Abstract
The importance of personalized medicine has been growing, mainly due to a more urgent need to avoid unnecessary and expensive treatments. In nuclear medicine, the theranostic approach is an established tool for specific molecular targeting, both for diagnostics and therapy. The visualization of potential targets can help predict if a patient will benefit from a particular treatment. Thanks to the quick development of radiopharmaceuticals and diagnostic techniques, the use of theranostic agents has been continually increasing. In this article, important milestones of nuclear therapies and diagnostics in the context of theranostics are highlighted. It begins with a well-known radioiodine therapy in patients with thyroid cancer and then progresses through various approaches for the treatment of advanced cancer with targeted therapies. The aim of this review was to provide a summary of background knowledge and current applications, and to identify the advantages of targeted therapies and imaging in nuclear medicine practices.
Introduction: goals of theranostics in nuclear medicine
Challenges in modern oncology include the fact that patients are getting older and are typically unfit for conventional chemotherapy regimens because of comorbidities or poor performance status.Citation1 Furthermore, the occurrence of side effects may aggravate treatment compliance in both young and elderly patients.Citation2 To manage these problems, it is important to improve patient selection, reduce side effects, and enhance therapeutic efficacy. Taking these factors into consideration, the combination of targeted cancer imaging and therapy is a considerable achievement for personalized medicine.
The theranostic approach in nuclear medicine couples diagnostic imaging and therapy using the same molecule or at least very similar molecules (), which are either radiolabeled differently or given in different dosages. For example, iodine-131 and lutetium-177 are gamma and beta emitters; thus, these agents can be used for both imaging and therapy. Furthermore, different isotopes of the same element, for example, iodine-123 (gamma emitter) and iodine-131 (gamma and beta emitters), can also be used for theranostic purposes.Citation3,Citation4 Newer examples are yttrium-86/yttrium-90 or terbium isotopes (Tb): 152Tb (beta plus emitter), 155Tb (gamma emitter), 149Tb (alpha emitter), and 161Tb (beta minus particle).Citation5,Citation6
Figure 1 The theranostic principle in nuclear medicine involves combining diagnostic imaging and therapy with the same molecule, which is radiolabeled differently, or administered in other dosages. In case of radioiodine therapy (RAI), the radioisotope (131I or 123I) can be directly mediated by the sodium-iodide symporter in the thyroid cells. In other cases, it can be more complex. The image shows a simplified model of a radiopharmaceutical, which consists of a binding molecule that binds the target, and a linking molecule, which binds the radioisotope. Examples of such theranostic molecules are DOTA-TOC, DOTA-TATE, and PSMA-617.
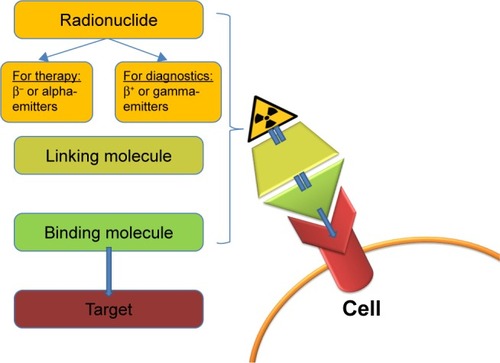
The detection of potential targets can help predict whether a patient will benefit from a particular treatment. Theranostics can be useful for estimating the potential response and eventual toxicity. During the treatment, theranostics can be applied in monitoring the therapy course. However, one cause of concern is the safety of high cumulative doses of radioactive agents after multiple repeated cycles. For instance, reports concerning irreversible high-grade toxicity followed soon after the first treatments with radioiodine (iodine-131).Citation7 However, there have been remarkable advances in nuclear medicine, especially in the field of targeted therapies. After a proper preselection of candidates, targeted nuclear therapies have proven to be effective in the majority of cases with a favorable safety profile.Citation8–Citation11 Recent studies have shown no evidence of grade 3/4 toxicity in patients with neuroendocrine tumors after repeated (≥8 cycles) radiopeptide therapy with a cumulative dose of up to 97 GBq.Citation12 Good tolerability has also been observed in patients with prostate cancer (PC) after a cumulative dose of 36 GBq and up to six cycles of radioligand therapy.Citation13,Citation14
Nuclear imaging utilizes gamma and positron emitters (β+). Gamma emitters, such as technetium-99m (99mTc) or iodine-123 (123I), can be located using gamma cameras (planar imaging) or SPECT (single photon emission computed tomography).Citation15 However, better resolution can be achieved via PET (positron emission tomography) using positron emitters, such as gallium-68 (68Ga) and fluor-18 (18F).Citation16
Most therapeutic radiopharmaceuticals are labeled with beta-emitting isotopes (β−). The tissue penetration of these particles is proportional to the energy of the radioisotopes.Citation17 Beta particles have a potential cytocidal effect, but they also spare the surrounding healthy tissue due to having a tissue penetration of only a few millimeters.Citation8 Commonly used beta emitters in routine nuclear oncology practices include lutetium-177 (177Lu, tissue penetration: 0.5–0.6 mm, maximum: 2 mm, 497 keV, half-life: 6.7 days) and yttrium-90 (90Y, tissue penetration: mean 2.5 mm, maximum: 11 mm, 935 keV, half-life: 64 hours).Citation8,Citation10,Citation13,Citation18–Citation20
The first theranostic radiopharmaceutical in nuclear medicine history was radioiodine, which was used for therapy and imaging in thyroid diseases.Citation21 Since then, the use of theranostic agents has been consistently increasing. Nuclear targeted therapies play an essential role, especially in patients with advanced neuroendocrine tumors, such as gastroenteropancreatic (GEP) tumors, bronchopulmonary neuroendocrine tumors, pheochromocytoma, and neuroblastoma.Citation10,Citation11,Citation20,Citation22–Citation27 Furthermore, there are positive results with radioligand therapies in metastatic PC and metastatic melanoma.Citation8,Citation13,Citation19,Citation28,Citation29
The aim of this review was to discuss the most important milestones of nuclear theranostics in current practice (), and to provide a summarized background and overview of current applications and advantages of targeted therapies and imaging.
Table 1 Overview of theranostic agents
Radioiodine therapy: “the gold standard” in thyroid diseases
Iodine (stable isotope: iodine-127) is taken up by the thyroid gland for the production of thyroid hormones, namely, thyroxine (T4) and triiodothyronine (T3).Citation30 Thyroid hormones are vital for the embryonic and neonatal development of the brain, normal growth, and metabolic balance.Citation9,Citation31–Citation33 In 1936, Dr Saul Hertz, director of the Thyroid Clinic in Massachusetts (1931–1943), developed the idea of administering radioactive iodine in patients with thyroid diseases. Iodine and external beam radiation were well-known tools in thyroid disease therapy, but the combination of these was a considerably innovative approach. This idea followed a few years of preclinical studies in collaboration with the Massachusetts Institute of Technology (MIT), where the first cyclotron for medical use had been built. The MIT Cyclotron produced 90% iodine-130 (130I, half-life: 12 hours) and 10% iodine-131 (131I, half time: 8 days). Subsequently, on March 31, 1941, Dr Hertz treated the first patient with radioiodine (130I).Citation21,Citation34,Citation35
The first radioiodine therapy (RAI) with 131I in patients with thyroid cancer (TC) was undertaken by Seidlin et al in 1946.Citation36 This group studied the use of RAI in patients with metastatic thyroid carcinomas.Citation36,Citation37 Seidlin et al also reported one of the first cases of acute myeloid leukemia after repeated RAI treatments.Citation7
To date, 131I is still the gold standard for the therapy and diagnosis of differentiated TC.Citation38 It is a low-cost nuclear reactor product from the neutron bombardment of tellurium-131. 131I combines the characteristics of a beta (β−, approximately 90% of the radiation, mean: 192 keV, mean tissue penetration: 0.4 mm) and gamma (approximately 10% of the radiation, mean: 383 keV) emitter. In this way, it irradiates the TC and the thyroid remnant from the inside and, at the same time, targeted lesions can be visualized using a gamma camera or SPECT.Citation9,Citation32,Citation33 Another radioisotope is 123I, which is a pure gamma emitter and is used for pre- and post-therapeutic diagnostics. The advantages of imaging with 123I include a higher quality of whole-body scans, which improves the sensitivity in detecting thyroid remnants.Citation3,Citation4
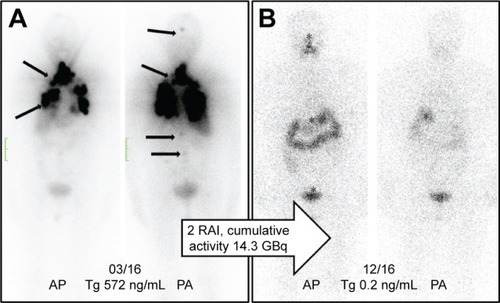
shows the initial 131I-planar images of a 74-year-old female with metastatic TC (lung, bone, and intracranial soft-tissue metastases). The level of tumor marker thyroglobulin (Tg) before RAI was 572 ng/mL. After two administrations of RAI (cumulative activity: 14.3 GBq), the patient was in complete remission with a Tg level of 0.2 ng/mL ().
Figure 2 (A) Initial 131I-planar images of a 74-year-old female with metastatic thyroid cancer (lung, bone, and intracranial soft-tissue metastases, marked with arrows). The tumor marker thyroglobulin (Tg) before radioiodine therapy (RAI) was 572 ng/mL. (B) After two administrations of RAI (cumulative activity: 14.3 GBq), the patient was in complete remission with the Tg at 0.2 ng/mL. The planar images show only a physiological uptake of the radiotracer in the gastrointestinal tract and pharyngeal mucosa (marked with an asterisk).
Diagnostics and therapy with metaiodobenzylguanidine
Metaiodobenzylguanidine (mIBG), or iobenguane, is a molecule similar to noradrenaline and enters neuroendocrine cells from the sympathetic nervous system, either by endocytosis or passive diffusion before being stored in neurosecretory granules.Citation39 Among the used radiolabeled molecules, [123I]I-mIBG has a lower gamma energy than [131I]I-mIBG (159 keV vs 360 keV), which makes it more suitable for planar imaging/SPECT. Furthermore, the pure gamma emitter [123I]I-mIBG consists of a shorter half-time of 13 hours compared to 8 days for the combined beta and gamma emitter [131I]I-mIBG, leading to a smaller radiation burden. Thus, higher quantities of [123I]I-mIBG can be injected.Citation40 Both [131I]I-mIBG and [123I]I-mIBG are used in mIBG scintigraphy for the detection of neuroendocrine tumors, such as neuroblastomas, pheochromocytomas, paragangliomas, medullary thyroid carcinomas, and other neuroendocrine neoplasias.Citation41 In patients with inoperable or advanced tumors with distant metastases, mIBG imaging plays an essential role in response assessment after therapy and in the evaluation of potential [131I]I-mIBG therapy.Citation42,Citation43 In patients with neuroblastoma and pheochromocytoma, [123I]I-mIBG has high sensitivity (97% and 94%) and specificity (up to 96% and 92%), respectively.Citation44–Citation46 If available, [124I]I-mIBG-PET can be equally used for the planning of mIBG targeted therapy.Citation47,Citation48
Targeted therapy with [131I]I-mIBG presents an encouraging efficacy with tolerable toxicity in relapsed or refractory neuroblastomas with response rates between 20% and 40% being used alone or in combination with high-dose chemotherapy followed by autologous stem cell transplantation.Citation23–Citation25,Citation49 Recently, the NB2004 Trial for Risk Adapted Treatment of Children with Neuroblastoma closed, and further analysis of the usage of mIBG therapy as a first-line therapy is outstanding.Citation23,Citation25
Palliation of bone metastases
Radiolabeled phosphonates have a high bone affinity and can be used for imaging and palliation of painful bone metastases. Depending on the degree of osseous metabolism, the tracer accumulates via adhesion to bones and, preferably, to osteoblastic bone metastases.Citation8
Therapy planning requires a bone scintigraphy with technetium-99m-hydroxyethylidene diphosphonate (HEDP) to estimate the metabolism and the extent of the metastases involvement. Bisphosphonate HEDP can be labeled for therapy either with rhenium-186 (beta-emitter, half-life: 89 hours, 1.1 MeV maximal energy, maximal range: 4.6 mm) or rhenium-188 (beta-emitter [to 85%, 2.1 MeV] and gamma-emitter [to 15%, 155 keV], half-life: 16.8 hours, maximal range in soft tissue: 10 mm).Citation50
Both agents induce pain relief in ≥90% of patients.Citation51 However, rhenium-188 is of particular interest because it can be cost-effectively produced using a tungsten-188/rhenium-188 generator. The gamma component of the agent allows post-therapeutic imaging of good quality.Citation8 Furthermore, patients with prostatic cancer have shown, after two [188Re]Re-HEDP injections, a significantly higher response rate (92%), a prostate-specific antigen (PSA) decline >50% (38%), and a longer PFS (7 months) compared to patients who have had a single injection.Citation52,Citation53 Biersack et al showed an improved OS of 15.7 months in patients after repeated injections (≥3 injections) compared to patients with a single therapy.Citation54 Besides transient bone marrow toxicity (thrombocytopenia grade III), the reported toxicity is low to moderate.Citation51
New promising radiopharmaceuticals for bone palliation therapy include radiolabeled complexes of zoledronic acid. Zoledronic acid belongs to a new, most potent generation of bisphosphonates with cyclic side chains. The bone affinity of zoledronic acid labeled with scandium-46 or lutetium-177 has shown excellent absorption (98% for [177Lu]Lu-zoledronate and 82% for [46Sc]Sc-zoledronate), which is much higher than of bisphosphonates labeled with samarium-153 (maximum: 67%).Citation55
Radiolabeled somatostatin analogs
Neuroendocrine neoplasia (NEN) of the GEP system originates most frequently from the pancreas, jejunum, ileum, cecum, rectum, appendix, and colon. The common characteristic of all GEP-NEN is the compound features of endocrine and nerve cells.Citation56–Citation58 Well-differentiated NEN overexpresses somatostatin receptors (SSTRs), especially the SSTR-2 subtype. Thus, SSTRs are theranostic targets in NEN that have been known for almost three decades and have become well established.Citation59–Citation61
SSTR imaging is necessary for staging, therapy planning, and follow-up. In the PET diagnostics, there are three routine somatostatin analog (SSA) tracers labelled with gallium-68: DOTA-TATE, DOTA-TOC, and DOTA-NOC. All three tracers bind specifically with SSTRs.Citation62,Citation63 Gallium-68-labeled SSTRs have high sensitivity (82%–97%) and specificity (80%–92%) in the detection of small primary tumors or metastases of GEP-NEN.Citation64–Citation66 Alternatively, a conventional whole-body scintigraphy (planat plus SPECT) or SPECT alone can be performed using indium-111-labeled SSTR, which is available at many institutions. Even if the 111In imaging does not have as good a quality as PET, this method has been shown to be superior to chromogranin A measurements alone for the management of GEP-NET patients and is better than computed tomography or ultrasound.Citation67,Citation68
Peptide receptor radionuclide therapy (PRRT) is a systemic therapy in patients with advanced metastatic NEN. PRRT requires a good tumor uptake in the SSTR imaging. For therapeutic purposes, the peptides DOTA-TATE and DOTA-TOC can be labeled with either 90Y or 177Lu. Because of the renal excretion and the proximal tubular reabsorption of these tracers, the kidneys are one of the primary limiting organs for this therapy.Citation69 Due to the smaller range (2 mm) and lower energy, 177Lu is less nephrotoxic than 90Y (range maximum: 11 mm, pure beta emitter, higher energy). 177Lu is also less hematotoxic.Citation70 This allows for the performance of several PRRT cycles without any relevant toxicity. Furthermore, the overall survival is not significantly different between the therapies. For that reason, [177Lu] Lu-DOTA-TATE and DOTA-TOC are, in many centers, the preferred agents for NEN therapy.Citation10,Citation11,Citation71,Citation72
PRRT has been increasingly gaining attention. The first randomized controlled phase III study, NETTER-1 (started 2012), compared the standard therapy of Sandostatin® LAR (somatostatin analog) with PRRT in patients with midgut NEN. The study showed a significant clinical benefit from the therapy, achieving a prolonged progression-free survival (median not reached, approximately 40 months, p<0.001), an overall response of 18%, and a presumably longer overall survival (median not reached, p=0.004).Citation11
represents the image of a male patient with a neuroendocrine tumor (unknown origin), with a recurrence of the disease nearly 2 years after the baseline PRRT. After a salvage therapy of PRRT, the patient had a partial response.
Figure 3 (A) [68Ga]Ga-DOTA-TOC-image* of a male patient with a neuroendocrine tumor (unknown origin) 23 months after the baseline PRRT (three cycles, cumulative activity: 19.6 GBq). The patient had a recurrence of the disease with multiple metastases in the bone, lung, liver, and lymph nodes (marked with arrows). (B) After another course of PRRT (three cycles, cumulative: 43.4 GBq), the PET images showed a partial response.
Abbreviations: PET, positron emission tomography; PRRT, peptide receptor radionuclide therapy.
![Figure 3 (A) [68Ga]Ga-DOTA-TOC-image* of a male patient with a neuroendocrine tumor (unknown origin) 23 months after the baseline PRRT (three cycles, cumulative activity: 19.6 GBq). The patient had a recurrence of the disease with multiple metastases in the bone, lung, liver, and lymph nodes (marked with arrows). (B) After another course of PRRT (three cycles, cumulative: 43.4 GBq), the PET images showed a partial response.](/cms/asset/4473e871-533c-40d5-877e-f63f0d61123b/dott_a_12195610_f0003_c.jpg)
Radiolabeled PSMA-ligands
PC is the most common cancer in men in Western countries.Citation73 Hormone- and chemotherapy-refractory patients with metastatic PC have a poor prognosis.Citation8,Citation74,Citation75 The main cause of death in these patients is progression to the androgen-independent stage.Citation8
PC cells overexpress prostate-specific membrane antigen (PSMA) on the cell surface.Citation76–Citation79 There are several available radiopharmaceuticals that target PSMA including [68Ga]Ga-PSMA-HBED-CC (also known as [68Ga]Ga-PSMA-11 [PET]), a monoclonal antibody (mAb) [177Lu]Lu/[90Y]Y-J591 (therapy), [123I]I-MIP-1072 (planar/SPECT), [131I]I-MIP-1095 (therapy), and the theranostic agents PSMA-I&T and DKFZ-PSMA-617 (PSMA-617), which are labeled with 68Ga for PET or with 177Lu for therapy.
The specificity of the two commercially available PET-tracers, [68Ga]Ga-PSMA-617 and [68Ga]Ga-PSMA-11, is similar – 99% and 100%, respectively.Citation80–Citation82 However, due to slower kinetics, PSMA-617 was suggested for labeling with the long-half-life Lutetium-177 for therapy and, therefore, there is sparse systematic evaluation of [68Ga]Ga-PSMA-617 for diagnostics.Citation83 The high kidney uptake of PSMA-11 in the kidneys makes it unsuitable for therapy and does not fit the definition “theranostics.”Citation84 Still, it is at issue if molecules with affinity to identical target structures might already present a “theranostic surrogate” even if more than the isotope differs between the diagnostics and the therapeutics.
The latest studies have shown that treatment with [177Lu] Lu-PSMA-617 is effective and well tolerated. In fact, nearly 70% of patients have benefited from this therapy.Citation13,Citation28,Citation85–Citation90 Dosimetry studies have shown that the most critical organs are the kidneys, with a maximum kidney radiation dose of 0.88 Gy/GBq for [177Lu]Lu-PSMA-617 and 0.93 Gy/GBq for [177Lu]Lu-PSMA-I&T.Citation91,Citation92 Okamoto et al stated that a cumulative activity of 40 GBq [177Lu]Lu-PSMA-I&T could be safely applied in patients.Citation91 Furthermore, [177Lu]Lu-PSMA-617 has been shown to produce no relevant increase in renal toxicity in the salvage setting or in patients with kidney radiation doses >19 Gy.Citation14
shows the pre-therapeutic [68Ga]Ga-PSMA-11 images of a patient with multiple lymph node, peritoneal, and bone metastases (arrows), and a history of chemotherapy (first and second line), enzalutamide, and abiraterone. After three cycles of [177Lu]Lu-PSMA-617, the follow-up images show a very good response with a substantial PSA decline ().
Figure 4 (A) Pre-therapeutic [68Ga]Ga-PSMA-11 images* of a patient with multiple lymph node, peritoneal, and bone metastases (arrows), and history of chemotherapy (first and second line) with enzalutamide and abiraterone. (B) After three cycles of [177Lu]Lu-PSMA-617, the follow-up images showed a very good response with a substantial PSA decline.
Abbreviations: PET, positron emission tomography; PSA, prostate-specific antigen.
![Figure 4 (A) Pre-therapeutic [68Ga]Ga-PSMA-11 images* of a patient with multiple lymph node, peritoneal, and bone metastases (arrows), and history of chemotherapy (first and second line) with enzalutamide and abiraterone. (B) After three cycles of [177Lu]Lu-PSMA-617, the follow-up images showed a very good response with a substantial PSA decline.](/cms/asset/8fea53dd-459d-4e09-b8ed-efd71061cd99/dott_a_12195610_f0004_c.jpg)
Melanin targeting in patients with metastatic melanoma
A promising approach in patients with metastatic melanoma is the specific targeting of melanin. The newly developed theranostic agents include [123I]I-/[131I]I-BA52 and [18F]F-/[131I]F-ICF15002, which may play a considerable role in the future.
BA52 is a melanin-binding benzamide. Labeled with 123I, it shows a specific binding of the pigmented metastases in planar imaging/SPECT and can help select patients who would probably benefit from the therapy. In a pilot study, [131I]I-BA52 was effective in three of five patients who were treated with more than 4.3 GBq.Citation29
ICF15002 is an arylcarboxamide derivative and, as a small molecule, can passively enter the cell and bind to melanin. The PET tracer is radiolabeled with 18F and [131I]I-ICF15002 is designed for the therapy. However, both tracers are still in the preclinical phase of their studies. One major problem may be the absorbed dose in melanin-rich tissues, such as skin, dark eyes, and the brain. For instance, in a murine model, there was a 30% decrease of the retinal thickness after two cycles of [131I]I-ICF15002.Citation93
Conclusion
In nuclear medicine, theranostics combine diagnostic imaging and therapy with the same, but differently radio-labeled, molecule, or the same agent in different dosages. The visualization of potential targets can help predict if a patient would benefit from a particular treatment. In properly preselected patients, targeted nuclear therapies have proven to be effective with a favorable safety profile. To conclude, the combination of targeted cancer imaging and therapy is a considerable contribution to personalized medicine and may play an increasingly important role in the future.
Disclosure
The authors report no conflicts of interest in this work.
References
- GridelliCDoes chemotherapy have a role as palliative therapy for unfit or elderly patients with non-small-cell lung cancer?Lung Cancer200238Suppl 2S45S5012431829
- RichardsonJLMarksGLevineAThe influence of symptoms of disease and side effects of treatment on compliance with cancer therapyJ Clin Oncol1988611174617523183704
- GerardSKCavalieriRRI-123 diagnostic thyroid tumor whole-body scanning with imaging at 6, 24, and 48 hoursClin Nucl Med20022711811805475
- AlzahraniASAlShaikhOTuliMAl-SugairAAlamawiRAl-RasheedMMDiagnostic value of recombinant human thyrotropin-stimulated 123I whole-body scintigraphy in the follow-up of patients with differentiated thyroid cancerClin Nucl Med201237322923422310247
- WalrandSHesseMRenaudLJamarFThe impact of image reconstruction bias on PET/CT 90Y dosimetry after radioembolizationJ Nucl Med2015563494495
- MüllerCZhernosekovKKösterUA unique matched quadruplet of terbium radioisotopes for PET and SPECT and for alpha- and beta-radionuclide therapy: an in vivo proof-of-concept study with a new receptor-targeted folate derivativeJ Nucl Med201253121951195923139086
- SeidlinSMSiegelEYalowAAMelamedSAcute myeloid leukemia following prolonged iodine-131 therapy for metastatic thyroid carcinomaScience1956123320180080113324088
- AhmadzadehfarHTargeted therapy for metastatic prostate cancer with radionuclidesMohanRProstate Cancer – Leading-edge Diagnostic Procedures and Treatments InTech201610.5772/64016 Available from: https://www.intechopen.com/books/prostate-cancer-leading-edge-diagnostic-procedures-and-treatments/targeted-therapy-for-metastatic-prostate-cancer-with-radionuclidesAccessed August 23, 2017
- BaumRPKulkarniHRTHERANOSTICS: from molecular imaging using Ga-68 labeled tracers and PET/CT to personalized radionuclide therapy – the Bad Berka experienceTheranostics20122543744722768024
- KwekkeboomDJde HerderWWKamBLTreatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0, Tyr3] octreotate: toxicity, efficacy, and survivalJ Clin Oncol200826132124213018445841
- StrosbergJEl-HaddadGWolinENETTER-1 Trial InvestigatorsPhase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumorsN Engl J Med2017376212513528076709
- YordanovaAMayerKBrossartPSafety of multiple repeated cycles of 177Lu-octreotate in patients with recurrent neuroendocrine tumourEur J Nucl Med Mol Imaging20174471207121428246882
- AhmadzadehfarHEppardEKurpigSTherapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancerOncotarget2016711124771248826871285
- YordanovaABeckerAEppardEThe impact of repeated cycles of radioligand therapy using [177Lu]Lu-PSMA-617 on renal function in patients with hormone refractory metastatic prostate cancerEur J Nucl Med Mol Imaging20174491473147928337529
- HolmanBLTumehSSSingle-photon emission computed tomography (SPECT): applications and potentialJAMA199026345615642403612
- EckelmanWCGibsonREThe design of site-directed radiopharmaceuticals for use in drug discoveryNuclear Imaging In Drug Discovery, Development, and ApprovalSpringer1993113134
- Kramer-MarekGCapalaJThe role of nuclear medicine in modern therapy of cancerTumour Biol201233362964022446937
- PillaiMRChakrabortySDasTVenkateshMRamamoorthyNProduction logistics of 177Lu for radionuclide therapyAppl Radiat Isot2003592–310911812941498
- AhmadzadehfarHBiersackHJEzziddinSRadioembolization of liver tumors with yttrium-90 microspheresSemin Nucl Med201040210512120113679
- TeunissenJJMKwekkeboomDJde JongMEsserJPValkemaRKrenningEPEndocrine tumours of the gastrointestinal tract. Peptide receptor radionuclide therapyBest Pract Res Clin Gastroenterol200519459561616183530
- HertzBDr. Saul Hertz (1905–1950) discovers the medical uses of radioactive iodine: the first targeted cancer therapyAhmadzadehfarHThyroid Cancer-Advances in Diagnosis and TherapyIntechopen20161
- KlingebielTBaderPBaresRTreatment of neuroblastoma stage 4 with 131I-meta-iodo-benzylguanidine, high-dose chemotherapy and immunotherapy. A pilot studyEur J Cancer1998349139814029849423
- FrenchSDuBoisSGHornB131I-MIBG followed by consolidation with busulfan, melphalan and autologous stem cell transplantation for refractory neuroblastomaPediatr Blood Cancer201360587988423024113
- MatthayKKYanikGMessinaJPhase II study on the effect of disease sites, age, and prior therapy on response to iodine-131-metaiodobenzylguanidine therapy in refractory neuroblastomaJ Clin Oncol20072591054106017369569
- YanikGAVillablancaJGMarisJM131I-metaiodobenzyl-guanidine with intensive chemotherapy and autologous stem cell transplantation for high-risk neuroblastoma. A new approaches to neuroblastoma therapy (NANT) phase II studyBiol Blood Marrow Transplant201521467368125639769
- StoelbenEYordanovaAGossmannACarcinoids of the lungsBest Practice Onkologie20161152228 German [with English abstract]
- YordanovaAAhmadzadehfarHGonzalez-CarmonaMA step-by-step clinical approach for the management of neuroendocrine tumoursHorm Metab Res2017492778528099977
- FerdinandusJEppardEGartnerFPredictors of response to radioligand therapy of metastatic castrate-resistant prostate cancer with 177Lu-PSMA-617J Nucl Med201758231231927587707
- MierWKratochwilCHasselJCRadiopharmaceutical therapy of patients with metastasized melanoma with the melanin-binding benzamide 131I-BA52J Nucl Med201455191424277756
- ZhangJLazarMAThe mechanism of action of thyroid hormonesAnnu Rev Physiol200062143946610845098
- PorterfieldSPHendrichCEThe role of thyroid hormones in prenatal and neonatal neurological development – current perspectivesEndocr Rev1993141941068491157
- BauerFKBarrettTFCassenBCScintigrams of the thyroid gland; the diagnosis of morphologic abnormalities with I131Calif Med195277638038213009497
- HänscheidHLassmannMDosimetrie bei der Radioiodtherapie benigner und maligner SchilddrüsenerkrankungenNuklearmediziner201235013036
- HertzBSchullerKSaul Hertz, MD (1905–1950): a pioneer in the use of radioactive iodineEndocrine Pract2010164713715
- HertzSRobertsARadioactive iodine as an indicator in thyroid physiology: V. The use of radioactive iodine in the differential diagnosis of two types of Graves’ diseaseJ Clin Invest1942211313216694888
- SeidlinSMRossmanIOshryESiegelERadioiodine therapy of metastases from carcinoma of the thyroid; a 6-year progress reportJ Clin Endocrinol Metab19499111122113715395978
- SeidlinSMRadioiodine in the treatment of metastatic thyroid carcinomaMed Clin North Am195236366368014928848
- LusterMClarkeSEDietleinMEuropean Association of Nuclear Medicine (EANM)Guidelines for radioiodine therapy of differentiated thyroid cancerEur J Nucl Med Mol Imaging200835101941195918670773
- NakajoMShapiroBCoppJThe normal and abnormal distribution of the adrenomedullary imaging agent m-[I-131]iodobenzylguani-dine [I-131 MIBG] in man: evaluation by scintigraphyJ Nucl Med19832486726826135764
- ShapiroBGrossMDRadiochemistry, biochemistry, and kinetics of 131I-metaiodobenzylguanidine (MIBG) and 123I-MIBG: clinical implications of the use of 123I-MIBGMed Pediatr Oncol19871541701773309602
- BombardieriEGiammarileFAktolunCEuropean Association for Nuclear Medicine131I/123I-metaiodobenzylguanidine (mIBG) scintigraphy: procedure guidelines for tumour imagingEur J Nucl Med Mol Imaging201037122436244620644928
- DecarolisBSchneiderCHeroBIodine-123 metaiodobenzylguanidine scintigraphy scoring allows prediction of outcome in patients with stage 4 neuroblastoma: results of the Cologne interscore comparison studyJ Clin Oncol201331794495123341514
- SchmidtMSimonTHeroBSchichaHBertholdFThe prognostic impact of functional imaging with (123)I-mIBG in patients with stage 4 neuroblastoma >1 year of age on a high-risk treatment protocol: results of the German Neuroblastoma Trial NB97Eur J Cancer200844111552155818424129
- JacobsonAFDengHLombardJLessigHJBlackRR123 I-metaiodobenzylguanidine scintigraphy for the detection of neuroblastoma and pheochromocytoma: results of a meta-analysisJ Clin Endocrinol Metab20109562596260620392867
- RufiniVFisherGAShulkinBLSissonJCShapiroBIodine-123-MIBG imaging of neuroblastoma: utility of SPECT and delayed imagingJ Nucl Med1996379146414688790194
- LeungAShapiroBHattnerRSpecificity of radioiodinated MIBG for neural crest tumors in childhoodJ Nucl Med1997389135213579293786
- OttRJTaitDFlowerMABabichJWLambrechtRMTreatment planning for 131I-mIBG radiotherapy of neural crest tumours using 124I-mIBG positron emission tomographyBr J Radiol1992657777877911393416
- HuangSYBolchWELeeCPatient-specific dosimetry using pretherapy [124I]m-iodobenzylguanidine ([124I]mIBG) dynamic PET/CT imaging before [131I]mIBG targeted radionuclide therapy for neuroblastomaMol Imaging Biol201517228429425145966
- ZhouMJDoralMYDuBoisSGVillablancaJGYanikGAMatthayKKDifferent outcomes for relapsed versus refractory neuroblastoma after therapy with (131)I-metaiodobenzylguanidine ((131)I-MIBG)Eur J Cancer201551162465247226254811
- PalmedoHRadionuclide therapy of bone metastasesBiersackHJFreemanLMClinical Nuclear MedicineBerlin, HeidelbergSpringer Berlin Heidelberg2007433442
- LiepeKKotzerkeJA comparative study of 188Re-HEDP, 186Re-HEDP, 153Sm-EDTMP and 89Sr in the treatment of painful skeletal metastasesNucl Med Commun200728862363017625384
- PalmedoHManka-WaluchAAlbersPRepeated bone-targeted therapy for hormone-refractory prostate carcinoma: tandomized phase II trial with the new, high-energy radiopharmaceutical rhenium-188 hydroxyethylidenediphosphonateJ Clin Oncol200321152869287512885803
- PalmedoHGuhlkeSBenderHDose escalation study with rhenium-188 hydroxyethylidene diphosphonate in prostate cancer patients with osseous metastasesEur J Nucl Med200027212313010755716
- BiersackHJPalmedoHAndrisAPalliation and survival after repeated (188)Re-HEDP therapy of hormone-refractory bone metastases of prostate cancer: a retrospective analysisJ Nucl Med201152111721172621976530
- MajkowskaANevesMAntunesIBilewiczAComplexes of low energy beta emitters 47Sc and 177Lu with zoledronic acid for bone pain therapyAppl Radiat Isot2009671111318929490
- KloppelGClassification and pathology of gastroenteropancreatic neuroendocrine neoplasmsEndocr Relat Cancer201118Suppl 1S1S1622005112
- KloppelGRindiGAnlaufMPerrenAKomminothPSite-specific biology and pathology of gastroenteropancreatic neuroendocrine tumorsVirchows Arch2007451Suppl 1S9S2717684761
- RindiGKloppelGCouvelardATNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading systemVirchows Arch2007451475776217674042
- LambertsSWBakkerWHReubiJCKrenningEPTreatment with Sandostatin and in vivo localization of tumors with radiolabeled somatostatin analogsMetabolism1990399 Suppl 21521552169572
- LambertsSWReubiJCBakkerWHKrenningEPSomatostatin receptor imaging with 123I-Tyr3-OctreotideZ Gastroenterol199028Suppl 220211980773
- KrenningEPBakkerWHBreemanWALocalisation of endocrine-related tumours with radioiodinated analogue of somatostatinLancet1989186322422442563413
- PoeppelTDBinseIPetersennS68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumorsJ Nucl Med201152121864187022072704
- WildDSchmittJSGinjMDOTA-NOC, a high-affinity ligand of somatostatin receptor subtypes 2, 3 and 5 for labelling with various radiometalsEur J Nucl Med Mol Imaging200330101338134712937948
- GabrielMDecristoforoCKendlerD68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CTJ Nucl Med200748450851817401086
- HaugAAuernhammerCJWanglerBIntraindividual comparison of 68Ga-DOTA-TATE and 18F-DOPA PET in patients with well-differentiated metastatic neuroendocrine tumoursEur J Nucl Med Mol Imaging200936576577019137293
- KayaniIBomanjiJBGrovesAFunctional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (DOTA-DPhe1, Tyr3-octreotate) and 18F-FDGCancer2008112112447245518383518
- ChitiAFantiSSavelliGComparison of somatostatin receptor imaging, computed tomography and ultrasound in the clinical management of neuroendocrine gastro-entero-pancreatic tumoursEur J Nucl Med19982510139614039818279
- CimitanMBuonadonnaACannizzaroRSomatostatin receptor scintigraphy versus chromogranin A assay in the management of patients with neuroendocrine tumors of different types: clinical roleAnn Oncol20031471135114112853358
- SabetAEzziddinKPapeUFAccurate assessment of long-term nephrotoxicity after peptide receptor radionuclide therapy with (177) Lu-octreotateEur J Nucl Med Mol Imaging201441350551024196919
- RomerASeilerDMarincekNSomatostatin-based radiopeptide therapy with [177Lu-DOTA]-TOC versus [90Y-DOTA]-TOC in neuroendocrine tumoursEur J Nucl Med Mol Imaging201441221422224085501
- WongFCKimEETherapeutic applications of radiopharmaceuticalsKimEEHandbook of Nuclear Medicine and Molecular Imaging: Principles and Clinical ApplicationsNew JerseyWorld Scientific2012401
- CwiklaJBSankowskiASekleckaNEfficacy of radionuclide treatment DOTATATE Y-90 in patients with progressive metastatic gastroenteropancreatic neuroendocrine carcinomas (GEP-NETs): a phase II studyAnn Oncol201021478779419833821
- FerlayJSoerjomataramIDikshitRCancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012Int J Cancer20151365E359E38625220842
- SonpavdeGPerimanPOBernoldDSunitinib malate for metastatic castration-resistant prostate cancer following docetaxel-based chemotherapyAnn Oncol201021231932419633050
- TolcherAWQuinnDIFerrariAA phase II study of YM155, a novel small-molecule suppressor of survivin, in castration-resistant taxane-pretreated prostate cancerAnn Oncol201223496897321859898
- GhoshAHestonWDWTumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancerJ Cell Biochem200491352853914755683
- Mhawech-FaucegliaPZhangSTerraccianoLProstate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: an immunohistochemical study using mutiple tumour tissue microarray techniqueHistopathology200750447248317448023
- SantoniMScarpelliMMazzucchelliRTargeting prostate-specific membrane antigen for personalized therapies in prostate cancer: morphologic and molecular backgrounds and future promisesJ Biol Regul Homeost Agents201428455556325620167
- SilverDAPellicerIFairWRHestonWDCordon-CardoCProstate-specific membrane antigen expression in normal and malignant human tissuesClin Cancer Res19973181859815541
- Afshar-OromiehAAvtziEGieselFLThe diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancerEur J Nucl Med Mol Imaging201542219720925411132
- HijaziSMellerBLeitsmannCPelvic lymph node dissection for nodal oligometastatic prostate cancer detected by 68Ga-PSMA-positron emission tomography/computerized tomographyProstate201575161934194026356236
- PereraMPapaNChristidisDSensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysisEur Urol201670692693727363387
- Afshar-OromiehAHetzheimHKratochwilCThe theranostic PSMA ligand PSMA-617 in the diagnosis of prostate cancer by PET/CT: biodistribution in humans, radiation dosimetry, and first evaluation of tumor lesionsJ Nucl Med201556111697170526294298
- Afshar-OromiehAHetzheimHKublerWRadiation dosimetry of (68)Ga-PSMA-11 (HBED-CC) and preliminary evaluation of optimal imaging timingEur J Nucl Med Mol Imaging20164391611162027260521
- RahbarKAhmadzadehfarHKratochwilCGerman multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patientsJ Nucl Med201758859027765862
- RahbarKBodeAWeckesserMRadioligand therapy with 177Lu-PSMA-617 as a novel therapeutic option in patients with metastatic castration resistant prostate cancerClin Nucl Med201641752252827088387
- RahbarKSchmidtMHeinzelAResponse and tolerability of a single dose of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: a multicenter retrospective analysisJ Nucl Med20165791334133827056618
- AhmadzadehfarHRahbarKKurpigSEarly side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre studyEJNMMI Res20155111426099227
- BeckerAEppardEKuerpigSNephro- and hepatotoxicity after radioligand therapy of metastatic castrate-resistant prostate cancer with 177Lu-PSMA-617J Nucl Med201657Suppl 21430
- ZimbelmannSEppardEHauserSHematotoxicity after radioligand therapy of metastatic castrate-resistant prostate cancer with 177Lu-PSMA-617J Nucl Med201657Suppl 21429
- OkamotoSThiemeAAllmannJRadiation dosimetry for 177Lu-PSMA I&T in metastatic castration-resistant prostate cancer: absorbed dose in normal organs and tumor lesionsJ Nucl Med201758344545027660138
- KabasakalLAbuQbeitahMAygunAPre-therapeutic dosimetry of normal organs and tissues of (177)Lu-PSMA-617 prostate-specific membrane antigen (PSMA) inhibitor in patients with castration-resistant prostate cancerEur J Nucl Med Mol Imaging201542131976198326227531
- Rbah-VidalLVidalABillaudEMFTheranostic approach for metastatic pigmented melanoma using ICF15002, a multimodal radiotracer for both PET imaging and targeted radionuclide therapyNeoplasia2017191172727987437
