Abstract
Background
Currently, precise predictors in gastric cancer patients undergoing neoadjuvant chemotherapy are lacking. The study aims to investigate the prognostic value of the monocyte to lymphocyte ratio (MLR) in patients with advanced gastric cancer receiving S-1 plus oxaliplatin (SOX) or oxaliplatin and capecitabine (XELOX) neoadjuvant chemotherapy regimen.
Methods
The data from Harbin Medical University Cancer Hospital from August 2008 to September 2015 were retrospectively collected. Ninety-one patients with advanced gastric cancer treated with neoadjuvant chemotherapy were included. The blood samples were collected before neoadjuvant chemotherapy. The MLR was divided into two groups: Low-MLR <0.27 group and high-MLR ≥0.27 group. Survival curves were performed using the Kaplan–Meier method and compared using the log-rank test. Univariate and multivariate Cox proportional hazards regression model were evaluated to determine independent prognostic factors.
Results
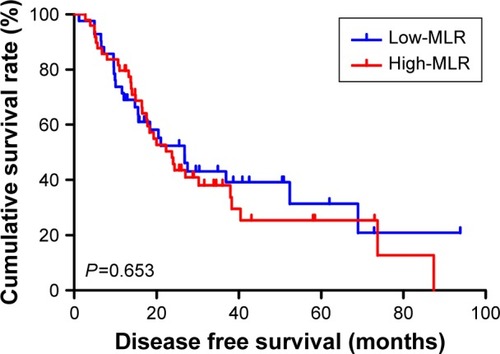
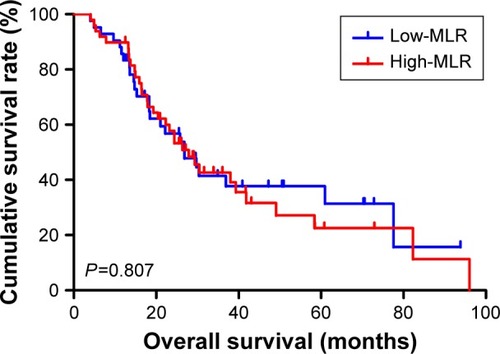
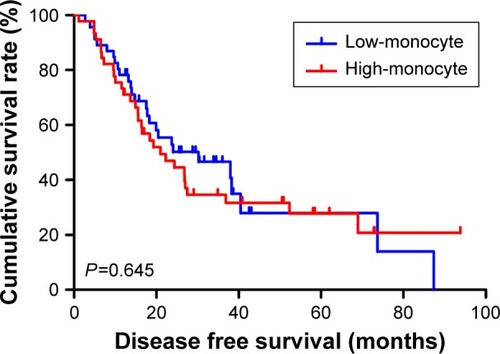
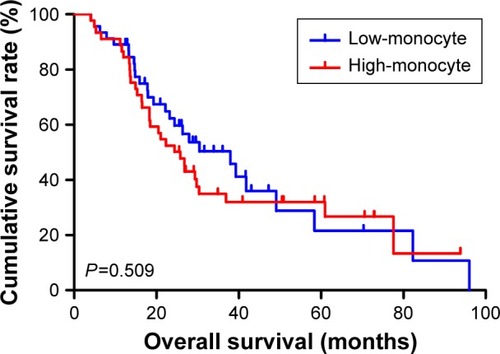
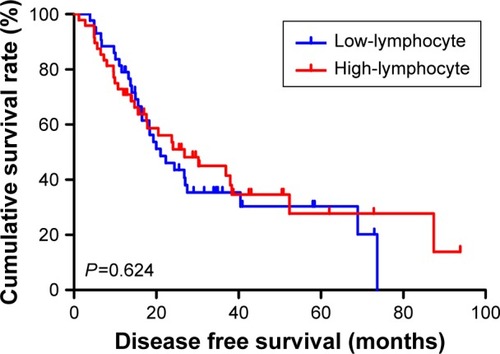
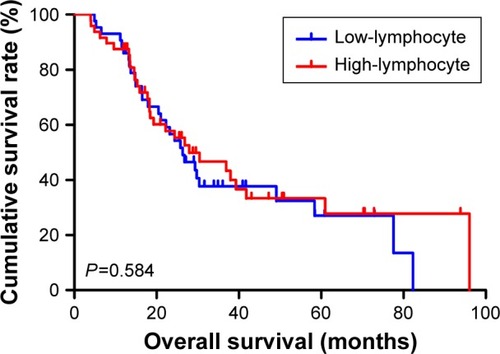
The univariate analysis showed that median disease free survival (DFS) and overall survival (OS) for all patients were better in low-MLR value group than high-MLR value group (median DFS 26.80 and 23.73 months, P=0.653, respectively; median OS 27.93 and 26.87 months, P=0.807, respectively). Multivariate analysis showed that MLR level was not an independent prognostic factor of DFS and OS. Nevertheless, median DFS and OS for all patients were better for patients with low monocyte values compared to those with high monocyte values (median DFS 30.23 and 21.03 months, P=0.645, respectively; median OS 37.97 and 25.83 months, P=0.509, respectively); in patients with high lymphocyte values compared with low lymphocyte values median DFS was 26.87 and 21.03 months, (P=0.624) respectively; median OS was 27.93 and 26.37 months, (P=0.584) respectively. However, the patients with low level MLR had better 5-year DFS and OS rates.
Conclusion
MLR may be used as a convenient and cheap prognostic marker in patients with advanced gastric cancer undergoing neoadjuvant chemotherapy with SOX or XELOX. Low level MLR as a prognostic marker may help doctors in terms of efficient measures to treat gastric cancer.
Introduction
Gastric cancer is the most common type of digestive system malignant tumors and the main cause of death in the whole world.Citation1 Surgical operation is the major treatment for advanced gastric cancer. However, recurrence and metastasis are common factors leading to low level 5-year survival rate in gastric cancer. In China, advanced gastric carcinoma accounts for the majority of all patients with gastric cancer, and the preoperative clinical tumor stage is usefulness to choose the better therapeutic strategy for patients. Nevertheless, it may not predict patients’ postoperative complications and prognosis. Gastric cancer death could be prevented if the disease was detected earlier. Hence, it is significant to explore actively the potential prognostic markers that could predict the survival of gastric carcinoma patients at high risk of recurrence and metastasis.
For several decades, neoadjuvant chemotherapy has been shown to be beneficial in the treatment of advanced gastric cancer. Many studies have demonstrated that the neoadjuvant chemotherapy may either decrease the tumor stage or increase the R0 resection rate without increasing surgical morbidity and mortality.Citation2 The neoadjuvant chemotherapy may result in increasing pathological complete response (CR) with tolerable side effects, and decreasing positive pathological nodes.Citation3 Many neoadjuvant chemotherapy regimens have been used for gastric cancer treatment, and the S-1 plus oxaliplatin (SOX) and oxaliplatin and capecitabine (XELOX) regimens are the major regimens in clinical practice.Citation4 In Asia, with these neoadjuvant chemotherapy regimens and surgical resection, it has remarkably improved survival of patients with advanced gastric cancer.Citation5 Hence, it is crucial to find new tumor indicators to improve better survival outcome.Citation6
Inflammation is the fundamental component of the tumor microenvironment, and the change of inflammatory cells might affect tumor progression, such as tumor cell proliferation, migration, invasion, metastasis, and so forth.Citation7,Citation8 In recent years, the relationship between inflammation and malignant tumor has been hotly studied. Tumor-inflammation interaction may be a potential therapeutic target for the neoplastic treatment, and the peripheral blood tests may influence and reflect the tumor inflammatory conditions. Many studies have shown that C-reactive protein (CRP), white blood cell (WBC), neutrophil, lymphocyte, monocyte, and platelet counts, as well as neutrophil to lymphocyte ratio (NLR), derived neutrophil to lymphocyte ratio (d-NLR), platelet to lymphocyte ratio (PLR), and monocyte to lymphocyte ratio (MLR) were used in many malignancies to quantify the survival outcome.Citation9,Citation10
As far as we know, the MLR has been identified as a useful predictor in gastric carcinoma.Citation11 However, the prognostic value of MLR in patients receiving the SOX or XELOX regimen for advanced gastric carcinoma is rarely reported. Therefore, the aim of our study was to evaluate the prognostic value of MLR in patients with advanced gastric cancer receiving SOX or XELOX neoadjuvant chemotherapy regimen.
Materials and methods
Ethics approval
This study was approved by the ethics committee of the Harbin Medical University Cancer Hospital, Harbin Medical University and all procedures were performed in accordance with the standards of the Declaration of Helsinki. All human materials were obtained with written informed consent.
Patient selection
We performed a retrospective analysis of a clinicopathological database at Harbin Medical University Cancer Hospital, Harbin Medical University. All patients with stage II/III gastric carcinoma and treated with neoadjuvant chemotherapy between August 2008 to September 2015 were included. All cases were diagnosed with pathological evidence, and the clinical stage was determined as II/III according to tumor-node-metastasis (TNM) staging system.Citation12 Patients were accepted into this study if they satisfied the following criteria: 1) all patients were diagnosed with locally advanced gastric cancer by pathological evidence; 2) Eastern Cooperative Oncology Group performance status with the range being 0–2, Karnofsky performance status must be ≥80; 3) a life expectancy survival of 3 months or longer; 4) no history of chemotherapy, radiotherapy, targeted therapy, and so forth. Patients were excluded if they met one of the following criteria: 1) patient was found to have distant metastases; 2) patient had some acute or chronic disease, such as diabetes mellitus, hypertension, atherosclerotic heart diseases, and difficult to control; 3) patients with obvious infections, active bleeding, intestinal obstruction; 4) patient received a blood transfusion within 1 month before neoadjuvant chemotherapy.
Treatment protocols
SOX regimen: oxaliplatin (Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, China) at 130 mg/m2 (intravenous infusion administered in 500 mL of 5% glucose over a period of 2 h) and S-1 (Taiho Pharmaceutical Co., Ltd., Tokyo, Japan) of 60 mg (orally administered twice a day on days 1–14). XELOX regimen: oxaliplatin at 130 mg/m2 (intravenous infusion administered in 500 mL of 5% glucose over a period of 2 h) and capecitabine (Shanghai Roche Pharmaceuticals Ltd., Shanghai, China) of 1,500 mg (orally administered twice a day on days 1–14). A cycle of the two regimens were repeated every 3 weeks.
Response evaluation
Response rates were determined according to the Response Evaluation Criteria In Solid Tumors (RECIST) guidelinesCitation13 and included the following categories: CR, partial response (PR), stable disease (SD), and progression of disease (PD). The clinical objective response rate was calculated as the sum of CR and PR, and non-clinical response was calculated as the sum of SD and PD.
Blood parameters
Blood samples were taken at the time of diagnosis before neoadjuvant chemotherapy. WBC differential counts were analyzed by XE-2100 hematology analyzer (Sysmex, Kobe, Japan).
Follow-up
After surgery, all patients were monitored every 3 months for the first 2 years, every 6 months thereafter, then annually, and until death. The patients were followed regularly every year thereafter with laboratory tests, multi-slice computed tomography (CT), and gastroscopy. Disease free survival (DFS) was measured from the date of surgery until date of relapse (local recurrence and distant metastases). Overall survival (OS) was measured from the date of surgery until date of death from any cause or the last follow-up. The last follow-up date was December 3, 2016.
Statistical analysis
Statistical analyses were performed using SPSS software (version 17.0; SPSS Inc., Chicago, IL, USA). The optimal cutoff value was determined using receiver operating characteristic (ROC) curve analyses. The area under the curve (AUC) was used to assess the predictive value. The categorical variables of the clinicopathological database of the patients were assessed by the χ2 test or Fisher’s exact test. The qualitative variables were expressed as the mean ± standard error, and compared using Student’s t-test. Survival curves were performed using the Kaplan–Meier method and compared using the log-rank test. Univariate and multivariate Cox proportional hazards regression model was evaluated to determine independent prognostic factors. All tests were two-sided, and a two-tailed P<0.05 was considered to indicate statistical significance.
Results
Patient characteristics
We used the ROC curve to determine an optimal cut-off value of the MLR. The AUC of MLR was 0.553, and the optimal cut-off value was 0.27. The patients were divided into two groups by the optimal cut-off value of MLR (low-MLR <0.27, high-MLR ≥0.27). We enrolled 91 patients in this retrospective study. Of the patients, 76.9% (70/91) were males and 23.1% (21/91) of the patients were females; the median age of all patients was 57 years (range: 32–73 years), with a median body mass index of 22.32 (range: 17.06–34.08). A low baseline MLR level (low-MLR <0.27) correlated with improved demographic and clinicopathological characteristics, including gender (χ2=4.622, P-value =0.032), primary tumor site (χ2=8.078, P-value =0.011), monocyte (χ2=15.072, P-value <0.001), lymphocyte (χ2=8.315, P-value =0.004) ().
Table 1 Demographic and clinicopathological characteristics of 91 patients with advanced gastric cancer
Survival analyses
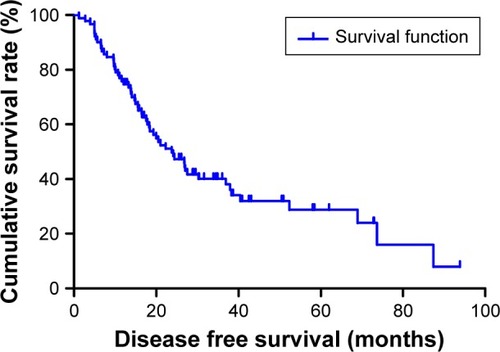
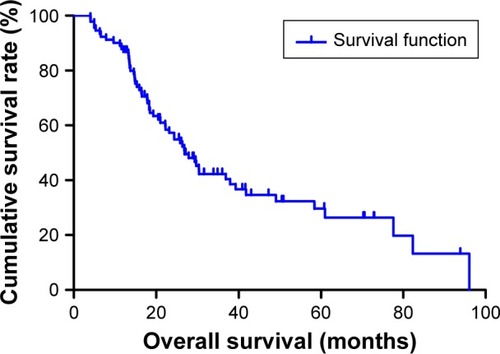
The median DFS for all the patients was 23.73 months (range 1.17–93.87 months), and the median OS for all the patients was 26.87 months (range 4.03–96.00 months) ( and ). Regarding DFS, high DFS factors were predicted for R0 resection, primary tumor site, pathological tumor in situ (Tis)/I stage. In multivariate Cox regression analysis, high DFS factors were predicted for R0 resection (P-value <0.001, HR: 2.059, 95% CI: 1.445–2.932), pathological Tis/I stage (P-value <0.001, HR: 2.782, 95% CI: 1.829–4.233) (). High OS factors were predicted for R0 resection, pathological Tis/I stage. In multivariate Cox regression analysis, high OS factors were predicted for R0 resection (P-value <0.001, HR: 2.494, 95% CI: 1.730–3.595), pathological Tis/I stage (P-value <0.001, HR: 3.041, 95% CI: 1.949–4.746) ().
Table 2 Univariate and multivariate Cox regression analyses of disease free survival in 91 patients with advanced gastric cancer
Table 3 Univariate and multivariate Cox regression analyses of overall survival in 91 patients with advanced gastric cancer
Moreover, we found that monocyte count, lymphocyte count, and MLR before neoadjuvant chemotherapy had no prognostic significance using the cut-off values of 0.44×109/L, 1.68×109/L, and 0.27 regarding DFS (P-value =0.365, 0.240, 0.938, respectively) and OS (P-value =0.191, 0.212, 0.904, respectively) in univariate analysis ( and ). Nevertheless, median DFS and OS for all patients were better for advanced gastric cancer patients with low MLR values than for those with high MLR values (median DFS 26.80 and 23.73 months, P=0.653, respectively; median OS 27.93 and 26.87 months, P=0.807, respectively) ( and ).
At the same time, we found that median DFS and OS for all patients were better for advanced gastric cancer patients with low monocyte values than for those with high monocyte values (median DFS 30.23 and 21.03 months, P=0.645, respectively; median OS 37.97 and 25.83 months, P=0.509, respectively) ( and ). Median DFS and OS for all patients were better for advanced gastric cancer patients with high lymphocyte values than for those with low lymphocyte values (median DFS 26.87 and 21.03 months, P=0.624, respectively; median OS 27.93 and 26.37 months, P=0.584, respectively) ( and ; ).
Table 4 Clinical and laboratory characteristics as well as disease free survival and overall survival of the 91 patients with advanced gastric cancer
The patients with low level MLR and low level monocyte count had better median DFS and OS (mean DFS 36.30 and OS 48.21 months, respectively); patients with low level MLR and high level lymphocyte count had better median DFS and OS (median DFS 36.93 and OS 39.93 months, respectively) ( and ).
Table 5 Clinical and laboratory characteristics as well as disease free survival of the 91 patients with advanced gastric cancer by MLR
Table 6 Clinical and laboratory characteristics as well as overall survival of the 91 patients with advanced gastric cancer by MLR
The 1-year, 3-year, and 5-year DFS and OS rates for all patients with advanced gastric cancer were 75.8%, 23.1%, 7.7%, 87.9%, 26.4%, and 11.0%, respectively. The patients with low MLR <0.27 had better 5-year DFS and OS rates; compared with those with high MLR ≥0.27, the 1-year and 3-year DFS and OS rates were similar ().
Table 7 The 1-year, 3-year, and 5-year DFS and OS rates of the 91 patients with advanced gastric cancer
The most common toxicities after neoadjuvant chemotherapy were hematologic toxicities. The National Cancer Institute Common Toxicity Criteria grade 1 and 2 anemia, leucopenia, neutropenia, thrombocytopenia were recorded in 33/91 (36.3%), 18/91 (19.8%), 21/91 (23.1%), and 4/91 (4.4%) of patients respectively ( and ). MLR had no significance using the cut-off value of 0.27 on toxicities of all patients.
Table 8 Main toxicities according to NCI-CTC scale of the 91 patients with advanced gastric cancer
Table 9 Main toxicities according to NCI-CTC scale of the 91 patients with advanced gastric cancer
Discussion
Gastric cancer is one of the most common types of malignant tumors all over the world. The mortality and morbidity rates of gastric cancer have declined in recent decades, however, it still has a poor prognosis and leads to hundreds of thousands of deaths annually.Citation14 Over the past 2 decades, with the rapid advances in surgical techniques and adjuvant therapy, survival and quality of life have been greatly improved for these patients with advanced gastric carcinoma.Citation15 Some immunological and histological biomarkers may influence the prognosis of patients with gastric cancer, which largely depends on the primary tumor samples, however, they are often time-consuming and expensive, which limits their use in clinical practice.Citation16 Therefore, we should find reliable and affordable prognostic factors in patients with advanced gastric cancer and help the clinicians choose the proper therapies. There is growing interest in a clinical interpretation of the cellular components of a systemic inflammatory response in peripheral venous blood. Nevertheless, the mechanisms by which inflammatory response induces a poor outcome are still ambiguous and poorly understood.
For all we know, a lot of research has indicated that monocyte count is associated with poor survival in patients with many types of cancer, but the potential mechanisms remain unknown.Citation17 The monocytes inhibit the immune system and promote tumor proliferation, angiogenesis, and progression.Citation18 Furthermore, monocytes can inhibit the immune system by releasing some cytokines and chemokines and regulates tumor microenvironment. The lymphocytes are known to play essential roles in defense of tumor cells and reflect the inability of the body to mediate effective cell-mediated immunity to initiate tumor cell death.Citation19 More often than not, increased lymphocyte levels are associated with better prognosis in some solid tumors.Citation20 Combined with these results, we found that an increase in the monocyte count and decrease of the lymphocyte count in the peripheral venous blood have been related to tumor growth and progression. Therefore, the MLR may be a good marker to reflect the degree of tumor progression and predict prognosis.
The clinicopathological database of the 91 enrolled patients with advanced gastric cancer was analyzed. Low MLR level may help improve the demographic and clinicopathological characteristics, including gender, primary tumor site, monocyte, and lymphocyte. And improved DFS and OS factors were predicted for R0 resection and pathological Tis/I stage. We found that low MLR, low monocyte, and high lymphocyte were all associated with better prognosis in advanced gastric cancer patients. Meanwhile, our study indicated that low level MLR and low level neutrophil or high level lymphocyte correlated with better median DFS and OS for all patients. The 5-year DFS and OS rates of patients with low level MLR were higher than those of patients with high level MLR. The MLR value lost its independent prognostic significance for DFS or OS, however, it still provided important information for clinical practice.
As far as we know, the MLR values with regard to DFS and OS in patients with advanced gastric cancer undergoing neoadjuvant chemotherapy are rarely reported. The present study indicates that the MLR level may be used to predict the prognosis of advanced gastric cancer patients. It is of importance to take into consideration the high gastric cancer morbidity and unbalanced medical condition in China, and these convenient and cheap biomarkers may be useful for the prevention and treatment of gastric cancer. Hence, a better understanding of hematologic parameters may help identify new targets for personal treatment. Therefore, this study may provide fundamental information for clinical practice.
To sum up, our study explains the reason for elevated MLR promoting tumor progression, and that low level MLR may represent a more beneficial prog nosis. However, the number of patients was small, and larger numbers of patients with advanced gastric cancer treated with neoadjuvant chemotherapy should be enrolled. The differences in the cut-off values of MLR among the studies may be due to the differences in the number of patients and disease stage. In our research, whether the cut-off value of 0.27 for MLR is correct needs further investigation.
Conclusion
In conclusion, MLR may be a convenient, easily measured prognostic indicator for patients with advanced gastric cancer treated with neoadjuvant chemotherapy. Low level MLR may help clinicians to identify those patients who will benefit from neoadjuvant chemotherapy. However, further investigations are needed to evaluate changes in inflammatory markers in larger groups of patients with advanced gastric cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2015CA Cancer J Clin201565152925559415
- ParkSCChunHJChemotherapy for advanced gastric cancer: review and update of current practicesGut Liver20137438539323898376
- PeraMGallegoRMontagutCPhase II trial of preoperative chemoradiotherapy with oxaliplatin, cisplatin, and 5-FU in locally advanced esophageal and gastric cancerAnn Oncol201223366467021652581
- BangYJKimYWYangHKAdjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trialLancet2012379981331532122226517
- QuéroLGuillermSHennequinCNeoadjuvant or adjuvant therapy for gastric cancerWorld J Gastrointest Oncol20157810211026306142
- el AzizLMBlood neutrophil-lymphocyte ratio predicts survival in locally advanced cancer stomach treated with neoadjuvant chemotherapy FOLFOX 4Med Oncol2014311231125367855
- GrivennikovSIGretenFRKarinMImmunity, inflammation, and cancerCell2010140688389920303878
- BalkwillFMantovaniAInflammation and cancer: back to Virchow?Lancet2001357925553954511229684
- LeeSOhSYKimSHPrognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapyBMC Cancer20131335023876227
- MohriYTanakaKOhiMIdentification of prognostic factors and surgical indications for metastatic gastric cancerBMC Cancer20141440924906485
- FengFSunLZhengGLow lymphocyte-to-white blood cell ratio and high monocyte-to-white blood cell ratio predict poor prognosis in gastric cancerOncotarget2017835281529128029656
- WashingtonK7th edition of the AJCC cancer staging manual: stomachAnn Surg Oncol201017123077307920882416
- EisenhauerEATherassePBogaertsJNew response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)Eur J Cancer200945222824719097774
- FockKMReview article: the epidemiology and prevention of gastric cancerAliment Pharmacol Ther201440325026024912650
- BrenkmanHJHaverkampLRuurdaJPvan HillegersbergRWorldwide practice in gastric cancer surgeryWorld J Gastroenterol201622154041404827099448
- ShiraiOOhmiyaNTaguchiAP53, P21 and P73 gene polymorphisms in gastric carcinomaHepatogastroenterology2010571041595160121443127
- EoWKJeongDWChangHJAbsolute monocyte and lymphocyte count prognostic score for patients with gastric cancerWorld J Gastroenterol20152192668267625759535
- GabrilovichDINagarajSMyeloid-derived suppressor cells as regulators of the immune systemNat Rev Immunol20099316217419197294
- MilneKAlexanderCWebbJRAbsolute lymphocyte count is associated with survival in ovarian cancer independent of tumor-infiltrating lymphocytesJ Transl Med2012103322369276
- QuigleyDAKristensenVPredicting prognosis and therapeutic response from interactions between lymphocytes and tumor cellsMol Oncol20159102054206226607741