Abstract
Purpose
To systematically investigate the efficacy and safety of the combination of FOLFOX (oxaliplatin, 5-fluorouracil, and leucovorin) regimen and cocultured dendritic cells and cytokine-induced killer cells (DC-CIK) immunotherapy for the treatment of colorectal cancer (CRC).
Methods
Publications reporting the clinical trials’ responses or safety of FOLFOX regimen combined with DC-CIK immunotherapy in treating CRC patients were searched in PubMed, Embase, Cochrane Library, China National Knowledge Internet, and Wanfang databases. Trials meeting the selection criteria were analyzed. The overall survival (OS), overall response rate (ORR), disease control rate (DCR), tumor markers, immune function, and adverse events were evaluated.
Results
Ten trials including 881 CRC patients were analyzed in this meta-analysis. The combined therapy showed advantages over FOLFOX treatment-alone in 2-year OS (odds ratio [OR] =2.77, confidence interval [CI] =1.58–4.86, P=0.0004), ORR (OR =1.85, CI =1.34–2.56, P=0.0002), and DCR (OR =2.54, CI =1.76–3.67, P<0.00001), with statistical significance. After immunotherapy, lymphocyte subset percentages of CD3+ (P=0.0006) and CD4+ (P=0.01), CD4+/CD8+ ratio (P=0.0003), and levels of cytokines IFN-γ (P=0.003) and IL-2 (P=0.01) were significantly increased, whereas analysis of CD8+, CD3−CD56+, CD3+CD56+, CD4+CD25+, IL-6, and TNF-α did not show any significant difference (P>0.05). Moreover, the level of carcinoembryonic antigen was also decreased significantly upon immunotherapy (P<0.00001).
Conclusion
The combination of FOLFOX regimen and DC-CIK immunotherapy was safe and effective for CRC patients.
Introduction
Colorectal cancer (CRC) is the second most frequent cause of death in Europe and North America with about 600,000 deaths per year.Citation1,Citation2 In recent years, the incidence of CRC has risen significantly with ~1.2 million new cases being diagnosed every year worldwide, while the 5-year survival rate of patients with stage IV CRC is <10%.Citation1–Citation4 Chemotherapy is the main treatment for CRC, and appropriate chemotherapy regimens effectively prolong patients’ lives and improve their quality of life.Citation2,Citation4 FOLFOX (oxaliplatin, 5-fluorouracil, and leucovorin) is one of the most common chemotherapy regimens and is a standard first-line therapy for CRC.Citation1,Citation5 However, many researchers have reported that chemotherapy alone was not able to completely eradicate small lesions and metastatic cells, which may raise the probability of cancer recurrence.Citation6 Comprehensive treatments are therefore taken as the future for CRC therapy, and the combination of chemotherapy and adoptive cell therapy is one of the options.
Adoptive cellular immunotherapy is achieved upon in vitro expansion of autologous immune effector cells, such as cytokine-induced killer cells (CIK),Citation7,Citation8 natural killer cellsCitation9 and other immune cells,Citation10–Citation12 and transfusion back to the patients. Since conventional therapies are not able to eradicate the tumor cells thoroughly, the killing effect of adoptive cellular immunotherapy on tumor cells will make it an important supplement.Citation8 CIK is a heterogeneous subset which consists primarily of CD3+CD56+ and can be easily induced from peripheral blood mononuclear cells.Citation7,Citation8 Compared with other immune cells, CIK show a broader antitumor spectrum and a stronger antitumor ability.Citation13 Dendritic cells (DC) are the most potent antigen-presenting cells.Citation14 Studies have shown that coculture with DC enhances the cytotoxicity of CIK, indicated by an increased proportion of CD3+CD56+ cells and improved levels of cytokines such as IL-2, IFN-γ, IL-12, and TNF-α.Citation15–Citation18 It has also been found that the combination of DC and CIK is more effective than CIK treatment.Citation8
Chemotherapy combined with adoptive immunotherapy has shown satisfactory therapeutic effects in treating patients with advanced CRC.Citation19–Citation21 In a meta-analysis of treating advanced CRC with CIK or DC-CIK combined with chemotherapy or chemotherapy alone, the former showed significantly improved overall survival (OS) and overall response rate (ORR), while safety, tumor markers, and levels of cytokines were not involved in this analysis.Citation22 Moreover, the therapeutic regimens among studies were different, which may have influenced the analysis of clinical therapy. In the present study, a more stringent inclusion criterion (only CRC patients treated by FOLFOX regimen with DC-CIK immunotherapy were included) was developed to exclude the impact of different treatments on the clinical efficacy. Meanwhile, a systematic review and meta-analysis were carried out to investigate the efficacy and safety of FOLFOX chemotherapy combined with DC-CIK immunotherapy in comparison with FOLFOX alone, in order to provide scientific evidence for future clinical trials.
Materials and methods
Search strategy and selection criteria
Literature was searched in PubMed, Embase, Cochrane Library, China National Knowledge Internet, and Wanfang databases with key terms “dendritic cells” and “cytokine-induced killer cells” combined with “colorectal cancer.” No language limits were applied. The initial search was performed in January 2016 and updated in July 2016.
The study inclusion criteria were as follows: 1) randomized controlled trials in CRC patients and 2) patients in the experimental group received FOLFOX regimen combined with DC-CIK immunotherapy, and patients in the control group were treated by FOLFOX alone.
Data collection and quality assessment
Data were extracted by two researchers independently (Yan Liu and Zhong Zheng), and discrepancy was resolved upon discussing with a third researcher (Qixin Zhang). Extracted information included first author’s names, years of publication, study locations, tumor stages, numbers of subjects, patient ages, in vitro cell culture conditions, and dosages of utilized immune cells. The quality of the included papers was evaluated based on Cochrane Handbook.Citation23
Definition of measurements
Treatment efficacy was assessed in terms of OS, complete response rate (CR), partial response rate (PR), stable disease rate (SD), progressive disease rate (PD), ORR (ORR = CR + PR), and disease control rate (DCR; DCR = CR + PR + SD). OS was defined as the duration from the initiation of treatment to death from any cause.Citation24 The immune function of CRC patients before and after treatment was determined by lymphocyte subsets (CD3+, CD4+, CD8+, CD3−CD56+, CD3+CD56+, and CD4+CD25+) and cytokines secretion (IFN-γ, IL-2, IL-6, and TNF-α). Adverse events during therapy were also taken into an assessment.
Statistical analysis
The analysis was performed using Review Manager 5.2 (Cochrane Collaboration). P<0.05 was considered statistically significant. Heterogeneity among studies was assessed to determine the most suitable analysis model.Citation25 Cochran’s Q test was performed to evaluate the homogeneity of the results; I2<50% or P>0.1 indicated that the studies were homogenous. Odds ratio (OR) was the principal measurement for therapeutic effects and is presented with a 95% confidence interval (CI).
Results
Search results
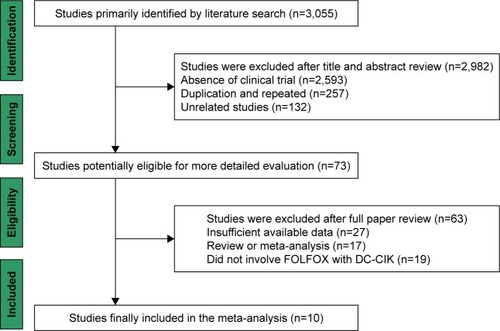
A total of 3,055 articles were identified with the initial retrieve. After reviewing the titles and abstracts, 2,982 articles were excluded because they were not clinical trials (n=2,593) were a duplication or repetition (n=257) or were unrelated studies (n=132), and 73 studies were selected as potentially relevant. After full-text assessment, 27 studies with insufficient data, 17 reviews or meta-analyses and 19 studies which did not involve FOLFOX regimen and DC-CIK immunotherapy were excluded. Finally, 10 trials including a total of 881 patients were found to be eligible for inclusion in this meta-analysis ().Citation26–Citation35
Patient characteristics
All the involved trials were conducted in mainland China. In total, 444 patients were treated by FOLFOX regimen in combination with DC-CIK immunotherapy, and 437 patients were treated by FOLFOX alone. DC and CIK used in the 10 trials were all obtained from autologous peripheral blood. All DC and CIK were examined and confirmed free of bacterial and fungal contamination, and negative for mycoplasma. Cells were cultured for amplification in vitro. The DC and CIK were suspended in saline containing 2% albumin (100 or 200 mL) and intravenously transfused into patients, except one trial in which DC were subcutaneously transfused.Citation33 DC-CIK were transfused for two or more treatment cycles. However, the accurate cell numbers were not provided in most studies. Detailed clinical information on the trials is presented in and .
Table 1 Clinical information from the eligible trials in the meta-analysis
Table 2 Information of DC-CIK immunotherapy
Quality assessment
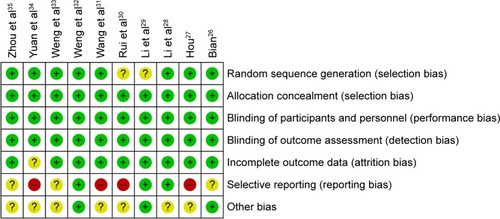
The assessment of bias risk is shown in . The overall qualities of the involved studies ranged from moderate to high. Eight studies were determined as of low risk, and the remaining two studies did not show a clear description of the randomization process. The risks of allocation, performance, and detection were low; one research was regarded as of unclear risk for lacking follow-up information; three trials were considered as of unclear risk for selective reporting, while the other four studies were considered as of high risk for missing primary outcome data.
Therapeutic efficacy assessments
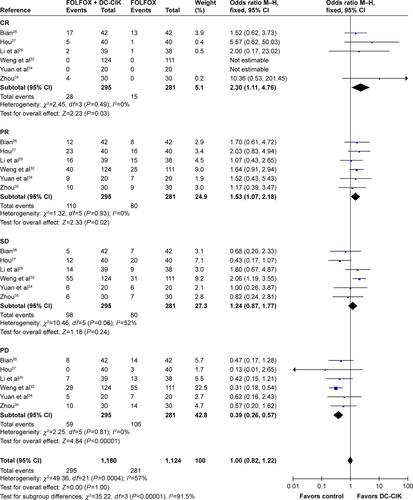
As shown in , , and S1 and , the pooled results indicated that patients in experimental group had significantly improved 2-year OS, CR, PR, ORR, and DCR (2-year OS: OR =2.77, CI =1.58–4.86, P=0.0004; CR: OR =2.30, CI =1.11–4.76, P=0.03; PR: OR =1.53, CI =1.07–2.18, P=0.02; ORR: OR =1.85, CI =1.34–2.56, P=0.0002; DCR: OR =2.54, CI =1.76–3.67, P<0.00001) and significantly decreased PD (PD: OR =0.39, CI =0.26–0.57, P<0.00001), whereas the 1-year OS and SD did not show significant difference from control group (1-year OS: OR =2.42, CI =0.86–6.79, P=0.09; SD: OR =1.24, CI =0.87–1.77, P=0.24). Fixed-effect models were used to analyze the OR rate because of low heterogeneity.
Figure 3 Forest plot of the comparison of OS between the patients who received FOLFOX + DC-CIK therapy and those who received FOLFOX alone. The fixed-effects meta-analysis model (Mantel–Haenszel method) was used.
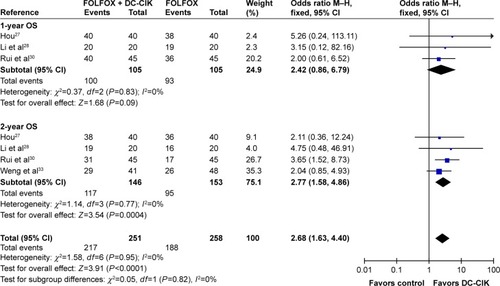
Figure 4 Forest plot of the comparison of ORR and DCR between the patients who received FOLFOX + DC-CIK therapy and those who received FOLFOX alone. The fixed-effects meta-analysis model (Mantel–Haenszel method) was used.
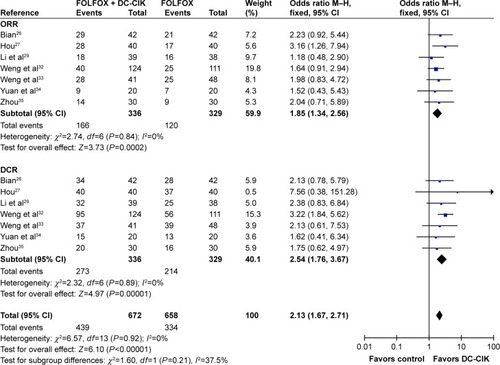
Table 3 Comparison of CR, PR, SD, PD, ORR, and DCR between the FOLFOX + DC-CIK and FOLFOX-alone groups
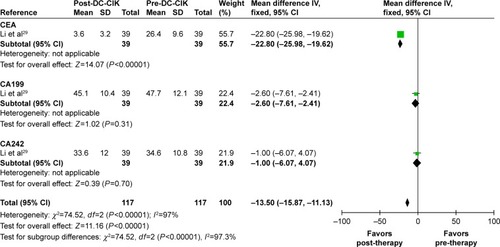
Tumor markers were also analyzed to evaluate the therapeutic effects of combination therapy (). Carcinoembryonic antigen (CEA) was significantly reduced after immunotherapy (OR =−22.80, CI =−25.98 to 19.62, P<0.00001), while no significant changes were found in carbohydrate antigen 199 (CA199) and carbohydrate antigen 242 (CA242) (CA199: OR =−2.60, CI =−7.61 to 2.41, P=0.31; CA242: OR =−1.00, CI =−6.07 to 4.07, P=0.70).
Figure 5 Forest plot of the comparison of tumor markers in pre- and post-DC-CIK immunotherapy. The patient data were obtained before (pre-DC-CIK) and after DC-CIK treatment (post-DC-CIK). The fixed-effects meta-analysis model (IV method) was used.
Immune function evaluation
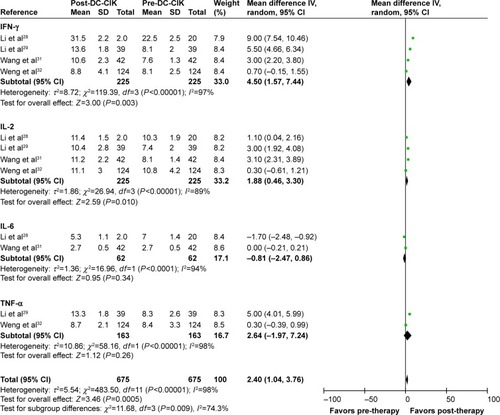
The immune status of patients was examined before and after treatment. After DC-CIK treatment, percentages of CD3+ and CD4+ cells and CD4+/CD8+ ratio were significantly increased (CD3+: OR =5.04, CI =2.18–7.90, P=0.0006; CD4+: OR =3.38, CI =0.73–6.02, P=0.01; CD4+/CD8+: OR =0.39, CI =0.18–0.60, P=0.0003) (), whereas no differences were found in the proportions of CD8+, CD3−CD56+, CD3+CD56+, and CD4+CD25+ cells (CD8+: OR =−0.30, CI =−3.46 to 2.86, P=0.85; CD3−CD56+: OR =4.04, CI =−0.36 to 8.45, P=0.07; CD3+CD56+: OR =1.11, CI =−1.64 to 3.85, P=0.43; CD4+CD25+: OR =−0.29, CI =−2.40 to 1.82, P=0.79). Moreover, the levels of IFN-γ and IL-2 were significantly increased after DC-CIK therapy (IFN-γ: OR =4.50, CI =1.57–7.44, P=0.003; IL-2: OR =1.88, CI =−0.46−3.30, P=0.01) (), while no obvious changes were identified in the expression of IL-6 and TNF-α (IL-6: OR =−0.81, CI =−2.47 to 0.86, P=0.34; TNF-α: OR =2.64, CI =−1.97 to 7.24, P=0.26).
Figure 6 Forest plot of the comparison of immunophenotype in pre- and post-DC-CIK immunotherapy. The patient data were obtained before (pre-DC-CIK) and after DC-CIK treatment (post-DC-CIK). The random-effects meta-analysis model (IV method) was used.
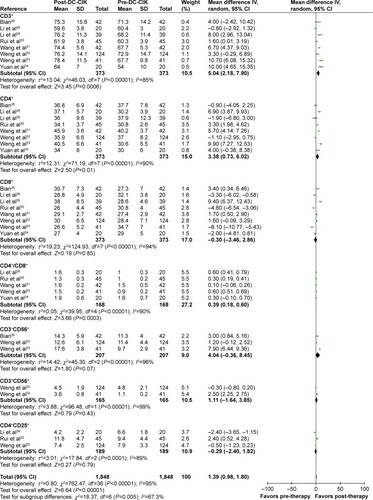
Figure 7 Forest plot of the comparison of cytokines in pre- and post-DC-CIK immunotherapy. The patient data were obtained before (pre-DC-CIK) and after DC-CIK treatment (post-DC-CIK). The random-effects meta-analysis model (IV method) was used.
Adverse events assessment
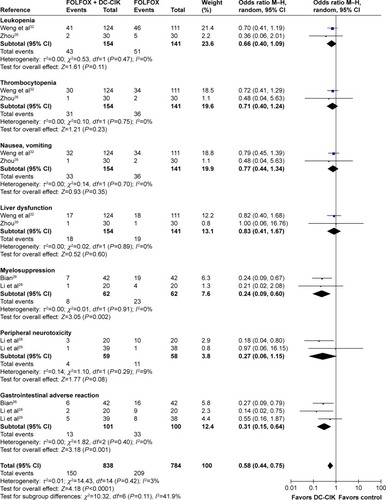
As shown in , compared with patients treated by FOLFOX alone, those treated by FOLFOX and DC-CIK combined therapy suffered milder myelosuppression and gastrointestinal adverse reactions, but the combined therapy was not able to alleviate leucopenia, thrombocytopenia, nausea and vomiting, liver dysfunction, and peripheral neurotoxicity (myelosuppression: OR =0.24, CI =0.09–0.60, P=0.002; gastrointestinal adverse reaction: OR =0.31, CI =0.15–0.64, P=0.001; leucopenia: OR =0.66, CI =0.40–1.09, P=0.11; thrombocytopenia: OR =0.71, CI =0.40–1.24, P=0.23; nausea and vomiting: OR =0.77, CI =0.44–1.34, P=0.35; liver dysfunction: OR =0.83, CI =0.41–1.67, P=0.60; peripheral neurotoxicity: OR =0.27, CI =0.06–1.15, P=0.08).
Figure 8 Forest plot of the comparison of adverse effects between the patients who received FOLFOX + DC-CIK therapy and those who received FOLFOX alone. The random-effects meta-analysis model (Mantel–Haenszel method) was used.
Discussion
To date, several clinical trials have been performed using chemotherapy plus DC-CIK immunotherapy for the treatment of CRC.Citation20,Citation21 However, the applied clinical protocols were unstandardized in these trials, which made therapeutic effects obscure for evaluation. In this study, a large number of comprehensive trials were investigated to achieve a higher statistical reliability. The meta-analysis revealed that the combination of FOLFOX regimen and DC-CIK immunotherapy improved OS, ORR, DCR, and immune function in CRC patients, without causing severe adverse events.
Safety is a key factor to be considered in clinical treatment. Patients in combination group showed milder adverse events than those treated by FOLFOX alone. FOLFOX caused myelosuppression and gastrointestinal adverse reactions which were alleviated by DC-CIK immunotherapy (P<0.05), but relief from other adverse events was not obtained. DC-CIK immunotherapy also enhanced the efficiency of FOLFOX in treating CRC. Compared to patients treated by FOLFOX alone, patients treated with combined therapy showed markedly increased 2-year OS, ORR, and DCR. Tumor markers, as the main factor for evaluation of therapeutic effects and prediction of recurrence and prognosis,Citation36–Citation39 also showed promising results with combined therapy. The levels of CEA, CA199, and CA242 were decreased after DC-CIK immunotherapy, although changes in the latter two were not statistically significant.
Immune reconstitution is important for treating malignancies.Citation6 CD4+ T cells assist in the recruitment of CD8+ T cells and activate macrophages through the secretion of immune-regulatory cytokines, including IL-2, IL-12, and IFN-γ, thereby inducing tumor cell death.Citation6,Citation40 This also indicates a synergistic relationship between CD4+ and CD8+ cells in immune responses.Citation40 It was found that the ratios of T-lymphocyte subsets were disordered in tumor patients.Citation6,Citation40 This analysis showed significantly increased percentages of CD3+ and CD4+ cells and CD4+/CD8+ ratio upon DC-CIK treatment, indicating that the immune function of CRC patients was improved upon DC-CIK immunotherapy. Moreover, the antitumor activity of immune cells was associated with higher percentages of CD3−CD56+ and CD3+CD56+ cellsCitation6 and lower percentages of CD4+CD25+ regulatory T cells.Citation41 In this meta-analysis, no significant difference was found in the percentages of CD8+, CD3−CD56+, CD3+CD56+, and CD4+CD25+ T cells between pre- and post-immunotherapy. This may be related to the various DC-CIK transfusion dosages and different time points of lymphocyte subset tests among these trials.Citation8 Furthermore, the balance between Th1 and Th2 cells is crucial in immunotherapy.Citation7 Th1 cytokines, including IFN-γ, IL-2, and TNF-α, enhance the cytotoxicity of CIK, whereas Th2 cytokines, such as IL-6 and IL-10, are associated with tumor immune escape.Citation42,Citation43 The present analysis showed that after DC-CIK immunotherapy, the levels of IFN-γ and IL-2 were significantly increased, whereas no significant differences were observed in TNF-α and IL-6, indicating an important role of IFN-γ and IL-2 in DC-CIK immunotherapy.
Limitations
There are some limitations in our study. The 10 trials meeting the selection criteria were all conducted on a Chinese population. This may be because the large number of Chinese CRC patients has made CRC a research focus in China and led to there being many papers on it. Although the effectiveness of DC-CIK immunotherapy in various malignant tumors has been wildly reported,Citation44–Citation46 its usage in CRC is still rare outside of China. One German trial was included in this meta-analysis originally, but it was excluded due to insufficient data.Citation47 We will keep up with the future research and carry outfurther analyses of studies conducted both in and outside of China. Moreover, this analysis did not go through an open external evaluation, which may have led to an overestimation of treatment effects. Finally, the different treatment dosages and cycles of DC-CIK immunotherapy may have led to different clinical responses,Citation19 but we were not able to obtain accurate information from some of the involved trials. Due to the above-mentioned limitations of this meta-analysis, further studies are needed to verify the safety and efficacy of the combined therapy strategy.
Conclusion
Taken together, this study confirms that FOLFOX regimen plus DC-CIK immunotherapy is a safe and effective treatment for CRC patients. It markedly prolongs patient’s survival time and improves the efficacy of CRC treatment by reconstructing the patient’s immune system. Therefore, the combination of FOLFOX chemotherapy regimen and DC-CIK immunotherapy is a promising treatment option for CRC patients.
Author contributions
All authors contributed toward data analysis, drafting, and critically revising the paper and agree to be accountable for all aspects of the work.
Supplementary material
Figure S1 Forest plot of the comparison of CR, PR, SD, and PD between the patients who received FOLFOX + DC-CIK therapy and those who received FOLFOX alone. The fixed-effects meta-analysis model (Mantel–Haenszel method) was used.
Abbreviations: CR, complete response rate; PR, partial response rate; SD, stable disease rate; PD, progressive disease rate; FOLFOX, oxaliplatin, 5-fluorouracil, and leucovorin; DC-CIK, dendritic cells and cytokine-induced killer cells; M–H, Mantel–Haenszel method; CI, confidence interval.
Disclosure
The authors report no conflicts of interest in this work.
References
- ChenYXYangQKuangJJEfficacy of adding bevacizumab in the first-line chemotherapy of metastatic colorectal cancer: evidence from seven randomized clinical trialsGastroenterol Res Pract2014201418
- GevaRVecchioneLTejparSPiessevauxHVan CutsemEPrenenHBevacizumab plus chemotherapy as salvage treatment in chemorefractory patients with metastatic colorectal cancerOnco Targets Ther20136535823378775
- KimJSKimYGParkEJCell-based immunotherapy for colorectal cancer with cytokine-induced killer cellsImmune Netw20161629910827162526
- GuoYXiongBHZhangTChengYMaLXELOX vs. FOLFOX in metastatic colorectal cancer: an updated meta-analysisCancer Invest20163429410426864862
- ColucciGGebbiaVPaolettiGPhase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia MeridionaleJ Clin Oncol200523224866487515939922
- MuYZhouCHChenSFEffectiveness and safety of chemotherapy combined with cytokine-induced killer cell/dendritic cell-cytokine- induced killer cell therapy for treatment of gastric cancer in China: a systematic review and meta-analysisCytotherapy20161891162117727421742
- MuYWangWHXieJPZhangYXYangYPZhouCHEfficacy and safety of cord blood-derived dendritic cells plus cytokine-induced killer cells combined with chemotherapy in the treatment of patients with advanced gastric cancer: a randomized Phase II studyOnco Targets Ther201694617462727524915
- ZhouCLLiuDLLiJChemotherapy plus dendritic cells co-cultured with cytokine-induced killer cells versus chemotherapy alone to treat advanced non-small-cell lung cancer: a meta-analysisOncotarget2016752865008651027863436
- ZhaoZLiaoHJuYEffect of compound Kushen injection on T-cell subgroups and natural killer cells in patients with locally advanced non-small-cell lung cancer treated with concomitant radiochemotherapyJ Tradit Chin Med2016361141826946613
- DudleyMEGrossCASomervilleRPRandomized selection design trial evaluating CD8+-enriched versus unselected tumor-infiltrating lymphocytes for adoptive cell therapy for patients with melanomaJ Clin Oncol201331172152215923650429
- ChiaWKTeoMWangWWAdoptive T-cell transfer and chemotherapy in the first-line treatment of metastatic and/or locally recurrent nasopharyngeal carcinomaMol Ther201422113213924297049
- ArteneSATurcu-StiolicaAHartleyRDendritic cell immunotherapy versus bevacizumab plus irinotecan in recurrent malignant glioma patients: a survival gain analysisOnco Targets Ther201696669667727877052
- JakelCESchmidt-WolfIGAn update on new adoptive immunotherapy strategies for solid tumors with cytokine-induced killer cellsExpert Opin Biol Ther201414790591624673175
- ZhongRHanBZhongHA prospective study of the efficacy of a combination of autologous dendritic cells, cytokine-induced killer cells, and chemotherapy in advanced non-small cell lung cancer patientsTumour Biol201435298799424006222
- WeiXCYangDDHanXRBioactivity of umbilical cord blood dendritic cells and anti-leukemia effectInt J Clin Exp Med2015810197251973026770637
- LiHRenXB,ZhangPAnXMLiuHHaoXSDendritic cells reduce the number and function of CD4+CD25+ cells in cytokine-induced killer cellsZhonghua Yi Xue Za Zhi2005854431343138 Chinese [with English abstract]16405818
- SchmidtJEisoldSBuchlerMWMartenADendritic cells reduce number and function of CD4+CD25+ cells in cytokine-induced killer cells derived from patients with pancreatic carcinomaCancer Immunol Immunother200453111018102615185013
- GeWLiCHZhangWCoculture of dendritic cell with cytokine-induced killer results in a significant increase in cytotoxic activity of CIK to tumor cells in vitro and in vivo]Zhonghua Xue Ye Xue Za Zhi2004255277280 Chinese [with English abstract]15182535
- ZhaoHWangYYuJPAutologous cytokine-induced killer cells improves overall survival of metastatic colorectal cancer patients: results from a phase II clinical trialClin Colorectal Cancer201615322823527052743
- LinTSongCChuoDYZhangHZhaoJClinical effects of autologous dendritic cells combined with cytokine-induced killer cells followed by chemotherapy in treating patients with advanced colorectal cancer: a prospective studyTumour Biol20163744367437226499782
- GaoDLiCXieXAutologous tumor lysate-pulsed dendritic cell immunotherapy with cytokine-induced killer cells improves survival in gastric and colorectal cancer patientsPLoS One201494e9388624699863
- WangZXCaoJXLiuZPCombination of chemotherapy and immunotherapy for colon cancer in China: a meta-analysisWorld J Gastroenterol2014204109524574784
- ZengXZhangYKwongJSThe methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic reviewJ Evid Based Med20158121025594108
- HanRXLiuXPanPJiaYJYuJCEffectiveness and safety of chemotherapy combined with dendritic cells co-cultured with cytokine-induced killer cells in the treatment of advanced non-small-cell lung cancer: a systematic review and meta-analysisPLoS One201499e10895825268709
- JacksonDWhiteIRRileyRDQuantifying the impact of between-study heterogeneity in multivariate meta-analysesStat Med201231293805382022763950
- BianJRStudy on chemotherapy combined with DC-CIK on colorectal cancerChin J Surg Oncol201355306309
- HouZYClinical study of DC-CIK combined with chemotherapy in the treatment of colon cancerGuide China Med201513356465
- LiSLiYLiangJLiuXLThe study of clinical application of DC-CIK combined with chemotherapy on colon cancerChin J Immunol2012289835839
- LiWWZhangHPWuMStudy on DC-CIK cells combined with intravenous chemotherapy for advanced colorectal cancer with diffuse hepatic metastasisMod J Integr Tradit Chin West Med2016251819421945
- RuiTWuGQXuJZhengAHWuHYeZYActivated DC combined with CIK immunotherapy for patients with advanced colorectal cancerZhejiang Med J20151815051509
- WangRYiMYangSRChaiLXHuaMThe influence of DC-CIK in advanced colorectal cancer patients on T lymphocyte subsets and cytokinesJ Hainan Med Univ2016221415801583
- WengHGShenDMaoWDHanLXClinical efficacy of DC-CIK immunotherapy combined with chemotherapy in treatment of advanced colorectal cancerZhejiang Med J20158626629
- WengJFYuLWLiYPShiYQPuYDCaoZYClinical observation of co-treatment with CIK cells and dendritic cells for advanced rectal cancerChin J Clin Oncol Rehabil201421910401043
- YuanJHPengDWLiJWWangMQClinical research of dendritic cells combined with cytokine induced killer cells therapy for advanced colorectal cancerChin Gen Pract20113641394141
- ZhouQMObservation effect of biological immune method in the treatment of patients with advanced colorectal cancerChina Mod Med201522343739
- LevyMVisokaiVLipskaLTopolcanOTumor markers in staging and prognosis of colorectal carcinomaNeoplasma200855213814218237252
- WangRFSongBRPengJJThe prognostic value of preoperative serum CEA and CA19-9 values in stage I–III colorectal cancerHepatogastroenterology20146113299499926158155
- YangXQChenCPengCWLiuSPLiYCarbohydrate antigen 242 highly consists with carbohydrate antigen 19-9 in diagnosis and prognosis of colorectal cancer: study on 185 casesMed Oncol20122921030103621553105
- ZhangSYLinMZhangHBDiagnostic value of carcinoembryonic antigen and carcinoma antigen 19-9 for colorectal carcinomaInt J Clin Exp Pathol2015889404940926464695
- WangMCaoJXPanJHAdoptive immunotherapy of cytokine-induced killer cell therapy in the treatment of non-small cell lung cancerPLoS One2014911e11266225412106
- LiuHLiJWangFComparative study of different procedures for the separation of peripheral blood mononuclear cells in cytokine-induced killer cell immunotherapy for hepatocarcinomaTumour Biol20153642299230725417201
- DulosJCarvenGJvan BoxtelSJPD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancerJ Immunother201235216917822306905
- WangZXCaoJXWangMAdoptive cellular immunotherapy for the treatment of patients with breast cancer: a meta-analysisCytotherapy201416793494524794183
- OliosoPGiancolaRDi RitiMContentoAAccorsiPIaconeAImmunotherapy with cytokine induced killer cells in solid and hematopoietic tumours: a pilot clinical trialHematol Oncol200927313013919294626
- LeeJHLimYSYeonJEAdjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinomaGastroenterology2015148713831391e625747273
- LinnYCNiamMChuSThe anti-tumour activity of allogeneic cytokine-induced killer cells in patients who relapse after allogeneic transplant for haematological malignanciesBone Marrow Transplant201247795796621986635
- Schmidt-WolfIGFinkeSTrojaneckBPhase I clinical study applying autologous immunological effector cells transfected with the interleukin-2 gene in patients with metastatic renal cancer, colorectal cancer and lymphomaBr J Cancer19998161009101610576658