of genes and molecular signaling pathways that are currently associated with the possible transition from endometriosis to ovarian cancer being regulated by an altered pattern of key for tumor suppressor genes or oncogenes,Citation5,Citation6 microsatellite instability and noncoding genes, especially miRNA alteration pattern.
Ovarian cancer is the cause of greatest number of cancer-related deaths worldwide according to the latest statistical data, with high mortality rate due to the asymptomatic profile in early stages and nonsensitive diagnostic methods.Citation7–Citation9 Ovarian cancer is frequently diagnosed in final stages when massive ascites and peritoneal spreading has already occurred, a stage that usually is nonresponsive to classic but to aggressive treatment methods,Citation10 and the survival rate remains low at 30%.Citation8,Citation11 In this regard, there is an urgent need not only for more sensitive and precise diagnostic methods but also for methods that are able to discriminate this type of malignant pathology in the early stages of development, or more preferably, before the installation of this asymptomatic disease, in the form of prevention biomarkers.Citation9,Citation12,Citation13 The recently discovered miRNAs may lead to early diagnostic and therapeutic approaches, due to their gene expression regulation ability of the genes that play crucial roles in the installation and development of ovarian cancer, being actively involved in the etiology of this pathology.Citation14
Ovarian epithelial tumors are classified as: type I tumors defined by a slow growth (comprising low-grade serous carcinoma, mucinous carcinoma, endometrioid carcinoma and clear-cell carcinomas) and type II tumors defined as a more aggressive form (including high-grade serous carcinoma, mixed mesodermal tumors and undifferentiated tumors).Citation15 The endometriomas or ovarian endometriosis cysts are retrieved in ~17%–44% of patients diagnosed with endometriosis, which are considered as the common precursor lesions to ovarian cancer. EAOC is especially noticeable and retrieved as endometrioid and clear cell ovarian tumor.Citation1
Another important concern in ovarian cancer biology consists in a low amount of information about the primary site of origin, due to the absence of glandular epithelia in the ovary which acts like a direct precedent for cancer.Citation5 The most suitable candidate for the primary source of ovarian cancer nowadays is considered to be the ovarian surface epithelium, tissue formed of a monolayer of cells that covers the exterior of the ovaries.Citation5,Citation10 However, this covering tissue is not the only origin for this malignant disease. Increased evidences show that endometriosis can be related to benign-malignant transition to ovarian cancer, particularly in the case of endometrioid and clear cell adenocarcinoma of the ovary (also named in the literature as EAOC); however, these conditions present discrete features which are difficult to be identifed.Citation16,Citation17 The malignant transformation of endometriosis into ovarian cancer, particularly in endometrioid, clear cell and low-grade serous histotypes, is related to genetic and nongenetic factors or a combination of these factors.Citation15
An increased number of studies incriminate miRNA in pathological status. miRNAs are presented today as key regulators involved in numerous processes in mammals and other multicellular organisms;Citation18–Citation20 they control biological pathways involved in proliferation, apoptosis, migration, cell cycle control, differentiation and processes that under aberrant functioning can lead to pathological status, including malignant transformation; hence, there is an increasing interest to use these short transcripts as biomarkers for prevention,Citation21 detection and prognostic application and also as therapeutic targets.Citation20,Citation22
Formalin-fixed paraffin-embedded (FFPE) tissue is a key source of biological material for retrospective analysis, evaluation of molecular profiling and developing novel biomarkers for personalized health care.Citation23,Citation24 Taking into bconsideration the crucial role of miRNA transcripts in the regulation of numerous signaling pathways,Citation19,Citation20,Citation25,Citation26 there is a possibility to associate the expression of miRNAs in endometriosis and ovarian cancer samples with the risk for malignant transformation.Citation21,Citation27,Citation28 Identification of a specific panel of miRNAs that are differentially expressed in these diseases, in particular among these two pathologies, can enable the discovery of precise biomarkers for diagnostic and prognosticCitation21,Citation25,Citation28 purpose and in particular for assessing the risk involved in the transition from benign to malignant status.
Materials and methods
Sampling procedures
FFPE samples were collected from patients diagnosed at the Institute of Oncology “Prof Dr Ion Chiricuta” in Cluj-Napoca, Romania, with endometriosis and ovarian cancer between the years 2007 and 2014. Approval for the study was obtained from the ethical committee of the same institution (no 72/26). All the procedures for sample selection and processing were done in agreement with the Romanian and international legislation. All the clinical data obtained from the Pathology Department were used anonymously, being in agreement with ethical and legal requirements. This study is in agreement with the institutional policy that imposes a signed informed consent, mentioning that the data can be used for additional future research focused on molecular profiling.
The patient cohort was selected based on clinical data prior to PCR-array or quantitative real-time PCR (qRT-PCR) evaluation. The control patient cohort was selected from a group of patients who had undergone benign surgical resection for uterine pathologies. PCR-array patient cohorts included two cases of endometrial polyps, one uterine fibroid and one leiomyoma. qRT-PCR control group included surgically resected tissue from three cases of ovarian or uterine fibroid, five cases of leiomyoma, two cases of salpingitis and four cases of benign endometrial polyps. The endometriosis patient cohort included cases with left/right adnexal endometriosis and bilateral endometriosis but showing no signs of malignity. The endometroid tissue represents over 90%.
Then the histopathological diagnosis was confirmed. The selection between endometriosis lesions and tumor area was done based on hematoxylin and eosin staining and macrodissected regions from serial unstained FFPE sections. All the cases were pathologically reviewed and the regions were isolated separately based on the tissue type. For ovarian cancer, only the endometriod carcinoma cases () with hyperplasia more than 80% were selected, using four slides with macrodissected tissue.
For extraction, we used an average of eight 10 µm FFPE sections from the tissue block for miRNA profiling and for qRT-PCR data validation. None of the selected patients included in this study had undergone preoperative chemotherapy or radiation therapy.
miRNA isolation from FFPE samples
Total RNA was extracted from FFPE tissue using miRCURY RNA Isolation Kit–FFPE (Exicon cat no 300115) based on the instructions furnished by the manufacturer’s protocol. Then the quantity of total RNA was evaluated using NanodropND-2000.
PCR array analysis
The cDNA synthesis was done using 100 ng of total RNA extracted from FFPE tissue, based on the recommended protocol, using the miScript HiSpec Buffer in a volume of 20 µL and the following program: 37 °C for 60 minutes and then at 95°C for 5 minutes. The cDNA was then diluted and used for PCR array based on a SybrGreen protocol and Human Ovarian Cancer miScript miRNA PCR array plate (MIHS-110Z; Qiagen NV, Venlo, the Netherlands) following the protocol recommended by the manufacturer. These arrays contain probes for 84 miRNAs whose expression is known or expected to be altered in ovarian cancer, three wells for miRNA reverse transcription control assay and a positive PCR control. The qRT-PCR reaction was performed in Roche LightCycler480 instrument.
qRT-PCR measurements
For the validation of the most relevant altered miRNAs, we selected a subset of 17 normal samples, 33 endometriosis samples and 28 ovarian cancer samples. We used a two-step qRT-PCR. cDNA was generated using miScriptII RT Kit and then was diluted 1:5. The diluted cDNA sample was used as a template for performing qRT-PCR using QuantiTect (QuantiTect SYBR Green PCR kit, Qiagen) with specific miScript Primer (Qiagen). qRT-PCR assays were performed on ViiA 7 Real Time PCR System using the recommended amplification protocol (45 cycles of 30 seconds at 95°C, 30 seconds at 55°C and 30 seconds at 72°C) and a melting curve step.
Data analysis
The analysis of miRNA relative expression was done using the data analysis portal furnished by Qiagen, USA (http://sabiosciences.com/pcr/arrayanalysis.php). For the normalization of data, we used the average Ct value of the cel-miR-39, SNORD68, SNORD95, SNORD96A and RNU6-6P. The quantification for determining the relative expression level of miRNAs was done in endometriosis (nine samples) versus normal tissue (five samples) and ovarian cancer tissue (eight samples).
The altered miRNA pattern in endometriosis versus control, in ovarian cancer versus normal tissue and in ovarian cancer versus endometriosis was analyzed using Ingenuity Pathways Analysis (www.ingenity.com) for biological significance identification.
The qRT-PCR data analysis was done using ΔΔCt method, as previously described by Berindan-Neagoe et al,Citation29 statistical analysis was done using Graphpad Software (https://www.graphpad.com), the ROC (receiving-operating characteristic) curve was used for evaluating the AUC (area under the curve) offering information for the sensitivity of evaluated miRNA transcript.
Results
Evaluation of miRNA relative expression in endometriosis and ovarian cancer
This retrospective molecular profiling study was conducted on nine samples of endometriosis tissue, eight samples of ovarian cancer tissue and five samples of normal ovarian tissue using macrodissected specimens. The average age of endometriosis patients was 40.3±5.57 years, for ovarian cancer patients was 59.33±10.36 years and for control group patients was 60±4.48 years.
First step in this study was to perform a comparative analysis of miRNA profile based on ΔΔCt method in endometriosis versus normal tissue, in ovarian cancer tissue versus normal tissue and then in endometriosis versus ovarian cancer tissue. summarizes the differentially expressed miRNAs in endometriosis tissue versus normal tissue, ovarian cancer tissue versus normal tissue and ovarian cancer tissue versus endometriosis tissue. Of considerable interest were the miRNAs that had 1.5-fold expression difference as a cut-off level and a P-value of <0.05. Four miRNAs were identified to be overexpressed in endometriosis tissue, and in ovarian cancer tissue, 15 miRNAs were differentially expressed (nine overexpressed and six downregulated). A higher number of altered miRNAs (14 overexpressed and 23 downregulated) were observed when endometriosis versus ovarian cancer tissue was analyzed. presents Venn diagram for the main miRNAs expressed across the analyzed subgroups.
Figure 1 Venn diagram presenting the altered expression levels of common and differentially expressed miRNAs in the analyzed subgroups.
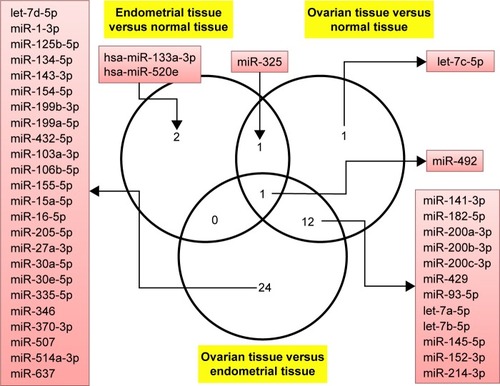
Table 1 Differentially expressed microRNAs considering as cut-off value a fold change ≤1.5 or ≥1.5 and P-value <0.05
The present study had identified a particular molecular signature based on the main oncogenic miRNAs overexpressed in endometriosis and ovarian pathology (Figure S1), and also we were able to identify an miRNA panel that was able to discriminate between ovarian cancer and endometriosis.
The differential expression of miRNA proved to be statistically significant, when analyzed using interpretative program Ingenuity Pathway Analysis (IPA), leading to summarization of their biological significance and to defining the molecular interaction between altered miRNAs and their related target gene (). The results are displayed as networks ( and ) and classified in terms of disease and cellular and molecular functions of the altered miRNAs ().
Figure 2 Network generated for the altered miRNAs in the analysis of endometriosis versus normal tissue. The upregulated miRNAs are displayed in red.
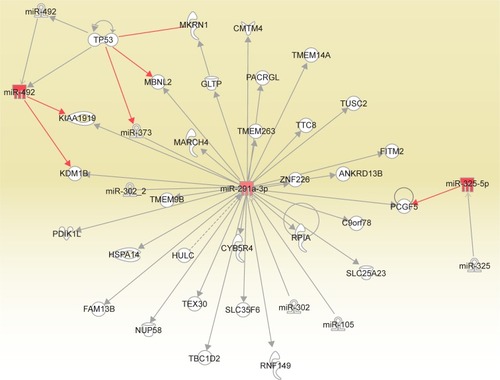
Figure 3 Network generated for the altered miRNAs in the analysis of ovarian cancer versus control. The upregulated miRNAs are displayed in red and the downregulated miRNAs in green.
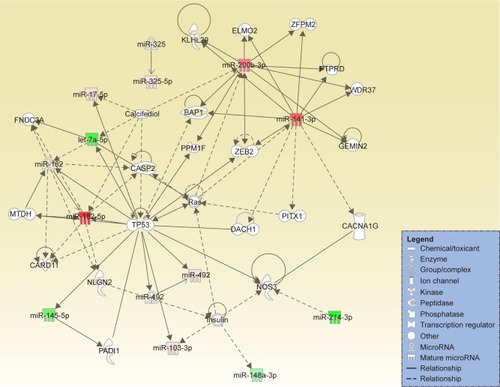
Table 2 IPA analysis based on altered miRNA pattern in the analyzed subgroups, focusing on diseases
Table 3 IPA analysis based on altered miRNA pattern in the analyzed subgroups, focusing on molecular and cellular functions
qRT-PCR data validation
qRT-PCR data validation comprises 17 control samples with an average age of 48.53±9.14 years, 33 endometriosis patients of 41.68±8.78 years, and 33 ovarian cancer patients of 55.75±10.98 years.
miR-93 was proved to be overexpressed in endometriosis and ovarian cancer patients, the expression level being much higher in ovarian cancer compared to endometriosis. Fold change (FC) for endometriosis was 2.42±1.63 and for ovarian cancer was 26.19±30.59, the results being statistically significant in agreement with the PCR-array data (). ROC curves for miR-92 reveal a value of AUC for endometriosis group of 0.69 and for ovarian cancer patients of 0.82.
Figure 4 qRT-PCR validation in endometriosis and ovarian cancer patient cohort for miR-93, miR-141, miR-155, miR-429, miR-200c, miR-205 and miR-492. ROC curve analysis of expression levels of miRNAs for endometriosis and ovarian cancer group. The figure displays AUC for each evaluated miRNA, a parameter that indicates the precision in discriminating the endometrial tissue and ovarian cancer tissue from the normal tissue. *P<0.05; ***P<0.001.
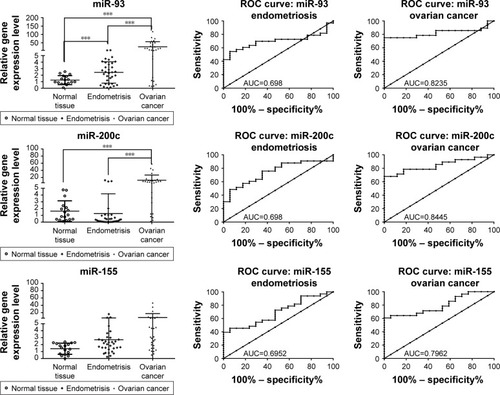
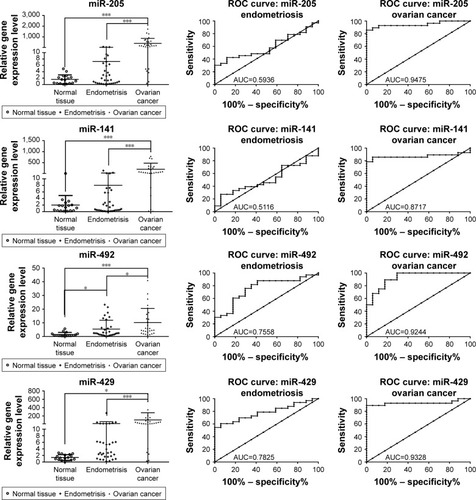
miR-200c was found to be overexpressed in ovarian cancer samples when they were analyzed versus control or versus endometriosis samples. The expression level for miR-200c in normal tissue was 1.63±1.53, in endometriosis 1.27±2.91 and in ovarian cancer 12.29±14.61. AUC values for miR-200c was 0.69 for endometriosis and 0.84 for ovarian cancer, displaying a good sensitivity and specificity for ovarian cancer. Similar expression level was observed for the other two representatives of the same family, namely miR-141 and miR-429, which have an expression level in endometriosis of 8.035±25.09 and 16.17±47.13 and in ovarian cancer of 203.1±275.6 and 104.6±172.6, respectively. A reduced sensitivity was observed for miR-141, whereas highest AUC values were obtained for miR-492 in both the analyses.
An increased level of miR-155 expression was observed in endometriosis and ovarian cancer tissues, but this difference was not statistically significant. For the endometriosis versus control group, FC was 1.416±0.7981 (P-value = 0.0507) and for ovarian cancer group, FC was 2.703±2.830 (P-value =0.0552). miR-205 expression level was 7.235±15.49 (AUC =0.69) in endometriosis and 390.2±466.3 (AUC=0.79) in ovarian cancer. miR-492 was overexpressed in both pathologies, with a fold change of 5.40±6.48 for endometriosis and 10.17±10.29 for ovarian cancer. The AUC values for miR-492 revealed a good sensitivity for both pathologies.
A good level of reproducibility was observed among the two patient cohorts used for PCR-array evaluation and qRT-PCR, with an exception for miR-155 which showed the same expression level but the P-value was not statistically significant, emphasizing the potential prognostic significance for the validated miRNA.
Discussion
In this study, we have demonstrated that miRNAs are aberrantly expressed in endometriosis versus normal tissue and ovarian cancer versus normal tissue, and we were also able to identify a panel of miRNAs that are differentially expressed in ovarian cancer versus endometriosis tissue, the details of which can be observed in and S1. Thus, miRNAs can be used as prognostic and diagnostic markers, emphasizing the functional differences that are able to regulate the key processes. The global miRNA expression pattern might undoubtedly discriminate endometriosis from normal tissue, ovarian cancer from normal tissue and also ovarian cancer from endometriosis tissue. The analysis has detected important number of miRNAs with an altered expression pattern being involved in malignant transformation. For qRT-PCR validated miRNAs, good sensitivity and specificity can be observed from ROC curves, in particular for ovarian cancer pathology. These panels of miRNAs can be taken into consideration for possible use as prognostic or diagnostic markers, especially for endometriosis patients undergoing surgical procedures.
One of the identified transcripts that was found to be overexpressed in endometriosis and ovarian cancer is miR-325, a transcript whose overexpression was confirmed even when comparing the degree of expression between the two pathologies using endometriosis samples as control group. The increased expression of miR-325 in ovarian cancer against endometriosis suggests a possible application for its use as a prognostic marker in regard to a possible transition from endometriosis to malignant pathologies. miR-325 has the potential of a tumor suppressor which is reflected by the promotion of tumor progression, increased angiogenesis, invasion and metastasis.Citation30 There is a lack of specific data regarding miR-325 expression in the context of ovarian cancer or endometriosis, but there are several studies reporting its role in other malignant pathologies.Citation31,Citation32 This way, the degree of expression of miR-325 can be utilized as a clinical parameter for detecting ovarian cancer invasion,Citation31 whose expression is inhibited in these tumors. miR-203, an inflammatory transcript,Citation33 was observed to be downregulated in the endometriosis group.
Regarding the expression of miR-200 family, our study has highlighted a significant overexpression value for miR-200a, miR-200b, miR-200c, miR-429, miR-141 and members of the ovarian tumor samples, these members topping the list for positive aberrant expression. Of the five members, miR-200a, miR-200b and miR-200c are currently proposed for use as biomarkers in ovarian cancer.Citation34–Citation38 The ROC data being in agreement with our studyCitation34 encourage their application in clinical practice. Furthermore, the expression level of miR-200a and miR-200c has been identified to be associated with the degree of progress for ovarian pathology, whereas the upregulation of miR-200a was found to be correlated with the histology and tumor stage; patients with metastasis in lymph nodes presented a significant elevation of mirR-200c.Citation38 Also, miR-200b has been characterized as a possible therapeutic target related with drug resistance.Citation35 With regard to the expression of miR-141, an increased resistance for cisplatin was found to be proportional to the grade of positive aberrancy of this transcript.Citation39 Members of the miR-200 family (miR-141 and miR-200a) have also been identified in the endometriosis samples but with an expression opposite to that found in ovarian cancer.Citation40 These two transcripts are currently proposed as potential noninvasive biomarkers, their expression from tissue samples being correlated with the values from blood samples.Citation41 Given the role of miR-200 family members in numerous biologic and pathologic processes, especially in epithelial–mesenchymal transition (EMT), we can assume that these transcripts may be involved in the transition from benign endometriosis to malignant pathologies such as ovarian cancer.
Another overexpressed transcript found with an altered expression level in endometriosis samples is miR-520e. This transcript has been studied in hepatocarcinomaCitation42 and breast cancer,Citation43 where its role as a tumor suppressor has been proven.Citation44 This feature places the transcript in a list of potential therapeutic elements. Given the similarity of endometriosis and malignant pathologies in terms of high levels of cell proliferation and also the presence of miR-520 in samples from endometriosis patients, there is a possibility of using this transcript as a therapeutic target for inhibiting cellular expansion.
miR-492 has been identified as overexpressed in both pathologies in the present study, with a more noticeable level in ovarian cancer; however, information on its exact role in progression of endometriosis and ovarian cancer is absent in literatures. miR-492 promotes the progression of hepatic cancer by targeting the PTEN gene and increasing the level of AKT activation in cancer cells, thus having an oncogenic role.Citation45 Patients with an overexpression of miR-492 and an inhibition of the PTEN gene show a lower rate of survival,Citation46 thus sustaining metastatic features.Citation47 Considering the different expression levels of miR-492 in endometriosis and ovarian cancers, with a higher level in the latter, follow-up studies could confirm the quality of miR-492 as a prognostic biomarker in terms of transition from benign to malignant; given its role in abnormal cellular proliferation, this transcript could be placed on the list of potential therapeutic targets for inhibiting aberrant cell growth.
miR-182 was proved to be overexpressed in ovarian cancer, sustaining the present findings. This transcript is presented as a prognostic marker for ovarian cancer,Citation48 as it plays a role in malignant transformation by sustaining cell proliferation, invasion and migration processes, as was emphasized by Liu et al.Citation49,Citation50
The let-7 family is composed of 13 distinct members found on nine different chromosomes.Citation51 Of these 13 members, our study has identified three of them (let-7a, let-7b and let-7c) as being overexpressed in ovarian cancer samples.Citation52 Because of their inhibiting activity on oncogenes associated with ovarian cancer, such as KRAS,Citation52 HRAS,Citation53,Citation54 c-MYCCitation54 and HMGA-2,Citation55 downregulation of the let-7 family members correlates with advanced tumor stages and a lower survival rate.Citation56 Together with the miR-200 family, the let-7 group represents an important potential prognostic and diagnostic biomarker for ovarian cancer as well as a therapeutic element involved in the regulation of key oncogenes.Citation54 An interesting study on miR-30a reported this to be related to decidualization and to endometriosis tissue transformation,Citation57 sustaining our finding related to malignant transformation and therapeutic target for ovarian serous adenocarcinoma.Citation58 miR-30a was found to have an oncogenic role and to modulate BCL2A1, IER3 and cyclin D2 expression via FOXL2,Citation59 the expression of which is related to drug resistance mechanisms.Citation60
miR-145 was found to be downregulated in ovarian cancer and thus has a prognostic role.Citation61 A functional study on ovarian cancer cell lines reveals that treatment with agomiR-145 caused inhibition of TRIM2 and leads to the inhibition of Bim, a proapoptotic Bcl-2 family member.Citation62 miR-145 is related to ovarian cancer chemoresistance by regulation of cell cycle-related proteins: Cdk6 and Sp1.Citation61 This transcript was not found to be statistically significant in endometriosis group but was downregulated in ovarian cancer versus control or ovarian cancer versus endometriosis analysis. Therefore, miR-145 can be involved in malignant transformation, due to the fact that miR-145 was related to an increased cell proliferation, invasiveness and stem cells features in endometrioid primary cell cultures and stabilized cell lines.Citation63
miR-205 is a highly controversial miRNA that was retrieved statistically significantly only in ovarian cancer versus endometriosis group. There are no data available in literature with regard to miR-205 implication in endometriosis, but in ovarian cancer, it is presented to be connected to the regulation of cell proliferation, EMT and invasion mechanism and metastasis processes.Citation64 miR-205 directly targets VEGF-ACitation65 or promotes cell motility via ZEB1,Citation66 which is related with the suppression of SMAD4 and PTEN, an event that favors ovarian cancer progression andCitation67 therefore sustaining malignant transition as showed in our data. miR-27a regulates cancer cell growth, EMT and migration in ovarian cancer.Citation68 miR-27a is an important transcript responsible for drug resistance by targeting MDR1/P-gp and HIPK2 in ovarian cancer cells.Citation69
The EMT-related miRNA has an important role in modulation and favoring proangiogenic mechanism; therefore, this miRNA can be considered as both a prognostic and a therapeutic target in the selected pathologies. These EMT-related miRNAs are prone for activation by angiogenic mechanimsCitation70,Citation71 and, in particular, can influence the implantation of endometrial cells on ectopic sites.Citation40
Conclusion
Our data have demonstrated that differentially expressed miRNAs might contribute significantly to regulating the functions of pathological processes sustaining cell proliferation. Endometriosis still remains an enigmatic disease, but now it is clear that miRNAs play an important role in this pathology and can be related to malignant transition. miRNAs that are related to inflammation (miR-325) and those regulating the EMT (miR-200a, miR-200b, miR-200c, miR-141, miR-429, miR-30a, miR-145 and miR-205) can contribute to malignant transformation.
The association between endometriosis and ovarian carcinoma remains debated but is sustained by the common miRNAs. At the same time, we have identified a particular miRNA signature that is capable of discriminating between endometriosis and ovarian cancer, this finding being the ground for additional functional studies needed to clarify the prospective role of miRNAs in endometriosis and its related malignancies.
This panel of identified miRNAs can enable the discovery of dysregulated miRNAs that can act as precise biomarkers for diagnostic and prognostic applications and in particular for assessing the risk involved in the transition from benign to malignant status.
Acknowledgments
This study was sustained by the Iuliu Hatieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania, for PhD research project (grant no 7690/13/15.04.2016).
Supplementary materials
Figure S1 Cluster analysis of endometriosis, ovarian cancer and normal tissues (statistically significant miRNAs were selected).
Abbreviations: E, endometriosis tissue; N, normal tissue; O, ovarian cancer tissue.
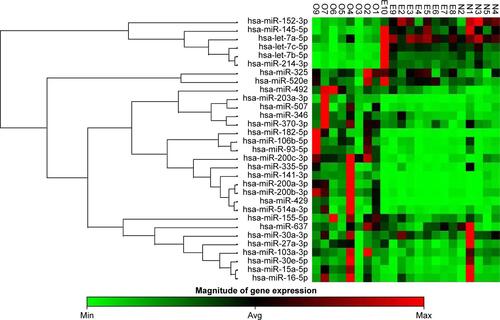
Table S1 Patient characteristics for PCR-array and qRT-PCR patient cohort
Disclosure
The authors report no conflicts of interest in this work.
References
- AznaurovaYBZhumataevMBRobertsTKAliperAMZhavoronkovAAMolecular aspects of development and regulation of endometriosisReprod Biol Endocrinol2014125024927773
- LiuFSMolecular carcinogenesis of endometrial cancerTaiwan J Obstet Gynecol2007461263217389185
- GuoSWEndometriosis and ovarian cancer: potential benefits and harms of screening and risk-reducing surgeryFertil Steril2015104481383026335131
- LaudanskiPCharkiewiczRTolwinskaASzamatowiczJCharkiewiczANiklinskiJProfiling of selected microRNAs in proliferative eutopic endometrium of women with ovarian endometriosisBiomed Res Int2015201576069826366419
- MandaiMYamaguchiKMatsumuraNBabaTKonishiIOvarian cancer in endometriosis: molecular biology, pathology, and clinical managementInt J Clin Oncol200914538339119856044
- PavoneMELyttleBMEndometriosis and ovarian cancer: links, risks, and challenges facedInt J Womens Health2015766367226170722
- BuisCCvan LeeuwenFEMooijTMBurgerCWOMEGA Project GroupIncreased risk for ovarian cancer and borderline ovarian tumours in subfertile women with endometriosisHum Reprod201328123358336924014607
- SiegelRLMillerKDJemalACancer statistics, 2017CA Cancer J Clin201767173028055103
- ManchandaRHalaskaMJPiekJMThe need for more workshops in laparoscopic surgery and surgical anatomy for European gynaecological oncology trainees: a survey by the European Network of Young Gynaecological OncologistsInt J Gynecol Cancer20132361127113223792606
- VladCKubelacPAlexandruIAchimas-CadariuPThe role of primary debulking in advanced ovarian cancer patientsJ BUON2016215132027837639
- SiegelRMaJZouZJemalACancer statistics, 2014CA Cancer J Clin201464192924399786
- BuysSSPartridgeEBlackAEffect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled TrialJAMA2011305222295230321642681
- IrimieAAchimas-CadariuPBurzCPuscasEMultiple primary malignancies – epidemiological analysis at a single tertiary institutionJ Gastrointestin Liver Dis2010191697320361078
- KinoseYSawadaKNakamuraKKimuraTThe role of microRNAs in ovarian cancerBiomed Res Int2014201424939325295252
- PavoneMELyttleBMEndometriosis and ovarian cancer: links, risks, and challenges facedInt J Womens Health2015766367226170722
- Kondi-PafitiAPapakonstantinouEIavazzoCGrigoriadisCSalakosNGregoriouOClinicopathological characteristics of ovarian carcinomas associated with endometriosisArch Gynecol Obstet2012285247948321717140
- SuryawanshiSVladAMLinH-MPlasma microRNAs as novel biomarkers for endometriosis and endometriosis-associated ovarian cancerClin Cancer Res20131951213122423362326
- Mahdian-ShakibADorostkarRTatMHashemzadehMSSaidiNDifferential role of microRNAs in prognosis, diagnosis, and therapy of ovarian cancerBiomed Pharmacother20168459260027694003
- BraicuCCalinGABerindan-NeagoeIMicroRNAs and cancer therapy – from bystanders to major playersCurrent Med Chem2013202935613573
- MuresanMZaharieFBojanAMicroRNAs in liver malignancies. Basic science applied in surgeryJ BUON201520236137526011324
- BraicuCCojocneanu-PetricRChiraSClinical and pathological implications of miRNA in bladder cancerInt J Nanomedicine20151079180025653521
- JanssonMDLundAHMicroRNA and cancerMol Oncol20126659061023102669
- ChenXLuPWangDDThe role of miRNAs in drug resistance and prognosis of breast cancer formalin-fixed paraffin-embedded tissuesGene2016595222122627746365
- IrimieAIBraicuCCojocneanu-PetricRBerindan-NeagoeICampianRSNovel technologies for oral squamous carcinoma biomarkers in diagnostics and prognosticsActa Odontol Scand201573316116825598447
- Berindan-NeagoeICalinGAMolecular pathways: microRNAs, cancer cells, and microenvironmentClin Cancer Res201420246247625325512634
- Berindan-NeagoeIMonroig PdelCPasculliBCalinGAMicroRNAome genome: a treasure for cancer diagnosis and therapyCA Cancer J Clin201464531133625104502
- BraicuCCatanaCCalinGABerindan-NeagoeINCRNA combined therapy as future treatment option for cancerCurr Pharm Des201420426565657425341933
- BraicuCTomuleasaCMonroigPCucuianuABerindan-NeagoeICalinGAExosomes as divine messengers: are they the Hermes of modern molecular oncology?Cell Death Differ2015221344525236394
- Berindan-NeagoeIChioreanRBraicuCQuantitative mRNA expression of genes involved in angiogenesis, coagulation and inflammation in multiforme glioblastoma tumoral tissue versus peritumoral brain tissue: lack of correlation with clinical dataEur Cytokine Netw2012232455522591734
- LiHHuangWLuoRThe microRNA-325 inhibits hepatocellular carcinoma progression by targeting high mobility group box 1Diagn Pathol20151011726194496
- YaoSZhaoTJinHExpression of MicroRNA-325-3p and its potential functions by targeting HMGB1 in non-small cell lung cancerBiomed Pharmacother201570727925776482
- BannoKYanokuraMIidaMApplication of microRNA in diagnosis and treatment of ovarian cancerBiomed Res Int2014201423281724822185
- WangYDongQGuYGroomeLJUp-regulation of miR-203 expression induces endothelial inflammatory response: potential role in preeclampsiaAm J Reprod Immunol201676648249027753461
- LeeHChoiHJKangCSLeeHJLeeWSParkCSExpression of miRNAs and PTEN in endometrial specimens ranging from histologically normal to hyperplasia and endometrial adenocarcinomaMod Pathol201225111508151522766795
- CaoQLuKDaiSHuYFanWClinicopathological and prognostic implications of the miR-200 family in patients with epithelial ovarian cancerInt J Clin Exp Pathol2014752392240124966949
- HuXMacdonaldDMHuettnerPCA miR-200 microRNA cluster as prognostic marker in advanced ovarian cancerGynecol Oncol2009114345746419501389
- ChenYZhangLHaoQCandidate microRNA biomarkers in human epithelial ovarian cancer: systematic review profiling studies and experimental validationCancer Cell Int20131318623978303
- ZuberiMMirRDasJExpression of serum miR-200a, miR-200b, and miR-200c as candidate biomarkers in epithelial ovarian cancer and their association with clinicopathological featuresClin Transl Oncol2015171077978726063644
- van JaarsveldMTHellemanJBoersmaAWmiR-141 regulates KEAP1 and modulates cisplatin sensitivity in ovarian cancer cellsOncogene201332364284429323045278
- YangRQTengHXuXHMicroarray analysis of microRNA deregulation and angiogenesis-related proteins in endometriosisGenet Mol Res2016152
- RekkerKSaareMRoostAMCirculating miR-200-family micro-RNAs have altered plasma levels in patients with endometriosis and vary with blood collection timeFertil Steril20151044938946.e93226206343
- LiB-AA novel tumor suppressor miRNA miR-520e contributes to suppression of hepatomaActa Pharmacol Sin20123313422212428
- YiMLiMLongXmiR-520e regulates cell proliferation, apoptosis and migration in breast cancerOncol Lett20161253543354827900034
- ZhangSShanCKongGDuYYeLZhangXMicroRNA-520e suppresses growth of hepatoma cells by targeting the NF-[kappa] B-inducing kinase (NIK)Oncogene201231313607362022105365
- ShenFCaiWSFengZMiR-492 contributes to cell proliferation and cell cycle of human breast cancer cells by suppressing SOX7 expressionTumour Biol20153631913192125407488
- JiangYHanYSunCRab23 is overexpressed in human bladder cancer and promotes cancer cell proliferation and invasionTumour Biol20163768131813826715272
- von FroweinJPagelPKapplerRvon SchweinitzDRoscherASchmidIMicroRNA-492 is processed from the keratin 19 gene and up-regulated in metastatic hepatoblastomaHepatology201153383384221319197
- Marzec-KotarskaBCybulskiMKotarskiJCMolecular bases of aberrant miR-182 expression in ovarian cancerGenes Chromosomes Cancer2016551187788927295517
- LiuZLiuJSeguraMFMiR-182 overexpression in tumourigenesis of high-grade serous ovarian carcinomaJ Pathol2012228220421522322863
- MengXJoosseSAMullerVDiagnostic and prognostic potential of serum miR-7, miR-16, miR-25, miR-93, miR-182, miR-376a and miR-429 in ovarian cancer patientsBr J Cancer201511391358136626393886
- RoushSSlackFJThe let-7 family of microRNAsTrends Cell Biol2008181050551618774294
- ZamanMSMaherDMKhanSJaggiMChauhanSCCurrent status and implications of microRNAs in ovarian cancer diagnosis and therapyJ Ovarian Res2012514423237306
- OhJJGrosshansDRWongSGSlamonDJIdentification of differentially expressed genes associated with HER-2/neu overexpression in human breast cancer cellsNucleic Acids Res199927204008401710497265
- IorioMVVisoneRDi LevaGMicroRNA signatures in human ovarian cancerCancer Res200767188699870717875710
- SlackFJWeidhaasJBMicroRNA in cancer prognosisN Engl J Med2008359252720272219092157
- YangNKaurSVoliniaSMicroRNA microarray identifies Let-7i as a novel biomarker and therapeutic target in human epithelial ovarian cancerCancer Res20086824103071031419074899
- AoyagiYNasuKKaiKDecidualization differentially regulates microRNA expression in eutopic and ectopic endometrial stromal cellsReprod Sci201724344545527412773
- ZhouJGongGTanHUrinary microRNA-30a-5p is a potential biomarker for ovarian serous adenocarcinomaOncol Rep20153362915292325962395
- WangTLiFTangSMiR-30a upregulates BCL2A1, IER3 and cyclin D2 expression by targeting FOXL2Oncol Lett20159296797125621074
- LiuJWuXLiuHExpression of microRNA-30a-5p in drug-resistant and drug-sensitive ovarian cancer cell linesOncol Lett20161232065207027602140
- ZhuXLiYXieCmiR-145 sensitizes ovarian cancer cells to paclitaxel by targeting Sp1 and Cdk6Int J Cancer201413561286129624510775
- ChenXDongCLawPTMicroRNA-145 targets TRIM2 and exerts tumor-suppressing functions in epithelial ovarian cancerGynecol Oncol2015139351351926472353
- AdammekMGreveBKassensNMicroRNA miR-145 inhibits proliferation, invasiveness, and stem cell phenotype of an in vitro endometriosis model by targeting multiple cytoskeletal elements and pluripotency factorsFertil Steril201399513461355.e134523312222
- ZhouJLiuHChenYWenJLiLWuXExpression and significance of VEGF, miR-205 and target protein Ezrin and Lamin A/C in ovarian cancerZhong Nan Da Xue Xue Bao Yi Xue Ban2014392142150 Chinese24608381
- LiJLiLLiZThe role of miR-205 in the VEGF-mediated promotion of human ovarian cancer cell invasionGynecol Oncol2015137112513325597268
- NiuKShenWZhangYZhaoYLuYMiR-205 promotes motility of ovarian cancer cells via targeting ZEB1Gene2015574233033626275944
- LiJHuKGongGUpregulation of MiR-205 transcriptionally suppresses SMAD4 and PTEN and contributes to human ovarian cancer progressionSci Rep201774133028145479
- XuLXiangJShenJOncogenic MicroRNA-27a is a target for genistein in ovarian cancer cellsAnticancer Agents Med Chem20131371126113223438830
- LiZHuSWangJMiR-27a modulates MDR1/P-glycoprotein expression by targeting HIPK2 in human ovarian cancer cellsGynecol Oncol2010119112513020624637
- OnisimAAchimas-CadariuAVladCKubelacPAchimas-CadariuPCurrent insights into the association of Nestin with tumor angiogenesisJ BUON201520369970626214620
- GhermanCBraicuOLZanoagaOCaffeic acid phenethyl ester activates pro-apoptotic and epithelial-mesenchymal transition-related genes in ovarian cancer cells A2780 and A2780cisMol Cell Biochem20164131–218919826838168
