Abstract
We previously reported that the soft coral-derived bioactive substance, sinuleptolide, can inhibit the proliferation of oral cancer cells in association with oxidative stress. The functional role of oxidative stress in the cell-killing effect of sinuleptolide on oral cancer cells was not investigated as yet. To address this question, we introduced the reactive oxygen species (ROS) scavenger (N-acetylcysteine [NAC]) in a pretreatment to evaluate the sinuleptolide-induced changes to cell viability, morphology, intracellular ROS, mitochondrial superoxide, apoptosis, and DNA damage of oral cancer cells (Ca9-22). After sinuleptolide treatment, antiproliferation, apoptosis-like morphology, ROS/mitochondrial superoxide generation, annexin V-based apoptosis, and γH2AX-based DNA damage were induced. All these changes were blocked by NAC pretreatment at 4 mM for 1 h. This showed that the cell-killing mechanism of oral cancer cells of sinuleptolide is ROS dependent.
Introduction
Oral cancer is the sixth most prevalent form of cancer worldwide.Citation1,Citation2 Treatment options for oral cancer include surgery and chemotherapy. Several clinically approved drugs such as cisplatin are getting ineffective due to drug resistance in oral cancer therapy.Citation3 Therefore, the discovery of new anti-oral cancer drugs becomes a challenging task.
Marine microbes, flora, and fauna provide promising sources of bioactive natural products, and they are used to develop well-received anticancer drugs.Citation4–Citation6 For example, peptides and roe protein hydrolysates derived from marine fish have been reported to inhibit the proliferation of oral cancer cells.Citation7 The methanolic extract of red alga Gracilaria tenuistipitata was found to inhibit oral cancer cell proliferation.Citation8 Luminacin, a marine microbial extract, was reported to induce autophagy and cell death in head and neck cancer cells.Citation9 Accordingly, marine resources feature abundant natural marine products with potential anticancer effects.
Recently, many soft coral-derived compounds have been reported as having potential applications as anticancer drugs.Citation10,Citation11 Studies have investigated Sinularia lochmodes-derived sinuleptolide for marine natural product identificationCitation12 and for use in treating inflammationCitation13 and skin cancer.Citation14 The structure of sinuleptolide was first derived from the soft coral Sinularia sp.Citation15 Alternatively, sinuleptolide was extracted from the soft corals Sinularia leptoclados and S. lochmodes in our laboratory.Citation16,Citation17 However, few studies have investigated the effects of sinuleptolide in the treatment of oral cancer.
In our recent study,Citation18 we reported that oxidative stress was associated with the sinuleptolide-induced killing of oral cancer cells. However, the dependence of oxidative stress in the cell-killing effect of sinuleptolide on oral cancer cells was not investigated. N-acetylcysteine (NAC), a glutathione precursor for replenishing cellular glutathione storage, is a well-known reactive oxygen species (ROS) scavenger.Citation19 NAC pretreatment can be used to investigate the role of oxidative stress dependence in drug and natural product-mediated cancer cell death.Citation20–Citation23 Therefore, the purpose of this study is to evaluate the role of oxidative stress in the cell-killing effects of sinuleptolide against oral cancer cells.
Materials and methods
Cell cultures and chemicals
Human oral cancer cells (Ca9-22), purchased from the Health Science Research Resources Bank (HSRRB) (Osaka, Japan), were incubated with DMEM medium (Gibco, Grand Island, NY, USA) and fetal bovine serum.Citation24 Human normal gingival fibroblast cells (HGF-1), purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA), were incubated with DMEM-F12 medium (Gibco, Grand Island, NY, USA).Citation25 These cells were maintained at 37°C in a humidified 5% CO2 atmosphere. The structure and preparation of soft corals Sinularia-derived sinuleptolide was isolated from S. lochmodes as described in our previous study.Citation17 It was freshly prepared in dimethyl sulfoxide (DMSO) for cell studies. All the DMSO concentrations of sinuleptolide treatments were unified at 0.24%. NAC (Sigma, St Louis, MO, USA) was pretreated with 4 mM for 1 h before sinuleptolide treatment.
Cell viability
CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) (Promega Corporation, Madison, WI, USA) was chosen to measure cell proliferation.Citation24 After plating overnight, Ca9-22 cells were incubated with sinuleptolide for 24 h with or without NAC pretreatment. Finally, the MTS response was measured by an ELISA reader (EZ Read 400 Research Reader; BioChrom, Cambridge, UK).
Intracellular ROS production
Intracellular hydrogen peroxide or other oxidizing ROS can react with 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) and generate fluorescence.Citation26,Citation27 The ROS level can be detected using flow cytometry.Citation8 In brief, after plating overnight, Ca9-22 cells were incubated with sinuleptolide for 3 h with or without NAC pretreatment. After washing with PBS, cells were incubated with 100 nM DCFH-DA in PBS at 37°C for 30 min. After harvesting, cells were resuspended in 1 mL PBS for flow cytometry analysis (BD Accuri™ C6; Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Mean intensity of ROS was collected from 1×104 cell counts.
Mitochondrial superoxide production
The mitochondrial superoxide was reacted with MitoSOX™ Red (Molecular Probes; Invitrogen, Eugene, OR, USA) and generated fluorescence.Citation28 MitoSOX Red was also applied to flow cytometry.Citation18 In brief, after plating overnight, Ca9-22 cells were incubated with sinuleptolide for 1 h with or without NAC pretreatment. Subsequently, cells were incubated with 5 µM MitoSOX 37°C for 30 min. After harvesting, cells were resuspended in 1 mL PBS for flow cytometer analysis (BD Accuri C6). Mean intensity of mitochondrial superoxide was collected from 1×104 cell counts.
DNA damage by γH2AX/propidium iodide
γH2AX is the marker of DNA double-strand breaks, and it can be detected by flow cytometry.Citation24 In brief, sinuleptolide-treated cells were fixed in 70% ethanol. After washing with BSA-T-PBS solution (1% bovine serum albumin and 0.2% Triton X-100 in PBS; Sigma), cells were incubated with p-Histone H2A.X (Ser 139) monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and BSA-T-PBS buffer in 1:50 dilution at 4°C for 1 h. After washing, Alexa Fluor 488-tagged secondary antibody (Jackson Laboratory, Bar Harbor, ME, USA) with BSA-T-PBS buffer in a 1:50 dilution was added for 30 min at room temperature. After the addition of 20 µg/mL of propidium iodide (PI), cells were resuspended for flow cytometry analysis (BD Accuri C6). Mean intensity of γH2AX was collected from 1×104 cell counts.
Statistical analysis
Experimental data were analyzed and expressed as mean ± SD. Data were analyzed using two-sample Student’s t-test with Bonferroni correction. The P-values <0.01 (=0.05/5) are considered as statistically significant.
Results
NAC effect on sinuleptolide-induced cell killing
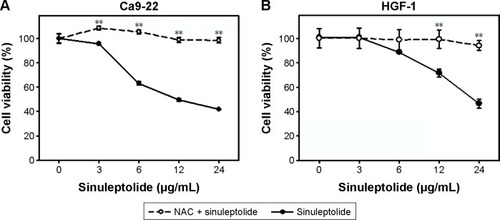
In the MTS assay (), sinuleptolide concentration responsively decreased the cell viability (%) of oral cancer cells (Ca9-22) and oral normal cells (HGF-1), but sinuleptolide selectively killed Ca9-22 cells and was less harmful to HGF-1 cells, which was consistent with our previous study.Citation18 The IC50 values of sinuleptolide in Ca9-22 and HGF-1 cells were 11.76 and 22.3 µg/mL, respectively. As NAC is a common ROS scavenger,Citation29,Citation30 this NAC effect was used in the present study to address the dependence of oxidative stress for the sinuleptolide effect on oral cancer compared with normal cells. We found that the sinuleptolide (3–24 µg/mL)-induced cell killing in Ca9-22 and HGF-1 cells was significantly reduced by a pretreatment with NAC (P<0.002) ().
Figure 1 NAC effect on cell viabilities of sinuleptolide-treated oral cancer and normal cells.
Abbreviation: NAC, N-acetylcysteine.
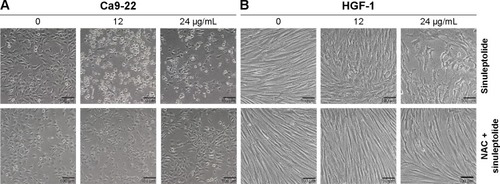
NAC effect on sinuleptolide-induced morphology change
The cell morphology was changed and became more abnormal in sinuleptolide-treated oral cancer cells (Ca9-22) () than in HGF-1 cells (). At higher concentrations of sinuleptolide (12 and 24 µg/mL), apoptosis-like morphological changes, such as apoptotic bodies and cell shrinkages, were observed in Ca9-22 cells. However, these sinuleptolide-induced apoptosis-like or abnormal morphologies were reduced by NAC pretreatment.
Figure 2 NAC effect on cell morphology of sinuleptolide-treated oral cancer and oral normal cells.
Abbreviation: NAC, N-acetylcysteine.
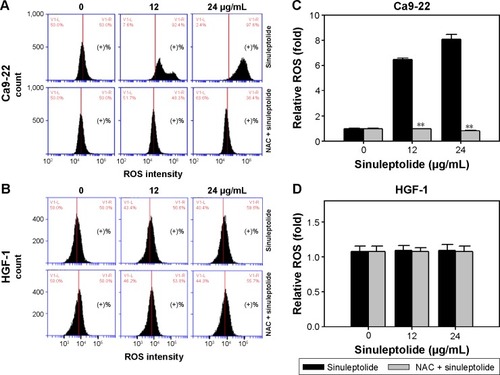
NAC effect on the sinuleptolide-induced ROS generation
shows the relative ROS intensity patterns of sinuleptolide-induced ROS generation of Ca9-22 and HGF-1 cells with or without NAC pretreatment. At higher concentrations of sinuleptolide (12 and 24 µg/mL) (), the ROS generation of Ca9-22 cells was upregulated, which was consistent with the results of our previous study.Citation18 After NAC pretreatment, the sinuleptolide-induced ROS generation of Ca9-22 cells was significantly reduced by NAC pretreatment (P<0.002) (). In contrast, the ROS generation of HGF-1 cells was maintained at a basal level with or without NAC pretreatment ().
Figure 3 NAC effect on ROS generation of sinuleptolide-treated oral cancer cells and oral normal cells.
Abbreviations: NAC, N-acetylcysteine; ROS, reactive oxygen species.
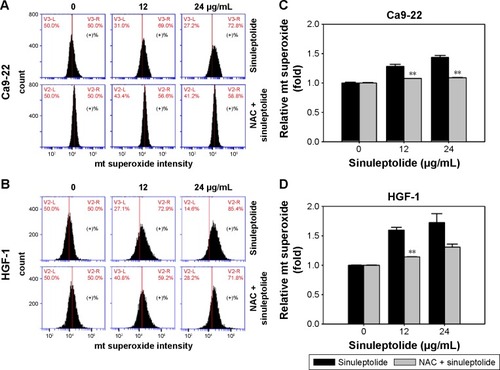
NAC effect on the generation of sinuleptolide-induced mitochondrial superoxide
Mitochondria-specific ROS staining dye (MitoSOX Red) was used to evaluate mitochondrial superoxide by flow cytometry. shows the relative mitochondrial superoxide intensity patterns of NAC pretreatment effects against sinuleptolide-treated oral cancer and normal cells. Higher concentrations of sinuleptolide (12 and 24 µg/mL) () induced the mitochondrial superoxide generation of Ca9-22 and HGF-1 cells. NAC pretreatment significantly reduced the sinuleptolide-induced mitochondrial superoxide generation of Ca9-22 and HGF-1 cells (P<0.002).
Figure 4 NAC effect on mitochondrial (mt) superoxide generation of sinuleptolide-treated oral cancer cells.
Abbreviation: NAC, N-acetylcysteine.
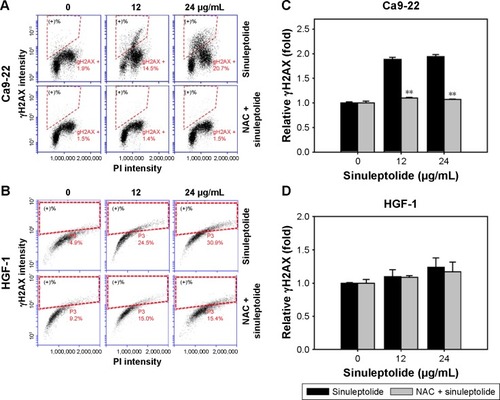
NAC effect on sinuleptolide-induced γH2AX/PI-based DNA damage
shows the relative γH2AX intensity patterns of sinuleptolide-induced DNA damage in Ca9-22 and HGF-1 cells with or without NAC pretreatment. The higher concentrations of sinuleptolide (12 and 24 µg/mL) () dramatically induced the γH2AX expression of Ca9-22 cells, which was consistent with our previous study.Citation18 After NAC pretreatment, the sinuleptolide-induced γH2AX expression in Ca9-22 cells was significantly reduced by NAC pretreatment (P<0.002). In contrast, sinuleptolide-induced γH2AX expression of HGF-1 cells was maintained at a basal level with or without NAC pretreatment ().
Figure 5 NAC effect on γH2AX-based DNA damage in sinuleptolide-treated oral cancer and oral normal cells.
Abbreviations: NAC, N-acetylcysteine; PI, propidium iodide.
Discussion
We could show here that NAC pretreatment inhibited sinuleptolide-induced cell killing, apoptosis-like morphology, and apoptosis of oral cancer cells. This indicated the role of oxidative stress in the cytotoxicity provided by sinuleptolide for oral cancer cells. Moreover, oxidative stress is involved in early apoptosisCitation31 and mitochondrial dysfunction.Citation32–Citation34 NAC can interact with ROS, such as hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid.Citation35 Consistently, we also found that NAC pretreatment inhibited sinuleptolide-induced ROS and the generation of mitochondrial superoxide. However, NAC is known to show other properties as well. For example, NAC also has anti-inflammatory effects.Citation36–Citation38 Since our study lacks experiments to exclude this possibility, sinuleptolide-induced cytotoxicity of oral cancer cells may also include other than an “ROS-dependent” mechanism.
DCFH-DA is used to detect intracellular ROS such as hydrogen peroxide, but does not specifically detect mitochondrial ROS. In contrast, MitoSOX Red dye has been reported to selectively detect superoxide in the mitochondria of live cells rather than other ROS.Citation39 The role of mitochondrial superoxide was first reported as being involved in sinuleptolide-induced cytotoxicity in the present study. However, sinuleptolide induces cell death in both kinds of cells with different IC50 values (Ca9-22=11.76 and HGF-1=22.3 µg/mL). The reason for some differences only observed with ROS and DNA damage in sinuleptolide-treated cells remains unclear as yet. One possibility is that mitochondrial and cytoplasmic ROS have diverse functions. For example, accumulating evidence suggests that mitochondrial ROS are important for normal cell functioning.Citation40 Mitochondrial and cytoplasmic ROS may play opposing effects throughout the life cycle. For Caenorhabditis elegans, the increase of mitochondrial ROS increases the lifespan of this invertebrate, whereas the increase of cytoplasm ROS decreases its lifespan.Citation41 However, the detailed function of mitochondrial and cytoplasmic ROS in sinuleptolide-induced cytotoxicity against cancer cells warrants further investigation.
p53 is reported to highly regulate redox homeostasis and to modulate several ROS-regulating genes.Citation42 In contrast, ROS can modify p53 conformation to adjust the transcription of p53.Citation42 In the current study, ROS was validated to play an important role in sinuleptolide-induced cell death of oral cancer cells. However, the role of p53 in sinuleptolide-treated cells was not investigated in the current study. Furthermore, the status of p53 in Ca9-22 cells is a mutant form,Citation43,Citation44 but it is wild-type (wt) in the HGF-1 cells. Cells with mutant or wt p53 may display different responses. For example, hyperthermia induced apoptosis in oral squamous cell carcinoma (OSCC) cells (wt p53) and decreased IL-12 expression, but it increased IL-12Rβ1 in OSCC (mutated p53).Citation45 Accordingly, the role of p53 in sinuleptolide-induced antiproliferation and DNA damage effects of oral cancer cells warrants further investigation in the future.
Mitochondria are commonly assumed to have a tubular form in healthy cells, but donut or blob forms increased with mitochondrial superoxide,Citation46 suggesting that mitochondrial shape may change at different conditions of mitochondrial ROS generation. The mitochondrial superoxide intensity increased upon oxidative stress with inhibitors of the mitochondrial complex I (rotenone) and mitochondrial complex II (antimycin).Citation46 NAC pretreatment has been reported to reduce mitochondrial superoxide levels and mitochondrial donut or blob formations.Citation46 This suggests further investigation of sinuleptolide-induced mitochondrial shape change.
Moreover, oxidative stress commonly induces DNA damage,Citation47 but we found that sinuleptolide-induced DNA damage was reduced by NAC pretreatment, suggesting that oxidative stress plays an important role in the DNA damaging effect of sinuleptolide in oral cancer cells. In addition, the sinuleptolide-induced DNA damage effect may have led to apoptosis in our previous study.Citation18 Our preliminary result also found that sinuleptolide-induced apoptosis may be reduced by NAC pretreatment as indicated by the cleaved Poly (ADP-ribose) polymerase (PARP) assay (data not shown).
Some oxidative stress modulating drugs have been reported to modulate the endoplasmic reticulum (ER) stress provided by oxidative stress.Citation48,Citation49 ROS has also been reported to induce autophagy when exposed to nonmarine drugs and marine drugs.Citation50 Because sinuleptolide-induced killing of oral cancer cells was shown to depend on oxidative stress, the possible responses of ER stress and autophagy in sinuleptolide-treated oral cancer cells warrant further investigation. Moreover, NAC was reported to inactivate c-Jun N-terminal kinase, p38 mitogen-activated protein kinase, activating protein-1, and nuclear factor kappa B.Citation51 These kinase signaling proteins need to be further investigated in terms of the sinuleptolide mediation in the future.
Conclusion
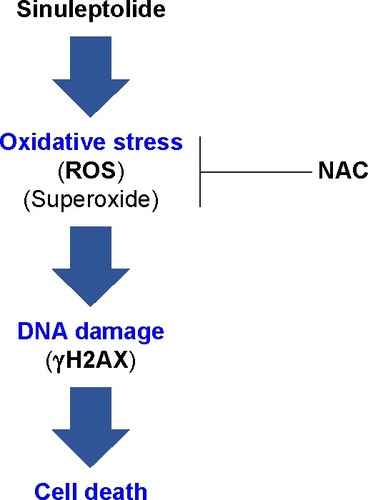
In conclusion, we demonstrated that sinuleptolide induces cell killing, apoptosis, and DNA damage in oral cancer cells by allowing for relatively high cellular ROS levels. This effect was significantly reduced by NAC pretreatment that allows ROS scavengers to reduce the actual ROS content (). This suggests that the marine bioactive compound sinuleptolide kills oral cancer cells by mediating oxidative stress.
Figure 6 Overview of the hypothesized mechanism of sinuleptolide-induced killing of human oral cancer cells (Ca9-22) involving oxidative stress.
Abbreviations: NAC, N-acetylcysteine; ROS, reactive oxygen species.
Acknowledgments
This work was supported by funds of the Ministry of Science and Technology (MOST 104-2320-B-037-013-MY3, MOST 104-2320-B-110-001-MY2, and MOST 105-2314-B-037-036), the National Sun Yat-sen University-KMU Joint Research Project (#NSYSU-KMU 106-p001), the Kaohsiung Municipal Ta-Tung Hospital (KMUH105-5R61), and the Health and welfare surcharge of tobacco products, the Ministry of Health and Welfare, Taiwan, Republic of China (MOHW105-TDU-B-212-134007). The authors thank Dr Hans-Uwe Dahms for help with English editing.
Disclosure
The authors report no conflicts of interest in this work.
References
- WarnakulasuriyaSGlobal epidemiology of oral and oropharyngeal cancerOral Oncol2009454–530931618804401
- PetersenPEOral cancer prevention and control – the approach of the World Health OrganizationOral Oncol2009454–545446018804412
- HagerSAckermannCJJoergerMGillessenSOmlinAAnti-tumour activity of platinum compounds in advanced prostate cancer-a systematic literature reviewAnn Oncol201627697598427052650
- Sithranga BoopathyNKathiresanKAnticancer drugs from marine flora: an overviewJ Oncol2010201021418621461373
- LeeJCHouMFHuangHWMarine algal natural products with anti-oxidative, anti-inflammatory, and anti-cancer propertiesCancer Cell Int20131315523724847
- KimJKKangKAPiaoMJGeneration of reactive oxygen species and endoplasmic reticulum stress by Dictyopteris undulata extract leads to apoptosis in human melanoma cellsJ Environ Pathol Toxicol Oncol201534319120026349602
- HanYCuiZLiYHHsuWHLeeBHIn vitro and in vivo anticancer activity of pardaxin against proliferation and growth of oral squamous cell carcinomaMar Drugs20161412
- YehCCYangJILeeJCAnti-proliferative effect of methanolic extract of Gracilaria tenuistipitata on oral cancer cells involves apop-tosis, DNA damage, and oxidative stressBMC Complement Altern Med201212114222937998
- ShinYSChaHYLeeBSAnti-cancer effect of luminacin, a marine microbial extract, in head and neck squamous cell carcinoma progression via autophagic cell deathCancer Res Treat201648273875226511816
- YenWHHuLCSuJHNorcembranoidal diterpenes from a For-mosan soft coral Sinularia spMolecules20121712140581406623187289
- LinYYLinSCFengCWAnti-inflammatory and analgesic effects of the marine-derived compound excavatolide B isolated from the culture-type Formosan Gorgonian Briareum excavatumMar Drugs20151352559257925923315
- el SayedKAHamannMTA new norcembranoid dimer from the red sea soft coral Sinularia gardineriJ Nat Prod19965976876898759167
- TakakiHKoganemaruRIwakawaYHiguchiRMiyamotoTInhibitory effect of norditerpenes on LPS-induced TNF-alpha production from the Okinawan soft coral, Sinularia spBiol Pharm Bull200326338038212612453
- LiangCHWangGHChouTH5-epi-Sinuleptolide induces cell cycle arrest and apoptosis through tumor necrosis factor/mitochondria-mediated caspase signaling pathway in human skin cancer cellsBiochim Biophys Acta2012182071149115722348919
- ShojiNUmeyamaAAriharaSA novel norditerpenoid from the Okinawan soft coral Sinularia spJ Nat Prod199356916511653
- AhmedAFShiueRTWangGHDaiCFKuoYHSheuJHFive novel norcembranoids from Sinularia leptoclados and S. parvaTetrahedron200359873377344
- TsengYJAhmedAFDaiCFChiangMYSheuJHSinulochmodins A-C, three novel terpenoids from the soft coral Sinularia lochmodesOrg Lett20057173813381616092882
- ChangYTHuangCYLiKTSinuleptolide inhibits proliferation of oral cancer Ca9–22 cells involving apoptosis, oxidative stress, and DNA damageArch Oral Biol20166614715426954095
- GibsonKRNeilsonILBarrettFEvaluation of the antioxidant properties of N-acetylcysteine in human platelets: prerequisite for bioconversion to glutathione for antioxidant and antiplatelet activityJ Cardiovasc Pharmacol200954431932619668088
- ChangHSTangJYYenCYAntiproliferation of Cryptocarya concinna-derived cryptocaryone against oral cancer cells involving apoptosis, oxidative stress, and DNA damageBMC Complement Altern Med20161619426955958
- ChenCYYenCYWangHRTenuifolide B from Cinnamomum tenuifolium stem selectively inhibits proliferation of oral cancer cells via apoptosis, ROS generation, mitochondrial depolarization, and DNA damageToxins (Basel)2016811319
- ShuCWChangHTWuCSRelA-mediated BECN1 expression is required for reactive oxygen species-induced autophagy in oral cancer cells exposed to low-power laser irradiationPLoS One2016119e016058627632526
- WuCHBaiLYTsaiMHPharmacological exploitation of the phenothiazine antipsychotics to develop novel antitumor agents-A drug repurposing strategySci Rep201662754027277973
- ChiuCCHaungJWChangFRGolden berry-derived 4beta-hydroxywithanolide E for selectively killing oral cancer cells by generating ROS, DNA damage, and apoptotic pathwaysPLoS One201385e6473923705007
- YenYHFarooqiAALiKTMethanolic extracts of Solieria robusta inhibits proliferation of oral cancer Ca9–22 cells via apoptosis and oxidative stressMolecules20141911187211873225405289
- ChanWHWuHJHsuuwYDCurcumin inhibits ROS formation and apoptosis in methylglyoxal-treated human hepatoma G2 cellsAnn N Y Acad Sci2005104237237815965083
- CarterWONarayananPKRobinsonJPIntracellular hydrogen peroxide and superoxide anion detection in endothelial cellsJ Leukoc Biol19945522532588301222
- MukhopadhyayPRajeshMYoshihiroKHaskoGPacherPSimple quantitative detection of mitochondrial superoxide production in live cellsBiochem Biophys Res Commun2007358120320817475217
- HseuYCLeeMSWuCRThe chalcone flavokawain B induces G2/M cell-cycle arrest and apoptosis in human oral carcinoma HSC-3 cells through the intracellular ROS generation and downregulation of the Akt/p38 MAPK signaling pathwayJ Agric Food Chem20126092385239722324429
- ShihHCEl-ShazlyMJuanYSCracking the cytotoxicity code: apoptotic induction of 10-acetylirciformonin B is mediated through ROS generation and mitochondrial dysfunctionMar Drugs20141253072309024857964
- Samhan-AriasAKMartin-RomeroFJGutierrez-MerinoCKaempferol blocks oxidative stress in cerebellar granule cells and reveals a key role for reactive oxygen species production at the plasma membrane in the commitment to apoptosisFree Radic Biol Med2004371486115183194
- OhSHLimSCA rapid and transient ROS generation by cadmium triggers apoptosis via caspase-dependent pathway in HepG2 cells and this is inhibited through N-acetylcysteine-mediated catalase upregulationToxicol Appl Pharmacol2006212321222316169029
- LiJJTangQLiYRole of oxidative stress in the apoptosis of hepatocellular carcinoma induced by combination of arsenic trioxide and ascorbic acidActa Pharmacol Sin20062781078108416867262
- EhlersRAHernandezABloemendalLSEthridgeRTFarrowBEversBMMitochondrial DNA damage and altered membrane potential (delta psi) in pancreatic acinar cells induced by reactive oxygen speciesSurgery1999126214815510455877
- AruomaOIHalliwellBHoeyBMButlerJThe antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acidFree Radic Biol Med1989665935972546864
- AtalayFOdabasogluFHaliciMN-acetyl cysteine has both gastro-protective and anti-inflammatory effects in experimental rat models: its gastro-protective effect is related to its in vivo and in vitro antioxidant propertiesJ Cell Biochem2016117230831925941092
- LasramMMLamineAJDhouibIBAntioxidant and anti-inflammatory effects of N-acetylcysteine against malathion-induced liver damages and immunotoxicity in ratsLife Sci20141071–2505824810974
- SadowskaAMManuelYKBDe BackerWAAntioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: discordant in vitro and in vivo dose-effects: a reviewPulm Pharmacol Ther200720192216458553
- MukhopadhyayPRajeshMHaskoGHawkinsBJMadeshMPacherPSimultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopyNat Protoc2007292295230117853886
- SenaLAChandelNSPhysiological roles of mitochondrial reactive oxygen speciesMol Cell201248215816723102266
- SchaarCEDuesDJSpielbauerKKMitochondrial and cytoplasmic ROS have opposing effects on lifespanPLoS Genet2015112e100497225671321
- HeZSimonHUA novel link between p53 and ROSCell Cycle201312220120223287470
- ImaiYOhnishiKYasumotoJGlycerol enhances radiosensitivity in a human oral squamous cell carcinoma cell line (Ca9–22) bearing a mutant p53 gene via Bax-mediated induction of apoptosisOral Oncol200541663163615975526
- KanedaYShimamotoHMatsumuraKRole of caspase 8 as a determinant in chemosensitivity of p53-mutated head and neck squamous cell carcinoma cell linesJ Med Dent Sci2006531576616722146
- YasumotoJKiritaTTakahashiAApoptosis-related gene expression after hyperthermia in human tongue squamous cell carcinoma cells harboring wild-type or mutated-type p53Cancer Lett20042041415114744533
- AhmadTAggarwalKPattnaikBComputational classification of mitochondrial shapes reflects stress and redox stateCell Death Dis20134e46123328668
- OrreniusSMechanisms of Oxidative Cell DamageBasel, SwitzerlandBirkhäuser Verlag1993
- FarooqiAALiKTFayyazSAnticancer drugs for the modulation of endoplasmic reticulum stress and oxidative stressTumour Biol20153685743575226188905
- KimJKKangKARyuYSInduction of endoplasmic reticulum stress via reactive oxygen species mediated by luteolin in melanoma cellsAnticancer Res20163652281228927127134
- FarooqiAAFayyazSHouMFLiKTTangJYChangHWReactive oxygen species and autophagy modulation in non-marine drugs and marine drugsMar Drugs201412115408542425402829
- ZafarullahMLiWQSylvesterJAhmadMMolecular mechanisms of N-acetylcysteine actionsCell Mol Life Sci200360162012613655
