Abstract
Background
Manifold data have demonstrated that the addition of bevacizumab to chemotherapy improved progression-free survival (PFS), while few trials have revealed its significant overall survival (OS) benefit. Furthermore, it still remains suspended how to maximize the benefits of bevacizumab as first-line therapy for human epidermal growth factor receptor 2 (HER2)-negative breast cancer. We sought to conduct a meta-analysis to assess the benefits of bevacizumab with chemotherapy and to identify the ideal chemotherapy partner of bevacizumab in the first-line setting for HER2-negative advanced breast cancer patients.
Methods
Computerized and manual searches were performed to identify randomized clinical trials evaluating the efficacy of bevacizumab plus chemotherapy versus chemotherapy alone or bevacizumab with different chemotherapy regimens as first-line therapy for HER2-negative locally recurrent or metastatic breast cancer patients. Risk ratios or odds ratios with their 95% CIs were used to estimate the association between multiple combinations of bevacizumab with chemotherapy and various clinical outcomes.
Results
With 7 trials identified, this analysis included 3,984 eligible patients. The addition of bevacizumab to chemotherapy resulted in a statistically significant improvement in PFS (P=0.019) and objective response rate (ORR; P<0.001) rather than in OS (P=0.783) when compared with chemotherapy alone. The greater benefits in PFS and ORR were achieved from bevacizumab plus taxane-based regimens compared with bevacizumab plus capecitabine-based regimens, while bevacizumab plus capecitabine had comparable OS with bevacizumab plus paclitaxel. Additionally, bevacizumab-based triplet therapy failed to improve the clinical outcomes when compared with doublet therapy.
Conclusion
This meta-analysis reveals that the addition of bevacizumab to chemotherapy yielded PFS and ORR benefits in HER2-negative advanced breast cancer. Additional studies are still prompted to further optimize the first-line treatment of bevacizumab.
Introduction
Breast cancer is the most common cancer that affects women worldwide. Although its prognosis has been greatly improved due to the rapid development of multidisciplinary therapy, breast cancer still remains the second leading cause of cancer-related deaths, the main reasons of which are disease progression and therapeutic resistance.Citation1 Recent investigations have revealed that angiogenesis contributes to the progression of breast cancer, and the overexpression of vascular epithelial growth factor (VEGF) promotes its proliferation and metastasis.Citation2–Citation8
Bevacizumab, a humanized recombinant monoclonal antibody that specifically targets VEGF receptors (VEGFRs), has become a promising agent for the treatment of metastatic breast cancer.Citation9 Indeed, this agent has attracted much spotlight because of several controversial issues. In 2008, the US Food and Drug Administration (FDA) granted accelerated approval to bevacizumab in combination with the paclitaxel as first-line therapy for human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer patients. This rapid approval was based on the positive results from the E2100 trial.Citation10 However, in 2010, the FDA finally decided to revoke the approval of bevacizumab for this indication. On the other hand, the European Medicines Agency reaffirmed its approval of the drug for the same indication.Citation11 Both agencies based their decisions on the same data from the subsequent AVADO and RIBBON-1 trials.Citation12,Citation13 These 2 trials demonstrated that the addition of bevacizumab to chemotherapy improved the progression-free survival (PFS) in HER2-negative metastatic breast cancer patients, while neither of them revealed statistically significant overall survival (OS) benefit. To date, the efficacy of bevacizumab is still hotly debated. Furthermore, there are no clear answers on how to maximize the benefits of bevacizumab as first-line therapy for HER2-negative breast cancer.
Therefore, we conducted a meta-analysis of all eligible studies to address the overall benefits of bevacizumab as first-line therapy in HER2-negative advanced breast cancer. Additionally, we aimed to determine the best combination of bevacizumab and chemotherapy in such setting.
Methods
Search strategy
The keywords “bevacizumab” or “Avastin”, “phase III” or “phase 3” and “breast cancer” were employed to search the PubMed, Web of Science, EMBASE and EBSCO databases from January 2000 to March 2017 for relevant randomized controlled clinical trials published in English. The selection of the included publications was independently performed by 2 experienced authors (Cunfu Li and Aizhai Xiang). The reference lists of textbooks, previous reviews and all retrieved articles were scrutinized to identify additional studies with potential eligibility. Abstracts, conference information, editorials, letters and case reports were excluded.
Eligibility criteria
We evaluated the full article of each relevant study and included 7 phase III randomized controlled trials that evaluated the efficacy of combining bevacizumab with chemotherapy versus chemotherapy alone or combining bevacizumab with different chemotherapy regimens as first-line therapy for HER2-negative locally recurrent or metastatic breast cancer. Phase I, phase II and nonrandomized studies were excluded. Trials were considered ineligible if they recruited patients to assess neoadjuvant or adjuvant bevacizumab or to evaluate bevacizumab with other target therapies. For multiple reports of the same cohort, the most recent publication was chosen for this analysis.
Data extraction
Data were extracted from eligible publications independently by 2 authors (Xianzhi Chen and Kai Yin). Discrepancies were discussed with a third author (Jinsong Lu), and agreements were reached with consensus according to the Quality of Reporting of Meta-Analyses guidelines.Citation14
The following data were extracted from all selected articles: first author, publication year, sample size, number of patients in each arm, bevacizumab and chemotherapy regimens, and either the number of endpoint events in each arm or hazard ratios (HRs) with 95% CIs in each arm.
Statistical analysis
In this meta-analysis, the primary endpoint was PFS, defined as the time from the date of randomization to the date of first documented disease progression or death. The secondary endpoints included OS and objective response rate (ORR), defined as the time from the date of randomization to the date of death from any cause and the percentage of patients who achieved a complete or partial response confirmed ≥28 days after initial documentation of response.
The risk ratio (RR) with its 95% CI was calculated for PFS and OS, while the odds ratio (OR) with 95% CI was calculated for ORR. For the trials with only HRs available, the HRs were used as RRs to facilitate the calculation. The heterogeneity of the study outcomes was calculated using Cochran’s Q statistics (χ2 test) and I2 test. Either a P-value <0.05 or I2>50% was considered as statistically significant heterogeneity, which indicated the use of a random-effects (DerSimonian and Laird method) model. If there was no between-study heterogeneity, a fixed-effects (Mantel–Haenszel method) model was applied.Citation15
Publication bias was evaluated using funnel plots and Begg’s test. Sensitivity analyses were performed to quantify the impact of individual trials on the overall effect. A two-sided P-value <0.05 was considered significant. All statistical analyses were conducted with Stata statistical software package (release 12.0; Stata Corporation, College Station, TX, USA).
Results
Characteristics of eligible trials
Considering the quality of the data, we included all the potentially eligible phase III randomized clinical trials with available information for the target population. Based on the search strategy, 7 eligible trials with a total of 3,984 patients were identified and included in this meta-analysis (Figure S1).Citation12,Citation13,Citation16–Citation20 The details of these included studies are summarized in . Three of these studies were used to evaluate the efficacy of adding bevacizumab to chemotherapy, including 1,558 women who received bevacizumab combined with chemo-therapy and 896 women who were administered chemotherapy alone.Citation12,Citation13,Citation17 The other 4 trials were obtained to assess the optimal chemotherapy partner of bevacizumab.Citation16,Citation18–Citation20 All of them were published as full articles and described as Phase III randomized controlled trials. The former 3 trials were double blinded,Citation12,Citation13,Citation17 and the latter 4 studies were open label.Citation16,Citation18–Citation20 Of note, the E2100 trial was considered ineligible for this meta-analysis despite its inclusion in the previous reports on this topic.Citation21,Citation22 It recruited both HER2-negative and HER-2 positive patients, but the data were only available for the overall population rather than the HER2-negative subgroup. Therefore, the E2100 trial was excluded in this meta-analysis.
Table 1 Characteristics of eligible studies
The AVADO trial was a 3-arm trial, in which HER2-negative locally recurrent or metastatic breast cancer patients were randomized on a 1:1:1 basis to receive bevacizumab 7.5 mg/kg plus docetaxel 100 mg/m2, bevacizumab 15 mg/kg plus docetaxel 100 mg/m2 or placebo plus docetaxel 100 mg/m2 on day 1 every 3 weeks as first-line therapy.Citation12 The evaluation of the 2 bevacizumab arms was separately compared with the control arm.
In the RIBBON-1 trial, HER2-negative locally recur-rent or metastatic breast cancer patients were randomly assigned in the 2:1 ratio to either bevacizumab 15 mg/kg plus chemotherapy or chemotherapy alone. Chemotherapy was chosen by investigators, including capecitabine (1,000 mg/m2 oral twice daily for 14 days), taxane-based (nab-paclitaxel 260 mg/m2, docetaxel 75–100 mg/m2) or anthracycline-based (fluorouracil 500 mg/m2, epirubicin 90–100 mg/m2 and cyclophosphamide 500 mg/m2; fluorouracil 500 mg/m2, doxorubicin 50 mg/m2 and cyclophosphamide 500 mg/m2; doxorubicin 50–60 mg/m2 and cyclophosphamide 500–600 mg/m2; or epirubicin 90–100 mg/m2 and cyclophosphamide 500–600 mg/m2) regimen administered every 3 weeks. All enrolled patients were separately analyzed in the capecitabine cohort and the pooled taxane/anthracycline cohort.Citation13
The MERiDiAN trial randomized locally recurrent or metastatic breast cancer patients to paclitaxel 90 mg/m2 on days 1, 8 and 15 with or without bevacizumab 10 mg/kg on days 1 and 15 every 4 weeks. The co-primary endpoints were investigator-assessed PFS in the intent-to-treat (ITT) and plasma VEGF-Ahigh populations.Citation17
The TABEA study compared taxanes (paclitaxel 80 mg/m2 on days 1, 8 and 15 or docetaxel 75 mg/m2 on day 1 every 3 weeks) plus bevacizumab (15 mg/kg on day 1 every 3 weeks) with (TBX group) or without capecitabine (1,800 mg/m2 daily on days 1–14 every 3 weeks; TB group) as first-line therapy in locally advanced or metastatic breast cancer patients.Citation16 In this meta-analysis, TBX and TB were referred to as capecitabine- and taxane-based regimens, respectively.
As to the SAKK 24/09 study, metastatic or locally recurrent inoperable HER2-negative breast cancer patients were randomized to bevacizumab 10 mg/kg every 2 weeks with either paclitaxel 90 mg/m2 on days 1, 8 and 15 every 4 weeks or cyclophosphamide 50 mg plus capecitabine 3×500 mg daily. Of note, the primary endpoint was the incidence of prespecified grade 3–5 adverse events (AEs), while PFS, OS and ORR were the secondary endpoints.Citation18
The TURANDOT study was a non-inferiority trial that randomly assigned HER2-negative locally recurrent or metastatic breast cancer patients to either bevacizumab 10 mg/kg on days 1 and 15 plus paclitaxel 90 mg/m2 on days 1, 8 and 15 every 4 weeks or bevacizumab 15 mg/kg on day 1 plus capecitabine 1,000 mg/m2 twice daily on days 1–14 every 3 weeks. The primary endpoint was OS, and the secondary endpoints included ORR and PFS. OS was compared in both ITT and per-protocol (PP) populations.Citation20 The OS data in the ITT and PP populations were separately included in the corresponding analysis of our study. On the other hand, the primary objective of this trial was to show non-inferior OS with bevacizumab plus capecitabine versus bevacizumab plus paclitaxel in the PP population by rejecting the null hypothesis of inferiority (HR $1.33) at a one-sided significant level of 0.025 using a stratified Cox proportional hazard model.Citation20 Therefore, the prespecified boundary for HR varied between this trial and the others, which might elicit misleading results. Accordingly, the OS data from this study were also excluded to avoid this confounding effect in this meta-analysis. Herein, there were altogether 3 types of pooled RRs for OS by using different datasets.
The CARIN trial randomized HER2-negative locally recurrent or advanced breast cancer patients to receive capecitabine 1,000 mg/m2 twice daily on days 1–14 plus bevacizumab 15 mg/kg on day 1 every 3 weeks with or without vinorelbine 25 mg/m2 on days 1 and 8 every 3 weeks. Despite its superior nature, the significant level was set at 0.05, two sided.Citation19
Efficacy of adding bevacizumab to chemotherapy
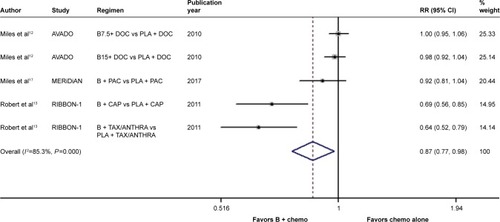
In terms of the comparison between bevacizumab combined with chemotherapy and chemotherapy alone, there was significant between-study heterogeneity in the RR for PFS (heterogeneity χ2, 27.15; I2, 85.3%; P<0.001; ). Through the random-effects model, we found a marked improvement in PFS with the addition of bevacizumab to chemotherapy (RR, 0.869; 95% CI, 0.772–0.977; P=0.019; ). On the other hand, no significant between-study heterogeneity was observed in both RR for OS (heterogeneity χ2, 3.12; I2, 0.0%; P=0.538; Figure S2) and OR for ORR (heterogeneity χ2, 5.03; I2, 20.5%; P=0.284; Figure S3). The fixed-effects model demonstrated that bevacizumab elicited great benefit in ORR (OR, 0.560; 95% CI, 0.475–0.661; P<0.001; Figure S3) rather than in OS (RR, 0.986; 95% CI, 0.891–1.090; P=0.783; Figure S2).
Figure 1 Forest plot of RR for the association between the addition of bevacizumab to chemotherapy and progression-free survival in human epidermal growth factor receptor 2-negative locally recurrent or metastatic breast cancer patients.
Abbreviations: B, bevacizumab; B7.5, bevacizumab 7.5 mg/kg; B15, bevacizumab 15mg/kg; CAP, capecitabine; chemo, chemotherapy; DOC, docetaxel; PAC, paclitaxel; PLA, placebo; TAX/ANTHRA, taxanes/anthracyclines; RR, risk ratio.
Efficacy of bevacizumab plus capecitabine-based chemotherapy compared with bevacizumab plus taxane-based chemotherapy
When it came to the comparison between bevacizumab with capecitabine- and taxane-based chemotherapy, significant between-study heterogeneity failed to be discerned in the RRs for PFS (heterogeneity χ2, 0.85; I2, 0.0%; P=0.654; Figure S4) and OS ([excluding the OS data from the TURANDOT trial: heterogeneity χ2, 0.05; I2, 0.0%; P=0.829; Figure S5]; [including the OS data from the TURANDOT trial in the ITT population: heterogeneity χ2, 0.17; I2, 0.0%; P=0.918; Figure S6]; [including the OS data from the TURANDOT trial in the PP population: heterogeneity χ2, 0.10; I2, 0.0%; P=0.951; Figure S7]) as well as in the OR for ORR (heterogeneity χ2, 1.31; I Citation2, 0.0%; P=0.519; Figure S8). Therefore, the fixed-effects model was used to analyze the data, and a deleterious effect of bevacizumab plus capecitabine-based chemotherapy on PFS (RR, 1.190; 95% CI, 1.103–1.283; P<0.001; Figure S4) and ORR (OR, 1.897; 95% CI, 1.535–2.344; P<0.001; Figure S8) compared with bevacizumab plus taxane-based regimen was found. Intriguingly, absolute gains in ORR and PFS translated into OS benefit when the data of the TURANDOT trial in the PP population were included (RR, 1.108; 95% CI, 1.005–1.221; P=0.040; Figure S7). However, these gains in ORR and PFS were lost no matter whether the data from the TURANDOT trial were excluded (RR, 1.139; 95% CI, 0.880–1.474; P=0.322; Figure S5), or its ITT population data were included (RR, 1.091; 95% CI, 0.992–1.201; P=0.074; Figure S6).
Efficacy of bevacizumab-based doublet therapy compared with bevacizumab-based triplet therapy
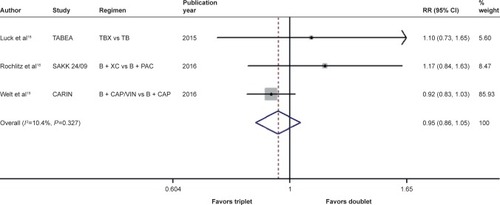
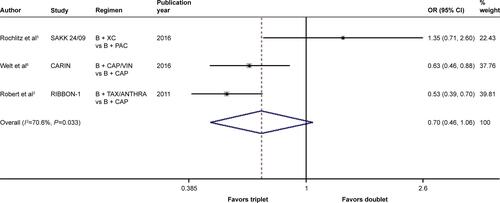
There was significant between-study heterogeneity in both RR for PFS (heterogeneity χ2, 8.43; I2, 76.3%; P=0.015; Figure S9) and OR for ORR (heterogeneity χ2, 6.80; I2, 70.6%; P=0.033; Figure S10) rather than in the RR for OS (heterogeneity χ2, 2.23; I2, 10.4%; P=0.327; ). Through the random-effects model, bevacizumab-based triplet therapy did not significantly improve the PFS (RR, 1.046; 95% CI, 0.856–1.279; P=0.658; Figure S9) or ORR (OR, 0.697; 95% CI, 0.459–1.058; P=0.090; Figure S10) when compared with bevacizumab-based doublet therapy. Similarly, no statistical significance was observed for OS with the random-effects model (RR, 0.951; 95% CI, 0.864–1.048; P=0.311; ).
Figure 2 Forest plot of RR for the association between bevacizumab plus different numbers of chemotherapy agents and overall survival in human epidermal growth factor receptor 2-negative locally recurrent or metastatic breast cancer patients.
Abbreviations: B, bevacizumab; CAP, capecitabine; PAC, paclitaxel; RR, risk ratio; TB, taxane/bevacizumab; TBX, taxane/bevacizumab/capecitabine; VIN, vinorelbine; XC, capecitabine/cyclophosphamide.
Publication bias and sensitivity analysis
To assess the association between the addition of bevacizumab to chemotherapy and various clinical outcomes in HER2-negative locally recurrent or metastatic breast cancer patients, either graphic inspection of funnel plots or quantitative evaluation using Begg’s test indicated the absence of publication bias in OS and ORR but not for PFS (P=0.014). As to the studies evaluating the efficacy of bevacizumab plus different types or different numbers of chemotherapy agents, no publication bias was detected with either graphic inspection of funnel plots or quantitative evaluation of Begg’s test in PFS, OS and ORR (data not shown). The sensitivity analyses clarified for each comparison that no individual study affected the overall RRs for PFS and OS as well as the overall ORs for ORR, because omission of any single study made no material difference.
Discussion
This meta-analysis is, to the best of our knowledge, the largest and latest study focusing on how to optimize the treatment of bevacizumab as first-line therapy in HER2-negative advanced breast cancer patients.
Our findings indicated that bevacizumab plus chemotherapy significantly improved the PFS and ORR, compared with chemotherapy alone. However, no significant advantage in OS was observed, which was similar to the results in each individual trial. Although the OS is regarded as a gold standard of endpoints and PFS as a surrogate endpoint in metastatic breast cancer, debates still continue for the importance of PFS.Citation23–Citation25 Advocates of OS argue that no therapeutic benefit is achieved without improving OS. This is also the reason why the FDA revoked bevacizumab’s approval for metastatic breast cancer. Using OS instead of PFS as a primary endpoint is relatively difficult and expensive in enrolling the patients and completing the study. Furthermore, it is clinically meaningful to get details on whether certain interventions are capable of delaying disease progression and improving quality of life, which is generally presented by PFS. On the other hand, patients in the control arm were admitted to cross over to bevacizumab after progression in some clinical trials,Citation12,Citation13 which might compromise and underestimate the OS benefit of bevacizumab. Additionally, OS is normally confounded by various post-protocol factors such as subsequent treatments.Citation23 By this token, advocates of PFS argue that PFS as a primary endpoint has its own merit and plays an important role in the evaluation of a new therapy for metastatic breast cancer, while it is not suitable for a promising agent to attach too much importance on OS.
Notwithstanding the ongoing arguments, researchers have long expected that substantial OS benefit could be obtained from bevacizumab-based regimens by identifying subgroups with specific biomarkers. A meta-analysis, which focused on the first-line bevacizumab therapy, revealed no significant improvement for OS in the triple-negative subgroup.Citation22 On the other hand, the subsequent retrospective biomarker analysis of the AVADO trial revealed that higher plasma concentration of either VEGF-A or VEGFR-2 was associated with more benefit from bevacizumab irrespective of the bevacizumab dose.Citation26 Unfortunately, the MERiDiAN trial, which was the first study to prospectively evaluate plasma VEGF-A, failed to verify the predictive effect of VEGF-A.Citation17 Furthermore, when the available samples from the AVADO trial were reassessed by using different VEGF assays, the predictive value of plasma VEGF-A disappeared.Citation27 Collectively, there is no solid evidence to support any biomarker as a predictive factor of bevacizumab efficacy.
This meta-analysis also demonstrated that bevacizumab combined with taxane-based chemotherapy greatly improved the PFS and ORR when compared with bevacizumab plus capecitabine-based regimen. Since the OS analysis of the TURANDOT trial was quite different from that of the other eligible trials, we separately pooled the RRs by excluding the data from the TURANDOT trial and including its data in the PP or ITT population. Only when its data in the PP population were included, did the OS comparison between the 2 groups exhibit statistical significance. Nevertheless, it is necessary for us to be aware of the prespecified boundary for HR in the TURANDOT trial. Given this situation, such advantage has to be interpreted with caution. If we take a close look at the OS data of each included trial, it is obvious that no significant OS benefit was verified in all but one of the included randomized phase III studies. The TURANDOT trial was the first study to compare bevacizumab plus paclitaxel and bevacizumab plus capecitabine.Citation20 Before this study, these 2 bevacizumab-containing regimens, compared with chemotherapy alone, had both revealed superior PFS and ORR in the previous reports.Citation10,Citation13,Citation17 Unfortunately, the oncologists had no idea whether patient tolerability could be improved by bevacizumab plus capecitabine without compromising the OS in comparison with bevacizumab plus paclitaxel at that time, so they might have been stuck in a dilemma between these two regimens. The TURANDOT trial was conducted to tackle this dilemma. Its final OS analysis met the primary endpoint of the trial, that is, the criterion for non-inferiority in the stratified analysis of the PP population. This finding was also supported by the stratified OS analysis of the ITT population despite the inferiority of bevacizumab plus capecitabine to bevacizumab plus paclitaxel for PFS.Citation10 Therefore, the TURANDOT trial provides the evidence that bevacizumab with either paclitaxel or capecitabine has comparable OS in the first-line setting for HER2-negative locally recurrent or metastatic breast cancer patients. Besides, the SAKK 24/09 trial also gives further support for the combined effect of capecitabine with bevacizumab. Although the primary endpoint in this trial was the incidence of pre-specified grade 3–5 AEs instead of ORR, PFS and OS, its results might also offer some clues. The SAKK 24/09 trial showed no statistical significance between bevacizumab plus paclitaxel and bevacizumab plus metronomic capecitabine-cyclophosphamide in terms of ORR, PFS and OS.Citation18 Taken together, these findings clarify that the combination of bevacizumab and capecitabine may function as a valid first-line treatment option. In Europe, this regimen, along with bevacizumab plus paclitaxel, has been identified as one of the first-line indications for HER2-negative locally recurrent or metastatic breast cancer patients.Citation28 However, bevacizumab combined with paclitaxel is the only regimen listed in the latest version of the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology for Breast Cancer.Citation29 Furthermore, Delaloge et al demonstrated on the basis of a large-scale real-life setting database that HER2-negative metastatic breast cancer patients who received paclitaxel plus bevacizumab as first-line chemotherapy had a significantly better OS and PFS than those receiving paclitaxel alone.Citation30 With the accumulation of evidence, we will get a clearer picture of how to choose the preferable chemotherapy partner of bevacizumab.
Last but not the least, when it came to the comparison between the doublet versus the triplet, the addition of a third agent appears to make no sense in the improvement of clinical outcomes. Similar findings have also been reported. The BCIRG 007 trial is a phase III randomized study conducted to compare docetaxel/carboplatin/trastuzumab (TCH) with docetaxel/trastuzumab (TH) as first-line therapy in HER2-amplified metastatic breast cancer. Interestingly, adding carboplatin to TH did not elicit any significant improvement in time to progression, response rate or OS.Citation31 In many cases, therefore, less is more.
Conclusion
This meta-analysis indicated that the combination of bevacizumab and chemotherapy as first-line treatment significantly improved the PFS and ORR in locally recurrent or metastatic breast cancer patients. Greater benefits in PFS and ORR were observed in bevacizumab plus taxane-based regimens compared with bevacizumab plus capecitabine-based ones. In view of the non-inferiority for OS, however, increasing evidence supports the use of bevacizumab plus capecitabine as a preferable first-line option in the USA. Nevertheless, it is far from the end of the story. Additional studies are necessary to further optimize the first-line treatment of bevacizumab.
Author contributions
WY and JL conceived and designed the work. CL and AX performed the publication search and the statistical analysis. XC and KY extracted the data. All authors interpreted the results. CL and AX wrote the manuscript draft. All authors participated in the revision. All authors approved the final version.
Acknowledgments
This study was supported by the grants from the National Natural Science Foundation of China (81302302), the Doctoral Programs Foundation of the Ministry of Education of China (20120071120105) and the Shanghai Natural Science Foundation (13ZR1452800).
Supplementary materials
Figure S1 Selection process for randomized controlled clinical trials included in the meta-analysis.
Abbreviation: RCTs, randomized controlled trials.
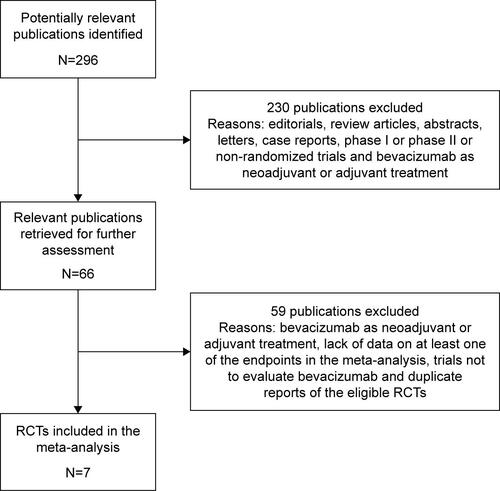
Figure S2 Forest plot of RR for the association between the addition of bevacizumab to chemotherapy and overall survival in human epidermal growth factor receptor 2-negative locally recurrent or metastatic breast cancer patients.
Notes: The size of the square box is proportional to the weight that each study contributes in the meta-analysis. The overall estimate and CI are marked by a diamond. Symbols on the right of the solid line indicate RR >1, and symbols on the left of the solid line indicate RR <1. The combined RR is calculated by the fixed-effects model.
Abbreviations: B, bevacizumab; B7.5, bevacizumab 7.5 mg/kg; B15, bevacizumab 15 mg/kg; CAP, capecitabine; chemo, chemotherapy; DOC, docetaxel; PAC, paclitaxel; PLA, placebo; RR, risk ratio; TAX/ANTHRA, taxanes/anthracyclines.
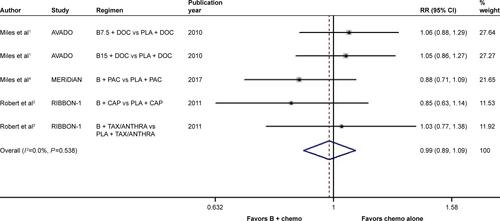
Figure S3 Forest plot of OR for the association between the addition of bevacizumab to chemotherapy and objective response rate in human epidermal growth factor receptor 2-negative locally recurrent or metastatic breast cancer patients.
Notes: The size of the square box is proportional to the weight that each study contributes in the meta-analysis. The overall estimate and CI are marked by a diamond. Symbols on the right of the solid line indicate OR >1, and symbols on the left of the solid line indicate OR <1. The combined OR is calculated by the fixed-effects model.
Abbreviations: B, bevacizumab; B7.5, bevacizumab 7.5 mg/kg; B15, bevacizumab 15 mg/kg; chemo, chemotherapy; CAP, capecitabine; DOC, docetaxel; OR, odds ratio; PAC, paclitaxel; PLA, placebo; TAX/ANTHRA, taxanes/anthracyclines.
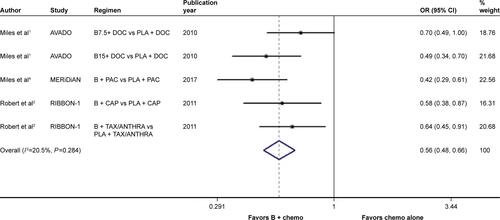
Figure S4 Forest plot of RR for the association between bevacizumab plus different types of chemotherapy agents and progression-free survival in human epidermal growth factor receptor 2-negative locally recurrent or metastatic breast cancer patients.
Notes: The size of the square box is proportional to the weight that each study contributes in the meta-analysis. The overall estimate and CI are marked by a diamond. Symbols on the right of the solid line indicate RR >1, and symbols on the left of the solid line indicate RR <1. The combined RR is calculated by the fixed-effects model.
Abbreviations: B, bevacizumab; CAP, capecitabine; PAC, paclitaxel; RR, risk ratio; TB, taxane/bevacizumab; TBX, taxane/bevacizumab/capecitabine; XC, capecitabine/cyclophosphamide.
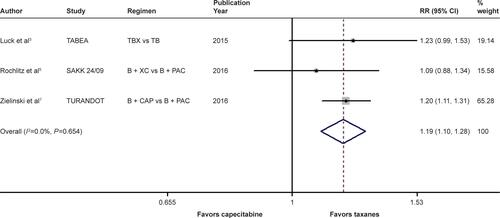
Figure S5 Forest plot of RR for the association between bevacizumab plus different types of chemotherapy agents and overall survival in human epidermal growth factor receptor 2-negative locally recurrent or metastatic breast cancer patients, excluding the data of the TURANDOT trial.
Notes: The size of the square box is proportional to the weight that each study contributes in the meta-analysis. The overall estimate and CI are marked by a diamond. Symbols on the right of the solid line indicate RR >1, and symbols on the left of the solid line indicate RR <1. The combined RR is calculated by the fixed-effects model.
Abbreviations: B, bevacizumab; PAC, paclitaxel; RR, risk ratio; TB, taxane/bevacizumab; TBX, taxane/bevacizumab/capecitabine; XC, capecitabine/cyclophosphamide.
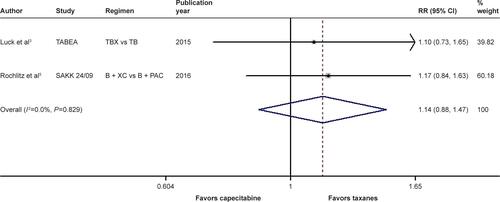
Figure S6 Forest plot of RR for the association between bevacizumab plus different types of chemotherapy agents and overall survival in human epidermal growth factor receptor 2-negative locally recurrent or metastatic breast cancer patients, including the data of the TURANDOT trial in the intent-to-treat population.
Notes: The size of the square box is proportional to the weight that each study contributes in the meta-analysis. The overall estimate and CI are marked by a diamond. Symbols on the right of the solid line indicate RR >1, and symbols on the left of the solid line indicate RR <1. The combined RR is calculated by the fixed-effects model.
Abbreviations: B, bevacizumab; CAP, capecitabine; PAC, paclitaxel; RR, risk ratio; TB, taxane/bevacizumab; TBX, taxane/bevacizumab/capecitabine; XC, capecitabine/cyclophosphamide.
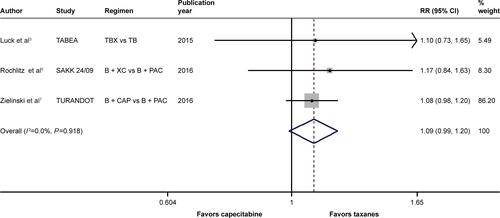
Figure S7 Forest plot of RR for the association between bevacizumab plus different types of chemotherapy agents and overall survival in human epidermal growth factor receptor 2-negative locally recurrent or metastatic breast cancer patients, including the data of the TURANDOT trial in the per-protocol population.
Notes: The size of the square box is proportional to the weight that each study contributes in the meta-analysis. The overall estimate and CI are marked by a diamond. Symbols on the right of the solid line indicate RR >1, and symbols on the left of the solid line indicate RR <1. The combined RR is calculated by the fixed-effects model.
Abbreviations: B, bevacizumab; CAP, capecitabine; PAC, paclitaxel; RR, risk ratio; TB, taxane/bevacizumab; TBX, taxane/bevacizumab/capecitabine; XC, capecitabine/cyclophosphamide.
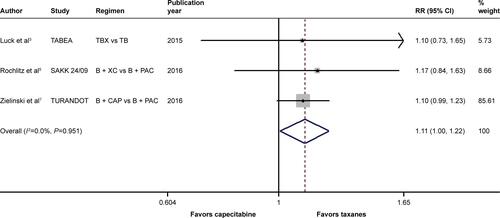
Figure S8 Forest plot of OR for the association between bevacizumab plus different types of chemotherapy agents and objective response rate in human epidermal growth factor receptor 2-negative locally recurrent or metastatic breast cancer patients.
Notes: The size of the square box is proportional to the weight that each study contributes in the meta-analysis. The overall estimate and CI are marked by a diamond. Symbols on the right of the solid line indicate OR >1, and symbols on the left of the solid line indicate OR <1. The combined OR is calculated by the fixed-effects model.
Abbreviations: B, bevacizumab; CAP, capecitabine; OR, odds ratio; PAC, paclitaxel; TAX/ANTHRA, taxanes/anthracyclines; XC, capecitabine/cyclophosphamide.
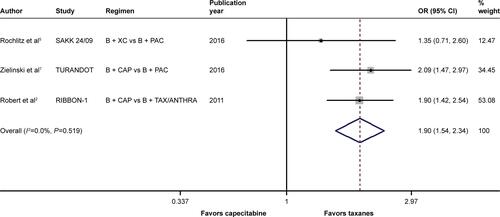
Figure S9 Forest plot of RR for the association between bevacizumab plus different numbers of chemotherapy agents and progression-free survival in human epidermal growth factor receptor 2-negative locally recurrent or metastatic breast cancer patients.
Notes: The size of the square box is proportional to the weight that each study contributes in the meta-analysis. The overall estimate and CI are marked by a diamond. Symbols on the right of the solid line indicate RR >1, and symbols on the left of the solid line indicate RR <1. The combined RR is calculated by the random-effects model. Weights are from the random-effects analysis.
Abbreviations: B, bevacizumab; CAP, capecitabine; PAC, paclitaxel; RR, risk ratio; TB, taxane/bevacizumab; TBX, taxane/bevacizumab/capecitabine; VIN, vinorelbine; XC, capecitabine/cyclophosphamide.
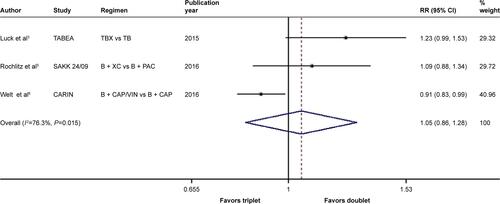
Figure S10 Forest plot of OR for the association between bevacizumab plus different numbers of chemotherapy agents and objective response rate in human epidermal growth factor receptor 2-negative locally recurrent or metastatic breast cancer patients.
Notes: The size of the square box is proportional to the weight that each study contributes in the meta-analysis. The overall estimate and CI are marked by a diamond. Symbols on the right of the solid line indicate OR >1, and symbols on the left of the solid line indicate OR <1. The combined OR is calculated by the random-effects model. Weights are from the random-effects analysis.
Abbreviations: B, bevacizumab; CAP, capecitabine; OR, odds ratio; PAC, paclitaxel; VIN, vinorelbine; TAX/ANTHRA, taxanes/anthracyclines; XC, capecitabine/cyclophosphamide.
References
- MilesDWChanADirixLYPhase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancerJ Clin Oncol2010283239324720498403
- RobertNJDierasVGlaspyJRIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancerJ Clin Oncol2011291252126021383283
- LuckHJLubbeKReinischMPhase III study on efficacy of taxanes plus bevacizumab with or without capecitabine as first-line chemotherapy in metastatic breast cancerBreast Cancer Res Treat201514914114925519041
- MilesDCameronDBondarenkoIBevacizumab plus paclitaxel versus placebo plus paclitaxel as first-line therapy for HER2-negative metastatic breast cancer (MERiDiAN): a double-blind placebo-controlled randomised phase III trial with prospective biomarker evaluationEur J Cancer20177014615527817944
- RochlitzCBiglerMvon MoosRSAKK 24/09: safety and tolerability of bevacizumab plus paclitaxel versus bevacizumab plus metronomic cyclophosphamide and capecitabine as first-line therapy in patients with HER2-negative advanced stage breast cancer – a multicenter, randomized phase III trialBMC Cancer20161678027724870
- WeltAMarschnerNLerchenmuellerCCapecitabine and bevacizumab with or without vinorelbine in first-line treatment of HER2/neu-negative metastatic or locally advanced breast cancer: final efficacy and safety data of the randomised, open-label superiority phase 3 CARIN trialBreast Cancer Res Treat20161569710726927446
- ZielinskiCLangIInbarMBevacizumab plus paclitaxel versus bevacizumab plus capecitabine as first-line treatment for HER2-negative metastatic breast cancer (TURANDOT): primary endpoint results of a randomised, open-label, non-inferiority, phase 3 trialLancet Oncol2016171230123927501767
Disclosure
The authors report no conflicts of interest in this work.
References
- AlvarezRHPresent and future evolution of advanced breast cancer therapyBreast Cancer Res201012Suppl 2S1
- LinderholmBGrankvistKWilkingNJohanssonMTavelinBHenrikssonRCorrelation of vascular endothelial growth factor content with recurrences, survival, and first relapse site in primary node-positive breast carcinoma after adjuvant treatmentJ Clin Oncol20001871423143110735889
- McLeskeySWTobiasCAVezzaPRFilieACKernFGHanfeltJTumor growth of FGF or VEGF transfected MCF-7 breast carcinoma cells correlates with density of specific microvessels independent of the transfected angiogenic factorAm J Pathol19981536199320069846989
- UzzanBNicolasPCucheratMPerretGYMicrovessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysisCancer Res20046492941295515126324
- WeidnerNFolkmanJPozzaFTumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinomaJ Natl Cancer Inst19928424187518871281237
- BonapaceLCoissieuxMMWyckoffJCessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesisNature2014515752513013325337873
- Sossey-AlaouiKPluskotaEDavuluriGKindlin-3 enhances breast cancer progression and metastasis by activating Twist-mediated angiogenesisFASEB J20142852260227124469992
- KongWHeLRichardsEJUpregulation of miRNA-155 promotes tumour angiogenesis by targeting VHL and is associated with poor prognosis and triple-negative breast cancerOncogene201433667968923353819
- ScottLJBevacizumab: in first-line treatment of metastatic breast cancerDrugs200767121793179917683175
- MillerKWangMGralowJPaclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancerN Engl J Med2007357262666267618160686
- European Medicines Agency: European Medicines Agency completes its review of Avastin used in breast cancer [Press release 16/12/2010]2010
- MilesDWChanADirixLYPhase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancerJ Clin Oncol201028203239324720498403
- RobertNJDierasVGlaspyJRIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancerJ Clin Oncol201129101252126021383283
- MoherDCookDJEastwoodSImproving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analysesLancet199935491931896190010584742
- HigginsJPThompsonSGQuantifying heterogeneity in a meta-analysisStat Med200221111539155812111919
- LuckHJLubbeKReinischMPhase III study on efficacy of taxanes plus bevacizumab with or without capecitabine as first-line chemotherapy in metastatic breast cancerBreast Cancer Res Treat2015149114114925519041
- MilesDCameronDBondarenkoIBevacizumab plus paclitaxel versus placebo plus paclitaxel as first-line therapy for HER2-negative metastatic breast cancer (MERiDiAN): a double-blind placebo-controlled randomised phase III trial with prospective biomarker evaluationEur J Cancer20177014615527817944
- RochlitzCBiglerMvon MoosRSAKK 24/09: safety and tolerability of bevacizumab plus paclitaxel versus bevacizumab plus metronomic cyclophosphamide and capecitabine as first-line therapy in patients with HER2-negative advanced stage breast cancer – a multicenter, randomized phase III trialBMC Cancer20161678027724870
- WeltAMarschnerNLerchenmuellerCCapecitabine and bevacizumab with or without vinorelbine in first-line treatment of HER2/neu-negative metastatic or locally advanced breast cancer: final efficacy and safety data of the randomised, open-label superiority phase 3 CARIN trialBreast Cancer Res Treat201615619710726927446
- ZielinskiCLangIInbarMBevacizumab plus paclitaxel versus bevacizumab plus capecitabine as first-line treatment for HER2-negative metastatic breast cancer (TURANDOT): primary endpoint results of a randomised, open-label, non-inferiority, phase 3 trialLancet Oncol201617191230123927501767
- FangYQuXChengBThe efficacy and safety of bevacizumab combined with chemotherapy in treatment of HER2-negative metastatic breast cancer: a meta-analysis based on published phase III trialsTumour Biol20153631933194125387808
- MilesDWDierasVCortesJDuenneAAYiJO’ShaughnessyJFirst-line bevacizumab in combination with chemotherapy for HER2-negative metastatic breast cancer: pooled and subgroup analyses of data from 2447 patientsAnn Oncol201324112773278023894038
- RobertNJOverall survival in metastatic breast cancer: always our quest but not necessarily a primary endpointBreast Cancer Res Treat2010122191020495865
- SharmaSPAvastin saga reveals debate over clinical trial endpointsJ Natl Cancer Inst20121041180080122673590
- RaphaelJVermaSOverall survival (OS) endpoint: an incomplete evaluation of metastatic breast cancer (MBC) treatment outcomeBreast Cancer Res Treat2015150347347825783185
- MilesDWde HaasSLDirixLYBiomarker results from the AVADO phase 3 trial of first-line bevacizumab plus docetaxel for HER2-negative metastatic breast cancerBr J Cancer201310851052106023422754
- BaisCRabeCWildNComprehensive reassessment of plasma VEGFA (pVEGFA) as a candidate predictive biomarker for bevacizumab (Bv) in 13 pivotal trials (seven indications)J Clin Oncol201432suppl 153040
- European Medicines Agency: Assessment Report For Avastin (bevacizumab)2011
- NCC NetworkNational Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for Breast cancer (Version 2. 2017)2017
- DelalogeSPérolDBrainEOverall survival of patients with HER2-negative metastatic breast cancer treated with a first-line paclitaxel with or without bevacizumab in real-life setting: results of a multicenter national observational study. 2016 ASCO Annual Meeting. McCormick Place, Chicago, IllinoisJ Clin Oncol201634Suppl abstr 1013
- ValeroVForbesJPegramMDMulticenter phase III randomized trial comparing docetaxel and trastuzumab with docetaxel, carboplatin, and trastuzumab as first-line chemotherapy for patients with HER2-gene-amplified metastatic breast cancer (BCIRG 007 study): two highly active therapeutic regimensJ Clin Oncol20112914915621115860
