Abstract
More recently, disease metastasis and relapse in many cancer patients several years (even some decades) after surgical remission are regarded as tumor dormancy. However, the knowledge of this phenomenon is cripplingly limited. Substantial quantities of reviews have summarized three main potential models that can be put forth to explain such process, including angiogenic dormancy, immunologic dormancy, and cellular dormancy. In this review, newly uncovered mechanisms governing cancer cell dormancy are discussed, with an emphasis on the cross talk between dormant cancer cells and their microenvironments. In addition, potential mechanisms of reactivation of these dormant cells in certain anatomic sites including lymph nodes and bone marrow are discussed. Molecular mechanism of cellular dormancy in head and neck cancer is also involved.
Introduction
Tumor relapse and metastasis in some cancers can arise years or even decades after treatment, causing huge damage to patients, and are responsible for the vast majority of cancer-related deaths. The inability to treat metastasis is the most important challenge faced by modern oncologists. Recently, the extensive period of time in which patients remain asymptomatic before metastasis and relapse represents the clinical observations known as tumor dormancy. This broadly defined phenomenon has now come into sharp focus. Tumor dormancy was first defined by WillisCitation1 and then redefined by HadfieldCitation2 as a temporary mitotic and growth arrest. The mitotic arrest precisely refers to cellular dormancy, suggesting that a G0–G1 arrest can exist in certain cancer cells.Citation3 The growth arrest means a dormant cancer mass, in which the constituent cancer cells are kept constant by the equilibrium between cell division and apoptosis. In addition, the current literature suggests that the latter process may be due to an angiogenic or/and immunologic dormancy.Citation4–Citation6 It is widely appreciated that residual cancer cells would continuously encounter different growth-constraining conditions during dissemination and tumorigenic progression, such as hypoxia, nutritional deprivation, and chemotherapy stimuli.Citation7,Citation8 These cancer cells can release certain factors to modulate their growth-related signaling pathways through the cross talk between residual cancer cells and their microenvironments, leading to a state of dormancy or proliferation. Residual cancer cells can escape immune surveillance and the lethal effect of chemotherapy in hard survival conditions via growth arrest. However, they could exit dormancy and proliferate again in distant organs.
Over the past 2 decades, constant findings have strived to clarify the sources, phenotypes, properties, hosting niches, and signaling pathways of disseminated tumor cells (DTCs) that predict the survival, dormancy, and reactivation of minimal residual disease in head and neck cancer (HNC). Large work has been performed to establish DTCs as selection markers and monitoring tools for identifying the early stage of cancers,Citation9 because of their increasing identification as the cause of metastatic relapse. DTCs are generally detected in the bone marrow (BM). The majority of DTCs remain a state of quiescence.Citation10 A subgroup of DTCs circulating in the blood is termed circulating tumor cells (CTCs), and some findings indicated that DTCs could hold a stem cell-like phenotype called cancer stem cells (CSCs; ). A significant body of evidence has demonstrated that DTCs and CTCs could be detected in asymptomatic patients with melanoma, breast cancer, HNC, etc.Citation11–Citation13 However, how DTCs and CTCs maintain the long-term survival and reactivate to form micrometastases in distant organs is poorly understood. Recently, the underlying mechanisms of DTCs in tumor dormancy have been revealed.
Figure 1 Schematic view of dormancy-related tumor cells and molecules in tumor development.
Abbreviations: BM, bone marrow; DTC, disseminated tumor cells; ECM, extracellular matrix; PT, primary tumor.
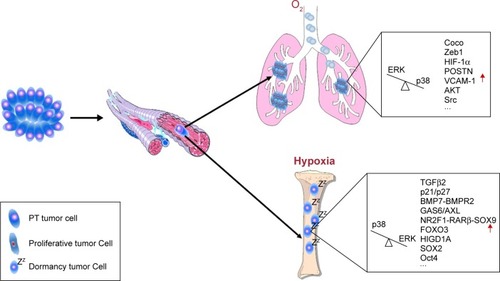
In this review, we focus primarily on mechanisms governing cancer cell dormancy and discuss how DTCs and their niche jointly modulate tumor dormancy in the metastatic progression of cancer. We also provide some insights into lymph node metastasis in patients with HNC based on recent evidence. Importantly, increasing studies of mechanisms of tumor dormancy could bring new hope to neoadjuvant chemotherapy and precision medicine for patients in the future.
Dormancy-related cancer cells
DTCs
Tumor dormancy is a critical step in the development of both primary tumor and metastatic disease. It is strongly conceived that there must be some cancer cells maintain and survive after an apparently successful treatment. In addition, they may even lodge in distant organs at the early stage of cancer and eventually contribute to late recurrence of disease.Citation14 Intriguingly, DTCs have been routinely detected in BM of patients with different cancers, since the pioneering work of Schlimok et alCitation15 and Riethmuller and JohnsonCitation16 was published in the 1980s. Moreover, current findings support the suggestion that certain DTCs retain dormant for an extended period of time, which is determined by the lack of proliferating markers (Ki-67, PCNA) accompanied by the lack of apoptotic markers terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay and (M30) and may be responsible for conventional chemoradiotherapy resistance.Citation17,Citation18 In addition, new markers including NR2F1, DEC2, and p27 have recently suggested a DTC dormancy.Citation19 BM is the most common organ for DTCs homing in different epithelial cancers including breast, prostate, colon, HNC, etc. ().Citation20 Also, substantial quantities of evidence suggest that BM niche is permissive to survival and maintenance of dormant DTCs (). However, there is an obvious distinction between the early DTCs and late DTCs. Late DTCs are validated to be more tumorigenic than early DTCs. In general, DTCs can be obtained through the BM aspiration. But this approach has not been universal in clinical practice due to being invasive. Up to now, the identification of dormant DTCs primarily depends on two main approaches, including immunological assays using monoclonal antibodies directed against histogenic proteins (GFP fluorescence) and polymerase chain reaction (PCR)-based molecular assays exploiting tissue-specific transcripts (quantitative PCR [qPCR]).
CTCs
CTCs are usually implicated in metastatic relapse and progression. The detection of CTCs in peripheral blood has been a routine program to indicate patients with cancer metastasis and poor prognosis in breast cancer, prostate cancer, and colorectal cancer.Citation22–Citation24 Although they have been detected in HNC, CTCs have not yet been widely acknowledged because of the limitation of the small patient cohorts, as well as the poor understanding of the impact of these cells. In addition, thus, CTCs are not in routine clinical practice for patients with HNC.Citation25,Citation26 It is reported that DTCs have more significant value compared to CTCs with respect to elucidating tumor dormancy. However, CTCs are more easily detected in peripheral blood, and the method brings merely damage to patients than DTCs. The convenience and acceptability of sequential peripheral blood analysis for CTCs are of great potential significance. Detection and enrichment of CTCs are based on the expression of EpCAMs and cytokeratins. CellSearch® System has been applied to noninvasive monitoring of CTCs in cancer patient samples as well as the isolation of single cell for genomic analysis with high accuracy.Citation27 Due to the low CTC counts in peripheral blood, advanced technologies and ultrasensitive methods need to be gradually developed to improve their clinical utility.
CSCs
CSCs have been demonstrated in several solid tumors, including HNC, melanoma, breast cancer, prostate cancer, colon cancer, and pancreas cancer.Citation28–Citation32 CSCs not only play a key role in cancer initiation and maintenance of tumor bulk but also reflect a more aggressive and poorer prognosis.Citation33 Specific CSC markers for solid tumors mainly include CD44, CD133, ALDH, and EpCAM.Citation34–Citation36 In addition, CD10 has been identified as a potential marker for CSCs in head and neck squamous cell carcinoma (HNSCC).Citation37 Increasing studies indicated that a subset of DTCs and CTCs undergoes an epithelial-to-mesenchymal transition (EMT) and obtain a stem-like phenotype. Also, the phenotype is associated with an increased capacity for migration and invasion, as well as resistance to anoikis and apoptosis. In HNSCC, epidermal growth factor receptor (EGFR), neurotrophin receptor B, and interleukin-1β are reported to be involved in EMT and an elevated population of CSCs.Citation38–Citation40 However, most of these cancer cells seem to be unable to undergo the reverse process of mesenchymal-to-epithelial transition (MET) to form metastases.Citation41 Chaffer and WeinbergCitation42 reported that CSCs have greatly enhanced tumor-initiating potential but a temporary growth arrest within a tumor. Dormancy and reactivation of CSCs are closely related to epigenetic reprogramming of these CSCs in HNC.Citation43,Citation44 A subset of miRNAs is demonstrated to be responsible for self-renewal and differentiation of CSCs in different types of cancers.Citation45 In HNC CSCs, miR-424, let-7a, miR-6836, and miR-6873 are lower expressed than miR-147b and miR-7152.Citation46 Especially, miRNA-34a is shown to repress EMT, aldehyde dehydrogenase activity, invasiveness, and clonogenicity of CSCs in HNSCC.Citation47 However, potential pathways involved in these processes have not been described in detail, and thus further work will be needed in the future. Strikingly, the production of CSCs in HNSCC may be orchestrated by stress-triggered atavistic reprogramming (STAR), and even the HNSCC evolution may be highly dependent on the STAR activities.Citation48
Molecular mechanisms of cancer cell dormancy
Cancer cell dormancy has been confirmed as residual cancer cells that lack proliferative and apoptotic markers and maintain in a state of quiescence without a continuous growth. In this paper, we reviewed the molecular mechanisms of dormant-related cancer cells, with an emphasis on the cross talk between cancer cells and their microenvironments.
Intracellular signals
Mitogen-activated protein kinase (MAPK) pathways
MAPK family (also known as Ras–Raf–MEK–ERK pathway) is of paramount importance in converting extracellular stimuli into a wide spectrum of cellular responses, and its functions in cancer development are complex. Some findings suggest that p38 MAPK signaling is a double-edged sword on cancer cell growth. P38α negatively regulates cell cycle progression like p38γ, while p38δ induces cell proliferation in squamous cell carcinoma.Citation49 Furthermore, Sosa et alCitation50 showed a low (ERK MAPK/p38 MAPK) signaling ratio in dormant HEp3 cells in head and neck carcinoma (). In addition, then, it has been confirmed in breast cancer, prostate cancer, melanoma, ovarian cancer, and fibrosarcoma, finding that ~90% of the dormant cell lines expressed a similar level of ERK MAPK/p38 MAPK. The alteration in ERK MAPK/p38 MAPK is demonstrated to be arranged by the uPA–uPAR complex (urokinase plasminogen activator binds to uPA receptor) in extracellular matrix (ECM).Citation51–Citation54 Activated p38 MAPK pathway could induce DTCs to enter into growth arrest via activating p53 and p16 signaling and downregulating cyclin D1 ().Citation50 Overall, the equilibrium of ERK MAPK and p38 MAPK is closely related to cancer cell dormancy and the ratio of ERK MAPK/p38 MAPK may be the key determining factor for tumor dormancy. Accordingly, p38 inhibitor should be used with caution as it may carry a potential risk for a cohort of patients with cancer for the reason that p38α/β inhibition after surgery can increase the burden of DTCs in the BM, liver, and spleen.Citation55
Table 1 Molecular mechanism of cancer cell dormancy and reactivation
Jun N-terminal kinase (JNK) is the third major MAPK pathway that has been reported to exert an inverse function compared with p38 MAPK signaling, as JNK can induce proliferation and tumorigenesis of cancer cells.Citation56 But extensive experiments suggested a contradictory scenario that active JNK was required for growth arrest and could induce dormancy in breast cancer ().Citation57 In addition, mitogen-activated protein kinase kinase 4 (MKK4) has been recently demonstrated to induce cancer cells into a transient growth arrest.Citation58,Citation59
PI3K-AKT pathways
PI3K-AKT-mTOR pathway is another well-studied signaling pathway in regulating the cell cycle. Jo et al described a cancer cell-secreted regulatory system that mediated the PI3K-AKT-mTOR pathway within nutritional deprivation stress and demonstrated that reduced PI3K-AKT signaling could result in quiescence and autophagy.Citation60 In addition, such phenomenon was further validated in dormant HNSCC cells.Citation61 Glucocorticoid-induced leucine zipper (GILZ) is an important upstream target of PI3K/AKT signaling pathway, and AKT can be downregulated by the repression of GILZ in dormant cancer cells. And FOXO3A (one key substrate of PI3K/AKT pathway) is consequently diminished and p21 increased and eventually the cancer cell maintain dormancy.Citation62 Inhibiting AKT can trigger EGFR autophosphorylation, which also can lead to a growth arrest in cancer cells ().Citation63
Extracellular signals
Transforming growth factor-β (TGF-β) family
TGF-β family is an intricate cytokine network that modulates an array of cell viabilities including cell proliferation, morphogenesis, migration, ECM production, cytokine secretion, and apoptosis. Over the past 2 decades, TGF-β family has been found in the process of EMT, angiogenesis, and cancer cell dormancy. Latent TGF-β-binding protein 2 (LTBP-2) has recently been suggested to promote dormancy against metastatic growth and restrain the proliferation in nasopharyngeal carcinoma and esophageal squamous cell carcinoma (ESCC). But, it can also augment cancer cell adhesion and migration in melanoma; therefore, the function of LTBP-2 is related to specific tumor types and environments.Citation64 TGF-β1 can suppress tumor progression in precancerous lesions and early stage of cancers, but it can promote tumor growth in advanced-stage cancers.Citation65 TGF-β3 has recently been demonstrated to accelerate the growth, migration, and invasion of HNC through inducing matrix-specific protein periostin (POSTN).Citation66 However, TGF-β2, upregulated within all-trans retinoic acid (atRA) niche can induce a dormant phenotype in HNSCC via p38 MAPK-dependent pathway.Citation67 In addition, TGF-β2-induced cancer cell dormancy also requires AXL and GAS6.Citation68 When Coco (an antagonist of TGF-β ligands) overexpresses in metastatic site, cancer cells can escape dormancy.Citation69 Therefore, it is concluded that TGF-β family paradoxically acts on tumor progression ().
Wendt et alCitation70 indicated that the multifunction of TGF-β largely reflects its ability to govern the expression levels of epithelial cadherin, a hallmark of a fully differentiated epithelium that scarcely proliferates or migrates which is implicated in the process of EMT. Nevertheless, the underlying mechanisms are masked by a complex interplay between diverse cytokines. For example, Denys et alCitation71 proved in a colon cancer model that cancer cells could remain dormant and did not proliferate on a basement membrane extract, which is composed mainly of various growth factors and other components.Citation72
Bone morphogenetic proteins (BMPs)
Bone morphogenetic protein 7 (BMP7) is one branch of the TGF-β family secreted from normal BM stromal cells. It exerts diverse functions of embryonic patterning and organogenesis and tissue remodeling and repair, especially skeletal tissue. Previously, BMP7 has been demonstrated to induce a low ERK MAPK/p38 MAPK signaling ratio in dormant HEp3.Citation50 Recently, BMP7 is also reported to induce CSC dormancy by activating p38 MAPK, p21, and N-myc downstream-regulated gene 1 (NDRG1) in a BMP receptor 2 (BMPR2)-dependent manner. Knockdown of BMPR2 inhibited BMP7 activation, and BMPR2 expression was found to be inversely connected with cancer relapse and bone metastasis.Citation73 Therefore, it is an attractive possibility that a BMPR2-dependent BMP7-induced dormancy exits in cancer cells (). Buijs et alCitation74 have demonstrated the hypothesis about an antagonistic role of BMP7 in Smad-mediated effects of TGF-β and suggested that BMP7 may be a promising target to inhibit local cancer progression and bone metastases. However, BMP7 also can induce MET, a process that has been validated to play a critical role in predicting cancer cells that grow into macrometastasis, Therefore, BMP7 might increase the growth of cancer cells in advanced metastasis.Citation75 Fang et alCitation76 found that the downregulation of BMP4 by elevated SOX2 could promote the growth of cancer cells. In addition, SOX2 silencing could mediate cancer cell dormancy, and this process was accompanied by the upregulation of BMP4.
BM-derived stromal cell niche
Hematopoietic stem cell (HSC) niche was first proposed by Schofield.Citation77 HSCs mainly reside in the BM, and HSC niche has been indicated as a fertile ground for the survival and development of DTCs. Recent work by Shiozawa et alCitation78 has demonstrated that DTCs can target and displace HSCs and establish pre-metastatic niche within this new home. Blood-vessel-derived signals could modulate the dormant phenotype of DTCs in certain tumor models. Vascular cell adhesion protein-1 (VCAM-1), for instance, has been demonstrated to predict the metastasis progression by interacting with integrin α4β1 expressed on osteoclasts ().Citation79 Obviously, HSC niche not only promotes the survival of DTCs but also could be a dangerous element for the reactivation of DTCs. It is suggested that atRA is abundant in the BM and perivascular niche and can regulate HSC renewal.Citation80 So, it is convinced that atRA may make coalescing contribution to cancer cell dormancy with TGF-β2 and BMP7 in the BM ().Citation81 Treatment of T-HEp3 cells with atRA induced an elevated expression of NR2F1-RARα-SOX9 signaling and TGF-β2, eventually pushing the cancer cells into a state of dormancy.Citation67
Osteoblasts and osteoclasts in the BM can secrete many ECM-related factors to manipulate metastatic dormancy and reactivation. Recent studies have shown that prostate cancer cell expresses the annexin II receptor, which can bind to annexin II expressed on osteoblast cell surface. In addition, this binding can induce the expression of AXL, Sky, and Mer and eventually induce cancer cell dormancy via AXL-GAS6 signaling pathway. It is validated that the conversion of solitary DTC to dormant cancer cell is regulated by AXL-GAS6 signaling pathway, whereas cancer cells, which grow rapidly in the BM milieu, can express less GAS6.Citation82–Citation84 However, Cackowski et alCitation85 have further investigated Mer tyrosine kinase (MERTK) in prostate cancer DTCs and noted that knockdown of MERTK could induce a low ratio of ERK MAPK/p38 MAPK, an increased expression of NR2F1 and p27 and a G0–G1 arrest. Comparing with AXL, Tyro3 (a subfamily of AXL) overexpresses in more proliferative cancer cells of the primary tumor, and high levels of Tyro3 is regarded as a marker for poor prognosis in prostate cancer.Citation86 Besides, Fra-1 that shares 50% correlation of coexpression with AXL highly expressed in multiple cancers, including HNC. But the overexpression of Fra-1 can directly deplete CSC dormancy and thereby promote cancer cell chemosensitivity.Citation87 Furthermore, Kim et alCitation88 found that binding with osteoblasts can induce the expression of TANK-binding kinase 1 (TBK1) in DTCs, which inhibits mTOR, and finally results in cell cycle arrest.
In vivo experiments reveal that dormant cancer cells are usually found near the perivascular niche. In addition, low levels of POSTN and TGF-β1 may contribute to DTCs to maintain a dormancy state ().Citation89 Mesenchymal stem/stromal cells (MSCs) are proposed to be first encountered with DTCs within the BM, as they are anatomically located at the abluminal surface of the central vasculature in the cavity.Citation90 Studies performed by Bliss et alCitation91 have shown that DTCs could instruct MSCs to release exosomes with distinct miRNAs, such as miR222/223 and miR23b, leading to cycling dormancy of certain DTCs ().Citation92 Intriguingly, MSC cannibalism in BM recently can be a unique mechanism supporting cancer cell dormancy via transfer of cell cycle inhibitory miRNA through gap junctions and/or exsomes.Citation93,Citation94 Consequently, MSCs may be a promising “vehicle” to modulate cancer cell dormancy within the BM.
Hypoxia and cancer cell dormancy
Hypoxia occurs frequently in human solid tumors. The environment is associated with poor survival and increased metastatic incidence and tumor burden in patients with various cancer, including HNC, prostate cancer, cervical cancer, breast cancer, etc.Citation95–Citation97 Under low oxygen tensions, cancer cells have predilection for becoming invasive and disseminating to distant sites through hypoxia-associated transcriptional activation, including the hypoxia-inducible factor (HIF), transcriptional regulators, mTOR complex 1, autophagy and endoplasmic reticulum stress responses, etc.Citation98,Citation99 Also, it is increasingly suggested that hypoxia is responsible for therapeutic resistance to both chemotherapy and radiotherapy because cancer cells may remain a state of dormancy or growth arrest in such microenvironment. For instance, forkhead box M1 (FOXM1), which is demonstrated as a regulator of cellular redox reaction and radiation response, was detected much lower in quiescent cancer cells than proliferating cancer cells in HNSCC ().Citation100 These data provide an underlying mechanism by which cancer cells enter into dormancy or are reactivated by the hypoxic environment.Citation101 Msaki et alCitation21 found that HIF-1α in late DTCs of mammary cancer was highly expressed and suggested that HIF-1α favored to enhance tumorigenic abilities of DTCs (). Interestingly, it is reported that conditional hypoxia could induce the expression of endogenous LOXL2, and then promote EMT in dormant MCF-7 cells, drive these cells to express CSC-like phenotypes, and eventually escape from dormancy to metastatic outgrowth.Citation102 Hypoxia is suggested to induce autophagy activities in a HIF-1α-dependent manner, and the process may lead to a state of temporary dormancy in the early stage of cancer or therapy-induced microenvironments in HNSCC.Citation103–Citation105 The leukemia inhibitory factor (LIF) receptor, the ligand LIF belongs to the IL-6 family of pro-inflammatory cytokines, has been recently recognized as a cancer distant metastasis suppressor and a dormant cancer cell promoter.Citation106–Citation108 Hypoxia could induce DTCs in the BM to spontaneously exit dormancy and reactivate through downregulating LIFR:STAT3:SOCS3 signaling pathway ().Citation108 In addition, hypoxia can increase uPAR expression, which could trigger cancer cell dissemination, invasion, EMT, and release from dormancy via ERK MAPK/p38 MAPK signaling pathway.Citation109
In another scenario, however, hypoxia seems to suppress cancer invasion and progression via inducing cancer cells into dormancy. Hypoxia-inducible gene domain family member 1A (HIGD1A), for example, can promote survival and dormancy in a HIF-independent manner ().Citation110,Citation111 It has been confirmed that severe hypoxia also could induce protein phosphatase 2A (PP2A) activity that mediated growth inhibition, and PP2A was positively correlated with HIF-1 expression.Citation112 Furthermore, hypoxia was demonstrated to upregulate dormancy markers including NR2F1 and DEC2.Citation67 Therefore, whether hypoxia promotes or inhibits DTC dormancy needs further work to validate.
Lymph node metastasis and dormancy in HNC
Regional lymph node metastasis and distant metastasis are two major metastatic models in epithelial cancer patients. In addition, lymph node metastasis is suggested the most common and adverse event in patients with HNSCC that the presence reduces survival by 50%.Citation113 Tumor-induced lymph angiogenesis had been reported to be involved in tumor growth and metastasis, not only at the primary site but also in lymph nodes and distant sites.Citation114,Citation115 Beasley et alCitation116 provided evidence in HNC that active lymphatic formation occurred in more invasive tumors and a high intra-tumoral lymph vessel concentration was significantly related to cervical node metastasis. Similarly, tumor lymph angiogenesis had been observed to take part in the initiation of lymphatic metastasis.Citation117 Substantial quantities of evidence suggested that tumor cells lodged in BM of patients with breast cancer maintained in a state of dormancy, as well as in HNSCC.Citation15 Although obvious differences in biology between the two kinds of cancer cells exist, similar micro-metastasis rates (30%–40%) were reported within the BM of patients with breast cancer and HNSCC. Accordingly, we can raise the possibility that there are some dormant cancer cells reside in lymph nodes of HNC patients, and these cells may be responsible for lymph angiogenesis, lymph node metastasis, and even for distant metastasis. In addition, occult lymph nodes in HNC may be explained by such scenario as well.
Mobilization of BM-derived cells (BMDCs) and their recruitment to metastatic niches by tumor-derived factors are the potential mechanisms of lymph node metastasis and tumor-induced lymph angiogenesis after a period of dormancy in clinical observation. VEGF-A, for example, not only directly mobilizes BMDCs in the BM, but indirectly recruits BMDCs to metastatic sites via inducing the expression of the pro-inflammatory S100A8 and S100A9 cytokines. In addition, that in turn induces the expression of serum amyloid A proteins.Citation118,Citation119 VEGF-C and VEGF-D, another two members of the VEGF family, were also shown to induce proliferation of local lymphatic vessels. Such tumor-induced lymph angiogenesis then may be induced intra- or peri-tumorally, even remotely in the distant draining lymph systems.Citation120 However, whether such tumor-derived factors can reactivate the dormant cancer cells in lymph nodes of HNC patients remains unclear.
Lymph nodes are frequently glutted with an inflammation milieu because of their anatomic location, and recently a report by De Cock et alCitation121 has unveiled a potential effect of inflammation on dormant DTCs. He noted that the escape from dormancy is regulated by a Zeb1-dependent pathway, which is a key regulator of the EMT, so it is highly plausible that other inflammatory stimuli may similarly contribute to the escape process (). In addition, this mechanism may be implicated in the phenomenon that lymph node metastasis is most frequent in HNSCC patients.
CSCs also can contribute to tumor-induced lymph angiogenesis via direct trans-differentiating to lymphatic endothelial cells and generating various lymph-angiogenic factors. Understanding the underlying mechanisms of cancer cell dormancy in draining lymph nodes and distant metastasis would improve the prognosis and survival of patients with HNC. Inactivating DTCs in lymph nodes by driving them into dormancy through targeting these tumor-induced factors is a promising neoadjuvant chemotherapy, which may become one part of precision medicine. Even it is necessary to reemphasize or de-emphasize the therapeutic effect of preventive neck dissection in the future.
Therapeutic implications and outlooks
Tumor dormancy and dormancy-related cancer cells have now come into sharp focus as contributions to metastasis and relapse. The studies discussed earlier mainly involved in molecular mechanisms of dormant cancer cells and provided a part of the framework to understand the process of tumor dormancy. Dormancy-related cancer cells are the causes of cancer metastasis and relapse, including DTCs, CTCs, and CSCs. In addition, a bulk of evidence yields that the vast majority of them can enter into a state of quiescence, temporarily or permanently. Strikingly, fluorouracil – the most frequently medicine in cancer treatment – has been found to increase the burden of dormant cancer cells and enrich the population of CSCs, and these cancer cells in turn to be involved in chemotherapy resistance.Citation122,Citation123 Also, hormonal therapy has also been demonstrated to promote the generation of CSCs in luminal breast cancer.Citation124 Therefore, it is anxious to confirm the effect on certain chemotherapies.
In this mini-review, we discussed intracellular, extracellular, and BM-derived factors that are associated with dormancy regulation system and summarized a bulk of potential treatment targets. In addition, we discussed the interaction between cancer cells and their niche. Also, we hypothesized the underlying interplay between lymph node metastasis and tumor dormancy in HNSCC.
Mechanisms of cancer cell dormancy will provide new insights into the complex biology of relapse and metastasis with important implications for the clinical management of cancer patients – either eradicate dormant cancer cells or maintain them. Furthermore, maintaining the state of cancer cell dormancy may be insufficient, but it is also required to suppress their survival. A combination of Src inhibitor and ERK MEK inhibitor, for instance, has been recommended in preventing breast cancer recurrence.Citation125 However, some studies also suggest to awake dormant cancer cells into the cell cycle and eventually to eliminate more cancer cells. For instance, in HNSCC, LB1 (an inhibitor of PP2A) can enhance the cytotoxic sensitivity to radiation or chemotherapy in quiescent cancer cells via promoting them from dormancy into the cell cycle.Citation126 Knockdown of TBK1 also can decrease dormant cancer cells and diminish drug resistance.Citation86 In conclusion, to identify the critical players and more responsive molecules that regulate the cancer cell dormancy which eventually turn to novel therapeutic targets. Furthermore, it is thus promising to expect that cancer cell dormancy may be the “Trojan horse” of the cancer therapy.
Acknowledgments
This work was supported by the National Natural Science Foundation of China grants (Nos 81772891, 81372891, 81672672, 81572650, 81272961, and 81361120399), the Fundamental Research Funds of the Central Universities of China (2015), and the State Key Laboratory of Oral Diseases Special Funded Projects (2016).
Disclosure
The authors report no conflicts of interest in this work.
References
- WillisRAThe Spread of Tumours in the Human BodyLondonJ&A. Churchill1934
- HadfieldGThe dormant cancer cellBr Med J19542488860761013190204
- GossPEChambersAFDoes tumor dormancy offer a therapeutic target?Nat Rev Cancer20101087187721048784
- Aguirre-GhisoJAModels, mechanisms and clinical evidence for cancer dormancyNat Rev Cancer200771183484617957189
- RakhraKBachireddyPZabuawalaTCD4+T cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon oncogene inactivationCancer Cell201018548549821035406
- KarevaIEscape from tumor dormancy and time to angiogenic switch as mitigated by tumor-induced stimulation of stromaJ Theor Biol2016395112226826487
- OsisamiMKellerETMechanisms of metastatic tumor dormancyJ Clin Med20132313615026237067
- RankinEBGiacciaAJHypoxic control of metastasisScience2016352628217518027124451
- SynnestvedtMBorgenEWistEDisseminated tumor cells as selection marker and monitoring tool for secondary adjuvant treatment in early breast cancer. Descriptive results from an intervention studyBMC Cancer20121261623259667
- SosaMSBragadoPAguirre-GhisoJAMechanisms of disseminated cancer cell dormancy: an awakening fieldNat Rev Cancer20141461162225118602
- HanselGSchonlebeJHaroskeGLate recurrence (10 years or more) of malignant melanoma in south-east Germany (Saxony). A single-centre analysis of 1881 patients with a follow-up of 10 years or moreJ Eur Acad Dermatol Venereol201024783383620070457
- LianidouESMarkouAStratiAThe role of CTCs as tumor biomarkersAdv Exp Med Biol201586734136726530376
- WuXLTuQFaureGGalletPKohlerCBittencourt MdeCDiagnostic and prognostic value of circulating tumor cells in head and neck squamous cell carcinoma: a systematic review and meta-analysisSci Rep201662021026831813
- EylesJPuauxALWangXTumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanomaJ Clin Invest201012062030203920501944
- SchlimokGFunkeIHozmannBMicrometastatic cancer cells in bone marrow: in vitro detection with anticytokeratin and in vivo labeling with anti-17-1A monoclonal antibodiesProc Natl Acad Sci U S A19878423867286762446326
- RiethmullerGJohnsonJPMonoclonal antibodies in the detection and therapy of micrometastatic epithelial cancersCurr Opin Immunol1992456476551418733
- LohrJGAdalsteinssonVACibulskisKWhole-exome sequencing of circulating tumor cells provides a window into metastatic prostate cancerNat Biotechnol201432547948424752078
- MagbanuaMJDasRPolavarapuPParkJWApproaches to isolation and molecular characterization of disseminated tumor cellsOncotarget2015631307153072926378808
- FluegenGAvivar-ValderasAWangYPhenotypic heterogeneity of disseminated tumour cells is preset by primary tumour hypoxic microenvironmentsNat Cell Biol201719212013228114271
- PantelKBrakenhoffRHBrandtBDetection, clinical relevance and specific biological properties of disseminating tumor cellsNat Rev Cancer20088532934018404148
- MsakiAPastoACurtarelloMA hypoxic signature marks tumors formed by disseminated tumor cells in the BALB-neuT mammary cancer modelOncotarget2016722330813309527105499
- ZhangLRiethdorfSWuGMeta-analysis of the prognostic value of circulating tumor cells in breast cancerClin Cancer Res201218205701571022908097
- AggarwalCMeropolNJPuntCJRelationship among circulating tumor cells, CEA and overall survival in patients with metastatic colorectal cancerAnn Oncol201324242042823028040
- ZhangTArmstrongAJClinical utility of circulating tumor cells in advanced prostate cancerCurr Oncol Rep2016181326700506
- KulasingheAPerryCJovanovicLNelsonCPunyadeeraCCirculating tumour cells in metastatic head and neck cancersInt J Cancer2015136112515252325111594
- NicholsACLowesLESzetoCCDetection of circulating tumor cells in advanced head and neck cancer using the cell search systemHead Neck201234101440144422076949
- CamptonDERamirezABNordbergJJHigh-recovery visual identification and single-cell retrieval of circulating tumor cells for genomic analysis using a dual-technology platform integrated with automated immunofluorescence stainingBMC Cancer20151536025944336
- SchattonTMurphyGFFrankNYIdentification of cells initiating human melanomasNature2008451717634534918202660
- ChenXLiXZhaoBDormancy activation mechanism of oral cavity cancer stem cellsTumour Biol20153675551555925722113
- Ricci-VitianiLLombardiDGPilozziEIdentification and expansion of human colon-cancer-initiating cellsNature2007445712311111517122771
- AdikrisnaRTanakaSMuramatsuSIdentification of pancreatic cancer stem cells and selective toxicity of chemotherapeutic agentsGastroenterology2012143123424522510202
- MoghbeliMMoghbeliFForghanifardMMAbbaszadeganMRCancer stem cell detection and isolationMed Oncol20143196925064729
- DionneLKDriverERWangXJHead and neck cancer stem cells: from identification to tumor immune networkJ Dent Res201594111524153126253189
- CohenSJPuntCJIannottiNRelationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancerJ Clin Oncol200826193213322118591556
- YuMTingDTStottSLRNA sequencing of pancreatic circulating tumour cells implicates WNT signaling in metastasisNature2012487740851051322763454
- TamWLWeinbergRAThe epigenetics of epithelial-mesenchymal plasticity in cancerNat Med201319111438144924202396
- FukusumiTIshiiHKonnoMCD10 as a novel marker of therapeutic resistance and cancer stem cells in head and neck squamous cell carcinomaBr J Cancer2014111350651424874475
- ZuoJHZhuWLiMYActivation of EGFR promotes squamous carcinoma SCC10A cell migration and invasion via inducing EMT-like phenotype change and MMP-9-mediated degradation of E-cadherinJ Cell Biochem201111292508251721557297
- KupfermanMEJiffarTEI-NaggarATrkB induces EMT and has a key role in invasive of head and neck squamous cell carcinomaOncogene201029142047205920101235
- JohnMADohadwalaMLuoJProinflammatory mediators upregulate snail in head and neck squamous cell carcinomaClin Cancer Res200915196018602719789323
- KangYPantelKTumor cell dissemination: emerging biological insights from animal models and cancer patientsCancer Cell201323557358123680145
- ChafferCLWeinbergRAA perspective on cancer cell metastasisScience201133160241559156421436443
- MascréGDekoninckSDrogatBDistinct contribution of stem and progenitor cells to epidermal maintenanceNature2012489741525726222940863
- NaikPPDasDNPandaPKImplications of cancer stem cells in developing therapeutic resistance in oral cancerOral Oncol20166212213527865365
- ChakrabortyCChinKYDasSmiRNA-regulated cancer stem cells: understanding the property and the role of miRNA in carcinogenesisTumour Biol20163710130391304827468722
- YataKBederLBTamagawaSMicroRNA expression profiles of cancer stem cells in head and neck squamous cell carcinomaInt J Oncol20154741249125626323893
- SunZHuWXuJKaufmannAMAlbersAEMicroRNA-34a regulates epithelial-mesenchymal transition and cancer stem cell phenotype of head and neck squamous cell carcinoma in vitroInt J Oncol20154741339135026323460
- MasudaMWakasakiTTohSStress-triggered atavistic reprogramming (STAR) addiction: driving force behind head and neck cancer?Am J Cancer Res2016661149116627429838
- Oeztuerk-WinderFVenturaJJThe many faces of p38 mitogen-activated protein kinase in progenitor/stem cell differentiationBiochem J20114451110
- SosaMSAvivar-ValderasABragadoPWenHCAguirre-GhisoJAERK1/2 and p38α/β signaling in tumor cell quiescence: opportunities to control dormant residual diseaseClin Cancer Res201117185850585721673068
- CheryLLamHMColemanICharacterization of single disseminated prostate cancer cells reveals tumor cell heterogeneity and identifies dormancy associated pathwaysOncotarget20145209939995125301725
- BarkanDGreenJEChambersAFExtracellular matrix: a gatekeeper in the transition from dormancy to metastatic growthEur J Cancer20104671181118820304630
- MazarAPAhnRWO’HalloranTVDevelopment of novel therapeutics targeting the urokinase plasminogen activator receptor (uPAR) and their translation toward the clinicCurr Pharm Des201117191970197821711234
- XueAXueMJacksonCSmithRCSuppression of urokinase plasminogen activator receptor inhibits proliferation and migration of pancreatic adenocarcinoma cells via regulation of ERK/p38 signalingInt J Biochem Cell Biol2009418–91731173819433314
- BragadoPEstradaYParikhFTGF-β2 dictates disseminated tumor cell fate in target organs through TGF-β-RIII and p38α/β signalingNat Cell Biol201315111351136124161934
- SuiXKongNYeLp38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agentsCancer Lett2014344217417924333738
- ChattergjeeMvan GolenKLFarnesyl transferase inhibitor treatment of breast cancer cells leads to altered RhoA and RhoC GTPase activity and induces a dormant phenotypeInt J Cancer20111291616920824700
- LotanTHicksonJSourisJc-Jun NH2-terminal kinase activating kinase 1/mitogen-activated protein kinase kinase 4-mediated inhibition of SKOV3ip.1 ovarian cancer metastasis involves growth arrest and p21 up-regulationCancer Res20086872166217518381422
- KnopekeMTRitschdorffETClarkRBuilding on the foundation of daring hypotheses: using the MKK4 metastasis suppressor to develop models of dormancy and metastatic colonizationFEBS Lett2011585203159316521925502
- JoHJiaYSubramanianKKCancer cell-derived clusterin modulates the phosphatidylinositol 3′-kinase-Akt pathway through attention of insulin-like growth factor 1 during serum deprivationMol Cell Biol2008284285429918458059
- ScheweDMAguirre-GhisoJAATF6alpha-Rheb-mTOR signaling promotes survival of dormant tumor cells in vivoProc Natl Acad Sci U S A200810530105191052418650380
- TouilYSegardPOstynPMelanoma dormancy in a mouse model is linked to GILZ/FOXO3A-dependent quiescence of disseminated stem-like cellsSci Rep201663040527465291
- HumtsoeJOKramerRHDifferential epidermal growth factor receptor signaling regulates anchorage-independent growth by modulation of the PI3K/AKT pathwayOncogene20102981214122619935697
- ChenHKoJMWongVCLTBP-2 confers pleiotropic suppression and promotes dormancy in a growth factor permissive microenvironment in nasopharyngeal carcinomaCancer Lett20123251899822743615
- ZhuHLuoHShenZHuXSunLZhuXTransforming growth factor-β1in carcinogenesis, progression, and therapy in cervical cancerTumour Biol2016377075708327010470
- QinXYanMZhangJTGFβ3-mediated induction of Periostin facilitates head and neck cancer growth and is associated with metastasisSci Rep201662058726857387
- SosaMSParikhFMaiaAGNR2F1 controls tumour cell dormancy via SOX9- and RARβ-driven quiescence programmesNat Commun20156617025636082
- YumotoKEberMRWangJAxl is required for TGF-β2-induced dormancy of prostate cancer cells in the bone marrowSci Rep201663652027819283
- GaoHChakrabortyGLee-LimAPThe BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sitesCell2012150476477922901808
- WendtMKTaylorMASchiemannBJSchiemannWPDown-regulation of epithelial cadherin is required to initiate metastatic outgrowth of breast cancerMol Biol Cell201122142423243521613543
- DenysHDeryckeLHendrixADifferential impact of TGF-beta and EGF on fibroblast differentiation and invasion reciprocally promote colon cancer cell invasionCancer Lett2008266226327418423981
- BarkanDKleinmanHSimmonsJLInhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeletonCancer Res200868156241625018676848
- KobayashiAOkudaHXingFBone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in boneJ Exp Med2011208132641265522124112
- BuijsJTHenriquezNVvan OverveldPGvan der HorstGten DijkePvan der PluijmGTGF-beta and BMP7 interactions in tumour progression and bone metastasisClin Exp Metastasis200724860961718008174
- NaYRSeokSHKimDJBone morphogenetic protein 7 induces mesenchymal-to-epithelial transition in melanoma cells, leading to inhibition of metastasisCancer Sci2009100112218222519735263
- FangWTFanCCLiSMDownregulation of a putative tumor suppressor BMP4 by SOX2 promotes growth of lung squamous cell carcinomaInt J Cancer2014135480981924477565
- SchofieldRThe relationship between the spleen colony-forming cell and the haemopoietic stem cellBlood Cells197841–2725747780
- ShiozawaYPedersenEAHavensAMHuman prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrowJ Clin Invest201112141298131221436587
- LuXMuEWeiYVCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitorsCancer Cell201120670171422137794
- GhiaurGYegnasubramanianSPerkinsBRegulation of human hematopoietic stem cell self-renewal by the microenvironment’s control of retinoic acid signalingProc Natl Acad Sci U S A201311040161211612624043786
- LindeNFluegenGAguirre-GhisoJAThe relationship between dormant cancer cells and their microenvironmentAdv Cancer Res2016132457127613129
- JungYDeckerAMWangJEndogenous GAS6 and Mer receptor signaling regulate prostate cancer stem cells in bone marrowOncotarget2016718256982571127028863
- JungYShiozawaYWangJPrevalence of prostate cancer metastases after intravenous inoculation provides clues into the molecular basis of dormancy in the bone marrow microenvironmentNeoplasia201214542943922745589
- ShiozawaYPedersenEAPatelLRGAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow nicheNeoplasia201012211612720126470
- CackowskiFCEberMRRheeJMer tyrosine kinase regulates disseminated prostate cancer cellular dormancyJ Cell Biochem2017118489190227753136
- TaichmanRSPatelLRBedenisRGAS6 receptor status is associated with dormancy and bone metastatic tumor formationPLoS One201384e6187323637920
- LuDChenSTanXFra-1 promotes breast cancer chemosensitivity by driving cancer stem cells from dormancyCancer Res201272143451345622586064
- KimJKJungYWangJTBK1 regulates prostate cancer dormancy through mTOR inhibitionNeoplasia20131591064107424027431
- GhajarCMPeinadoHMoriHThe perivascular niche regulates breast tumor dormancyNat Cell Boil2013157807817
- CorselliMChinCJParekhCPerivascular support of human hematopoietic stem/progenitor cellsBlood2013121152891290123412095
- BlissSASinhaGSandifordOAMesenchymal stem cell-derived exosomes stimulate cycling quiescence and early breast cancer dormancy in bone marrowCancer Res201676195832584427569215
- OnoMKosalaNTominagaNExosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cellsSci Signal20147332ra6324985346
- BartoshTJUllahMZeitouniSBeaverJProckopDJCancer cells enter dormancy after cannibalizing mesenchymal stem/stromal cells (MSCs)Proc Natl Acad Sci U S A20161134264476456
- LimPKBlissSAPatelSAGap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cellsCancer Res20117151550156021343399
- van den BeuckenTKochEChuKHypoxia promotes stem cell phenotypes and poor prognosis through epigenetic regulation of DICERNat Commun20142955203
- HoskinPJHypoxia dose painting in prostate and cervix cancerActa Oncol20155491259126226148050
- GilkesDMImplications of hypoxia in breast cancer metastasis to boneInt J Mol Sci20161710E166927706047
- JohnsonRWSchipaniEGiacciaAJHIF targets in bone remodeling and metastatic diseasePharmacol Ther201515016917725681658
- QiuBSimonMCOncogenes strike a balance between cellular growth and homeostasisSemin Cell Dev Biol20154331026277544
- EckersJCKalenALSarsourEHForkhead box M1 regulates quiescence-associated radioresistance of human head and neck squamous carcinoma cellsRadiat Res2014182442042925229973
- BragadoPSosaMSKeelyPCondeelisJAguirre-GhisoJAMicroenvironments dictating tumor cell dormancyRecent Results Cancer Res2012195253922527492
- WeidenfeldKSchif-ZuckSAbu-TayehHDormant tumor cells expressing LOXL2 acquire a stem-like phenotype mediating their transition to proliferative growthOncotarget2016744713627137727655685
- GammonLBiddleAHeywoodHKSubsets of cancer stem cells differ intrinsically in their patterns of oxygen metabolismPLoS One201384e6249323638097
- ZhangHBosch-MarceMShimodaLAMitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxiaJ Biol Chem200828316108921090318281291
- GammonLMackenzieICRoles of hypoxia, stem cells and epithelial-mesenchymal transition in the spread and treatment resistance of head and neck cancerJ Oral Pathol Med2016452778225952002
- ChenDSunYWeiYLIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic markerNat Med201218101511151723001183
- IornsEWardTMDeanSWhole genome in vivo RNAi screening identifies the leukemia inhibitory factor receptor as a novel breast tumor suppressorBreast Cancer Res Treat20121351799122535017
- JohnsonRWFingerECOlcinaMMInduction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrowNat Cell Biol201618101078108927642788
- GoniasSLHuJUrokinase receptor and resistance to targeted anticancer agentsFront Pharmacol2015615426283964
- AmeriKMaltepeEHIGD1A-mediated dormancy and tumor survivalMol Cell Oncol201524e103053727308509
- AmeriKJahangiriARajahAMHIGD1A regulates oxygen consumption, ROS production, and AMPK activity during glucose deprivation to modulate cell survival and tumor growthCell Rep2015 pii:S2211-1247150003300039
- HofstetterCPBurkhardtJKShinBJProtein phosphatase 2A mediates dormancy of glioblastoma multiforme-derived tumor stem-like cells during hypoxiaPLoS One201271e3005922253878
- ArosioADPignataroLGainiRMGaravelloWNeck lymph node metastases from unknown primaryCancer Treat Rev2017531928027480
- SleemanJPThieleWTumor metastasis and the lymphatic vasculatureInt J Cancer2009125122747275619569051
- AchenMGStackerSAMolecular control of lymphatic metastasisAnn N Y Acad Sci2008113122523418519975
- BeasleyNJPrevoRBanerjiSIntratumoral lymphangiogenesis and lymph node metastasis in head and neck cancerCancer Res20026251315132011888898
- OkadaYRelationship of cervical lymph node metastasis to histopathological malignancy grade, tumor angiogenesis, and lymphatic invasion in tongue cancerOdontology201098215315920652794
- HiratsukaSWatanabeASakuraiYThe S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phaseNat Cell Biol200810111349135518820689
- AchyutBRShankarAIskanderASBone marrow derived myeloid cells orchestrate antiangiogenic resistance in glioblastoma through coordinated molecular networksCancer Lett2015369241642626404753
- SleemanJSchmidAThieleWTumor lymphaticsSemin Cancer Biol200919528519482087
- De CockJMShibueTDongreAKeckesovaZReinhardtFWeinbergRAInflammation triggers Zeb1-dependent escape from tumor latencyCancer Res201676236778678427530323
- DaiYWangLTangJActivation of anaphase-promoting complex by p53 induces a state of dormancy in cancer cells against chemotherapeutic stressOncotarget2016718254782549227009858
- KleffelSSchattonTTumor dormancy and cancer stem cells: two sides of the same coin?Adv Exp Med Biol201373414517923143979
- SansonePCeccarelliCBerishajMSelf-renewal of CD133(hi) cells by IL6/Notch3 signalling regulates endocrine resistance in metastatic breast cancerNat Commun201671044226858125
- El TounyLHVieiraAMendozaAKhannaCHoenerhoffMJGreenJECombined SFK/MEK inhibition prevents metastatic outgrowth of dormant tumor cellsJ Clin Invest2014124115616824316974
- ZhuDWYuanYXQiaoJKEnhanced anticancer activity of a protein phosphatase 2A inhibitor on chemotherapy and radiation in head and neck squamous cell carcinomaCancer Lett20153562 pt B77378025449438
