Abstract
Background
The potential benefits and possible risks associated with combined nimotuzumab and concurrent chemoradiotherapy in patients with advanced nasopharyngeal carcinoma (NPC) have yet to be determined.
Methods
The databases PubMed, Web of Science, China National Knowledge Infrastructure, and Wanfang were systematically searched through February 2017 for studies comparing combined nimotuzumab and chemoradiotherapy versus chemoradiotherapy alone in the treatment of NPC. Primary outcomes were complete and partial responses, and the secondary outcome was adverse reactions. The random-effect model was used to pool relative risks (RRs) and 95% confidence intervals (CIs).
Results
Nine randomized control trials and six cohort studies were included in the final analysis (n=1,015 patients). Compared with chemoradiotherapy alone, chemoradiotherapy combined with nimotuzumab was associated with an increased response rate (RR =1.11, 95% CI: 1.01–1.22). Combined treatment further reduced the occurrence rate of erythropenia (RR =0.11, 95% CI: 0.05–0.28) and neutropenia (RR =0.12, 95% CI: 0.05–0.27). The differences in the rates of other complications were not significant.
Conclusion
Nimotuzumab combined with concurrent chemoradiotherapy is more effective in patients with advanced NPC than chemoradiotherapy alone. Patients receiving combination therapy did not have a higher rate of adverse reactions. Nimotuzumab can thus be recommended as an adjunct therapy in patients with advanced NPC.
Introduction
Nasopharyngeal carcinoma (NPC) is a common malignant tumor of the head and neck, especially in China. The World Health Organization estimates that >80% of NPCs occur in China.Citation1 In recent years, the use of intensity-modulated radiation therapy has increased the efficacy of treatment. The survival rate of patients with stage I or II NPC is 90%, and the rates of recurrence and metastasis are 8.2% and 15.4%, respectively.Citation2,Citation3 However, the primary lesion of NPC is often occult such that early detection, diagnosis, and treatment are challenging. Thus, at presentation, >70% of NPC patients have stage III or IV disease. The most common form of treatment for these patients with advanced disease is radiotherapy, but its efficacy is poor and severe adverse reactions occur at high rates.Citation4 Moreover, the rates of local recurrence and distant metastasis are as high as 50%, and the 5-year survival rate for patients treated with radiotherapy alone is only 20%. Indeed, the main reasons for treatment failure are recurrence and metastasis. Therefore, patients with advanced NPC are currently treated with concurrent chemotherapy or neoadjuvant therapy.Citation5 Emerging randomized controlled trials (RCTs) recommend cisplatin, paclitaxel, or both for use in concurrent chemoradiotherapy.Citation6,Citation7 However, despite the considerable therapeutic benefits, concurrent chemoradiotherapy considerably increases the occurrence of severe toxicities, including nausea and vomiting, gastrointestinal reactions, and skin injury.Citation8 Thus, there is an urgent need to identify more effective and tolerable agents for the treatment of advanced NPC.
Many types of cancers overexpress growth factors on the tumor cell surface. In the non-keratinizing form of NPC, 90% of the tumor cells are positive for epidermal growth factor receptor (EGFR).Citation9 Altered EGFR signaling has been widely implicated in processes such as apoptosis, proliferation, metastasis, and invasion.Citation10,Citation11 Thus, targeted therapy against EGFR has been proposed as an adjunct to radiotherapy to improve the curability of NPC. Nimotuzumab is a humanized anti-EGFR mouse monoclonal antibody that reduces immunoreactivity and promotes radiosensitivity. In clinical trials, nimotuzumab combined with concurrent radiotherapy seemed to facilitate radiosensitivity and thus increase treatment efficacy while maintaining low toxicity.Citation12,Citation13 These findings indicate a synergistic effect of nimotuzumab combined with radiotherapy. The efficiency of this combined approach versus chemoradiotherapy alone has been examined in several RCTs and cohort studies, but no conclusions were reached regarding the potential benefits and possible risks in patients with advanced NPC. We therefore conducted a meta-analysis of the recent literature to assess the efficacy and safety of combined nimotuzumab with traditional concurrent chemoradiotherapy.
Methods
Ethical approval was not needed for the present study since it was a meta-analysis of previously published studies. The analysis was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analysis Statement (PRISMA; Figure S1) guidelines and the Cochrane Handbook for Systematic Reviews of Interventions. Ethical approval was also not needed for this secondary study.
Literature search
We performed systematic electronic and manual searches of the relevant literature in PubMed, Web of Science, China National Knowledge Infrastructure, and Wanfang databases from their inception to February 28, 2017. The searches were conducted using the following medical subject headings and key words: “Nimotuzumab OR h-R3” AND “nasopharyngeal carcinoma OR NPC”, “targeted therapy” AND “nasopharyngeal carcinoma OR NPC”, “nasopharyngeal carcinoma OR NPC” AND “radiotherapy OR chemotherapy”. Literature lists of reviews and relevant articles were also retrieved for potentially eligible studies. Only articles published in English and Chinese languages were included.
Inclusion and exclusion criteria
Two investigators independently performed the initial searches, excluding duplicates, and scanning the titles and abstracts of potentially eligible publications. The full text was then obtained for further screening. Any disagreement regarding the appropriateness of the study was solved by discussion and consensus. The criteria for study inclusion were as follows: (1) study design: RCT or cohort study; (2) population: patients with moderate or advanced NPC (stage ≥ III) as primarily confirmed by the pathological results (gold standard); (3) intervention: nimotuzumab combined with concurrent chemoradiotherapy in the experimental group and concurrent chemoradiotherapy alone in the control group; and (4) outcome: complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), or adverse reactions.
Data extraction
The collected data were entered into a standardized Excel sheet, extracted by ZZL, and checked by LFS. The following information was extracted: first author, year of publication, mean ages of the two study groups, numbers of male and female patients, sample size, clinical setting (inclusion criteria for the study population), clinical stage, therapeutic schedules of the trial and control groups, and outcomes. The primary outcomes were CR, PR, SD, and PD, and the secondary outcome was adverse reactions. Any discrepancies were resolved by consensus with the other investigators involved in this study.
Assessment of quality
Our study included RCTs and prospective cohort studies. The Newcastle–Ottawa Scale was used to assess the quality of non-randomized studies based on a “star system” ranking selection, comparability, and outcome. Selection included the exposed cohort, nonexposed cohort, and ascertainment of the exposure and outcome of interest. The outcomes included assessment, length of follow-up, and adequacy of follow-up. Studies with ≥7 stars were considered to be of priority quality, those with ≥5 stars of high quality, and those with ≤5 stars of low quality.Citation14
The Cochrane Collaboration’s tool was used to assess the risk of bias in the RCTs.Citation15 The risk was evaluated as high, low, or unclear according to the following items: random sequence generation, allocation concealment, blinding of participants and outcome assessment, incomplete outcome data, selective reporting, and other biases. Because blinding of the patients and clinicians in these trials was not possible, the studies were considered to have a high risk of bias when another risk item besides blinding was identified.
Statistical analysis
Relative risks (RRs) and 95% confidence intervals (CIs) were used to calculate estimates of dichotomous outcomes. A value of 1 was considered to indicate beneficial effects for patients because the primary outcome was either CR or PR. The heterogeneity of the studies was assessed using the Cochran Q and I2 statistics.Citation16 For the I2 statistic, a value >50% was considered to indicate a high level of heterogeneity. The outcome data were pooled using the random-effect model for significant heterogeneity. The fixed-effect model was used for less significant heterogeneity. The risk of occurrence of 15 adverse reactions was also determined: nausea and vomiting, gastrointestinal reactions, skin injury, leucopenia, radiation dermatitis, mucosa reaction, declining hemoglobin, erythropenia, thrombocytopenia, liver injury, renal injury, neutropenia, myelosuppresion, hair loss, and fever. A sensitivity analysis was performed by sequentially excluding individual studies to assess the stability of the results. Publication bias was assessed by visually inspecting a funnel plot and using Egger’s linear regression test.Citation17–Citation20 All statistical analyses were performed using Stata 14.0 (Stata Corp., College Station, TX, USA) and Review Manager 5.3 (Cochrane Center). P-value <0.05 was considered to indicate statistical significance.
Results
Study selection
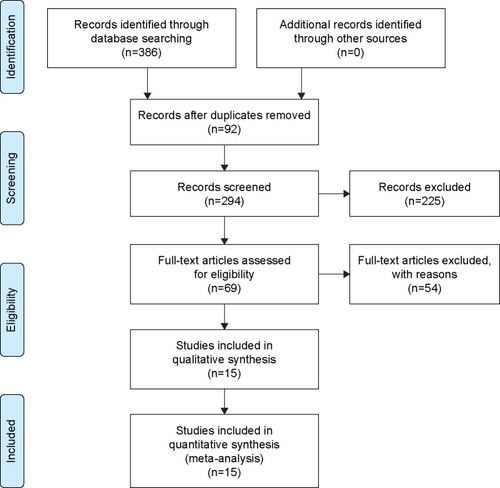
A flow chart of the study selection process is presented in . Our initial search returned 386 results. No record was obtained from additional sources. After the removal of 92 duplicate records, 294 articles were screened for their titles and abstracts. After the full text of 69 articles eligible for inclusion was reviewed, nine RCTs and six cohort studies were finally included in the meta-analysis.Citation4,Citation21–Citation34
General characteristics of the included studies
The general characteristics of the included studies are summarized in . The studies were published from 2012 to 2016, and the sample sizes ranged from 31 to 183, with a total of 1,015 patients. In three of the 15 studies, nimotuzumab was combined with cisplatin and paclitaxel, in six studies with cisplatin and radiotherapy, in three studies with nedaplatin and radiotherapy, in two studies with paclitaxel and radiotherapy, and in one study with carboplatin and radiotherapy. All studies were conducted in adult patients. All outcomes were assessed within 1 year. Consistent with the finding of the World Health Organization that 80% of NPCs occur in China, all of the included studies were from this location.Citation35
Table 1 Characteristics of included study comparing nimotuzumab combined with three-dimensional radiotherapy for nasopharyngeal carcinoma
Quality assessment
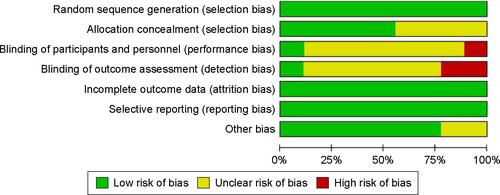
Quality was assessed based on an assessment of the risk of bias (Figures S2 and S3). Among the nine RCTs, three were considered to have a low risk of bias, five an unclear risk, and one a high risk. The main sources of bias were related to allocation concealment, the blinding of participants and personnel, and outcome assessment. Each of the six cohort studies received >5 stars. The overall quality of the cohort studies was high ().
Table 2 Methodological quality assessment (risk of bias) of included studies by Newcastle–Ottawa Scale
Nimotuzumab and chemoradiotherapy versus chemoradiotherapy alone
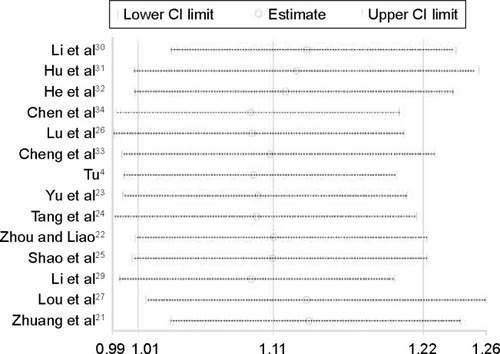
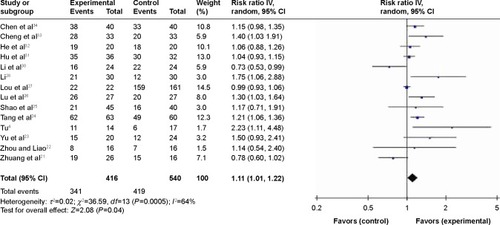
Fourteen studies with 877 NPC patients provided data on CRs and PRs. Compared with chemoradiotherapy alone, patients treated with nimotuzumab combined with chemoradiotherapy had a significantly improved response rate (RR =1.11, 95% CI: 1.01–1.22). The heterogeneity of the studies was 75% ().
Figure 2 Forest plot of nimotuzumab combined with concurrent chemoradiotherapy versus chemoradiotherapy alone in advanced NPC.
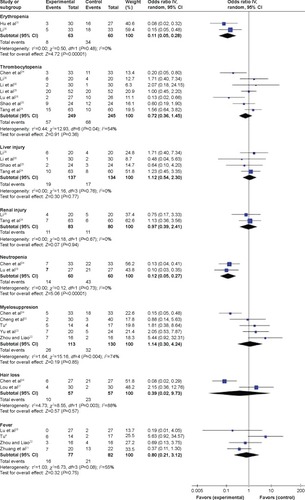
– present the pooled results for adverse reactions. Compared with chemoradiotherapy alone, the combination of nimotuzumab and chemoradiotherapy did not lower the occurrence rate of nausea and vomiting (RR =0.74, 95% CI: 0.39–1.42), gastrointestinal reactions (RR =1.04, 95% CI: 0.42–2.36), skin injury (RR =0.82, 95% CI: 0.39–1.70), leucopenia (RR =1.43, 95% CI: 0.79–2.57), radiation dermatitis (RR =1.08, 95% CI: 0.15–7.74), mucosal reactions (RR =0.79, 95% CI: 0.31–2.02), declining hemoglobin (RR =0.99, 95% CI: 0.62–1.59), thrombocytopenia (RR =0.72, 95% CI: 0.36–1.45), liver injury (RR =1.12, 95% CI: 0.54–2.30), renal injury (RR =0.97, 95% CI: 0.39–2.41), myelosuppression (RR =1.14, 95% CI: 0.30–4.24), hair loss (RR =0.39, 95% CI: 0.02–9.73), or fever (RR =0.80, 95% CI: 0.21–3.12). The results of the overall effect test were not significant (P>0.05). However, the occurrence rates of erythropenia and neutropenia were significantly lower in the combined nimotuzumab group than in the chemoradiotherapy-alone group (RR =0.11, 95% CI: 0.05–0.28; RR =0.12, 95% CI: 0.05–0.27).
Figure 3 Forest plot of adverse reactions between nimotuzumab group and chemoradiotherapy alone.
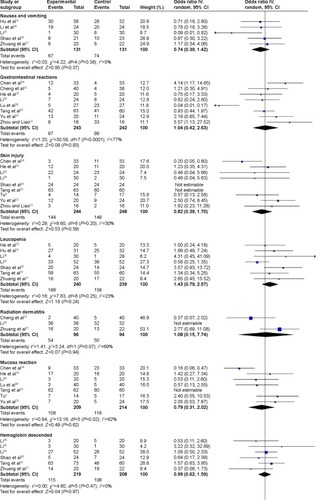
Figure 5 Sensitivity analysis of the pooled estimation.
Sensitivity analyses were conducted by sequentially excluding one study at a time. shows that the pooled estimation was not altered; therefore, the results were statistically robust.
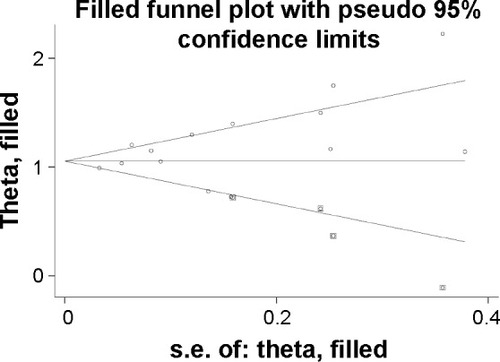
Begg’s and Egger’s tests were used to test for publication bias. Begg’s test did not detect publication bias (Z=1.480, P=0.155), but Egger’s test was based on an inspection of the funnel plot and formal statistical tests (t=2.17, P=0.050). A trim-and-fill analysis showed that four studies were required to balance the asymmetry of the funnel plot ().
Discussion
A comprehensive and systematic literature retrieval and meta-analysis of the eligible articles suggested that nimotuzumab combined with concurrent chemoradiotherapy benefits patients with advanced NPC and is better than chemoradiotherapy alone. The two treatment methods did not differ significantly in the occurrence rates of most of the adverse reactions. However, combined therapy lowered the occurrence rates of erythropenia and neutropenia compared with chemoradiotherapy alone.
Several studies have examined the efficacy of combined nimotuzumab therapy, but ours is the first to comprehensively evaluate the efficacy of nimotuzumab combined with chemoradiotherapy. In previous studies of head and neck carcinomas and NPC, concurrent chemoradiotherapy was shown to be more effective than radiotherapy or chemotherapy alone. However, these cases are rare, as most patients with these cancers do not need concurrent chemoradiotherapy. The 5-year overall survival rate of patients with stage II NPC treated with concurrent chemoradiotherapy is 94.5%, and the treatment plan is effective and feasible. The addition of nimotuzumab to the standard treatment protocol increases the CR rate without increasing the occurrence of adverse reactions. Our results support these findings.
Platinum has always been the main chemotherapeutic agent used to treat NPC, serving as a sensitizer during chemotherapy, radiotherapy, or both. Although patients with NPC greatly benefit from concurrent chemoradiotherapy, relapses and metastases are frequent, especially for patients with advanced NPC.Citation6 Thus, there is an urgent need for adjunct therapies for advanced disease. Nimotuzumab is a human monoclonal antibody that specifically recognizes EGFR-binding sites and blocks other factors from binding to them. The Chinese government approved clinical trials of nimotuzumab in 2002. By binding to extracellular segments of EGFR, nimotuzumab blocks its activation pathways, including those linked to tumor angiogenesis factors, and potently inhibits tumor proliferation and metastasis.Citation36,Citation37 Our results suggest that nimotuzumab synergizes with chemoradiotherapy, as reported in clinical populations. In the study of Nabid et al, the CR of patients treated with nimotuzumab for locally advanced squamous cell cancer of the head and neck after radiotherapy was 70%.Citation38 Crombet et al reported a 3-year survival rate for nimotuzumab combined with radiotherapy of 66.7%, which is significantly higher than that for radiotherapy alone, and fewer adverse reactions.Citation39 The Chinese Academy of Medical Sciences conducted a multicenter Phase II RCT to assess the efficacy and adverse reactions of nimotuzumab combined with radiotherapy in patients with advanced NPC. In that study, the 3-year survival rate was significantly higher in the treated than in the control group (84.3% versus 77.6%), a result attributed to the binding ability of nimotuzumab.Citation40 The affinity of nimotuzumab for EGFR is several times higher than that for the endogenous ligand. Moreover, the occurrence of erythropenia and neutropenia was lower.Citation31 However, these results were specifically reported in only two studies and remain to be confirmed.
A major strength of our meta-analysis is that it was conducted in accordance with PRISMA guidelines and the recommendations outlined in the Cochrane Center handbook. Another strength is the study population, which consisted of first-visit patients with advanced NPC. Our sensitivity analyses confirmed the findings of the included studies. However, there are several limitations that still need to be addressed. First, the studies included in our meta-analysis were performed in different clinical settings, and the standard treatment plans were different (nimotuzumab was combined with cisplatin, paclitaxel, or nedaplatin). This potential heterogeneity must be taken into account in interpreting the results of our analysis. Second, most of the included RCTs were not blinded, which may have caused performance and detection biases. Third, some adverse reactions were examined in only two or three studies; thus, the results may be unstable. Finally, the included studies used experimental arms, including cisplatin and paclitaxel, as the control arms. While their findings address these patients, for others the results remain to be determined.
In conclusion, the use of nimotuzumab combined with concurrent chemoradiotherapy was shown to benefit patients with advanced NPC and was better than chemoradiotherapy alone. Moreover, combined therapy did not increase the rates of adverse reactions. Nimotuzumab can therefore be recommended as adjunct therapy in patients with advanced NPC.
Acknowledgments
The authors thank all their colleagues working in the Department of Oncology, Xiangya Hospital, Central South University. This research was funded by National Natural Science Foundation (81602683) and Hunan Provincial Natural Science Foundation (2015JJ4055).
Supplementary materials
Figure S1 PRISMA checklist.
Notes: Reproduced from Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(6):e1000097.Citation10 For more information, visit: www.prisma-statement.org.
Figure S2 Risk of bias and applicability concerns graph: review authors’ judgments about each domain presented as percentages across included studies.
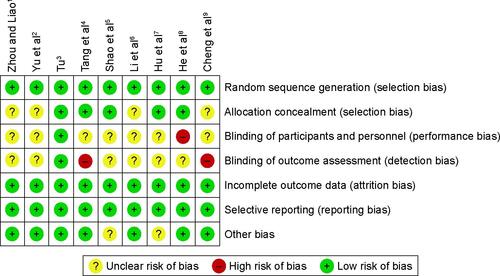
Figure S3 Risk of bias and applicability concerns summary: review authors’ judgments about each domain for each included study.
References
- ZhouTZLiaoZWClinical study of nimotuzumab and three dimensional conformal radiotherapy in the treatment of recurrent nasopharyngeal carcinomaInter Med Health Guidance News2013192132573260
- YuHWZhuagYJYuFLiaoZWZhouTCClinical study of nimotuzumab combined with cisplatin and three-dimensional conformal radiotherapy in treatment of nasopharyngeal carcinomaGuangxi Med J2014367882884
- TuBJClinical studies of Montezuma combined with cisplatin and three-dimensional conformal radiotherapy chemotherapy in the treatment of nasopharyngeal carcinomaGuangzhou University2013 Available from: http://d.g.wanfangdata.com.cn/Thesis_D375768.aspxAccessed November 1, 2017
- TangWBYangWCaoYEffect of nimotuzumab combined with radiotherapy and chemotherapy for locally advanced nasopharyngeal carcinomaGuangdong Med2012331116581662
- ShaoJFLiSPLianYNMaoJXMoYMClinical study of nimotuzumab plus nadaplatin combined with concurrent chemoradiotherapy for locally advanced nasopharyngeal carcinomaModern Med J Chin2014412326
- LiDBaiYXieKAoREfficacy and safety of nimotuzumab combined with nedaplatin and three-dimensional conformal concurrent radiotherapy for nasopharyngeal cancer chemotherapy and the impact of TGF-β1, S100A8 and S100A9J Med Res20154411136139
- HuJXGuWGHeZRLuoHTXuMShort-term effect of nimotuzumab combined with chemoradiotherapy for advanced nasopharyngeal carcinomaGuangdong Med J2013341320942096
- HeNSiYFSunYJEffect of nimotuzumab combined with concurrent chemoradiotherapy for locally advanced nasopharyngeal carcinomaGuangxi Med J2013357834837
- ChengJXunXLiGHClinical utility of nimotuzumab with cisplatin and chemoradiotherapy in advanced nasopharyngeal carcinomaHebei Med J2016222213215
- MoherDLiberatiATetzlaffJAltmanDGPRISMA GroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementPLoS Med200966e100009719621072
Disclosure
The authors report no conflicts of interest in this work.
References
- ChenWZhengRBaadePDCancer statistics in China, 2015CA Cancer J Clin201666211513226808342
- HuangTLChienCYTsaiWLLong-term late toxicities and quality of life for survivors of nasopharyngeal carcinoma treated with intensity-modulated radiotherapy versus non-intensity-modulated radiotherapyHead Neck201638Suppl 1E1026E103226041548
- SuSFHanFZhaoCLong-term outcome of nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapyChin J Cancer Prev Treat20132011853858
- TuBJClinical studies of Montezuma combined with cisplatin and three-dimensional conformal radiotherapy chemotherapy in the treatment of nasopharyngeal carcinomaGuangzhou University2013 Available from: http://d.g.wanfangdata.com.cn/Thesis_D375768.aspxAccessed November 1, 2017
- Ou YangPYXieCMaoYPSignificant efficacies of neoadjuvant and adjuvant chemotherapy for nasopharyngeal carcinoma by meta-analysis of published literature-based randomized, controlled trialsAnn Oncol20132482136214623613477
- LeeAWTungSYChuaDTRandomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinomaJ Natl Cancer Inst2010102151188119820634482
- ChanATLeungSFNganRKOverall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinomaJ Natl Cancer Inst200597753653915812080
- BrowmanGPHodsonDIMackenzieRJBesticNZurawLCancer Care Ontario Practice Guideline Initiative Head and Neck Cancer Disease Site GroupChoosing a concomitant chemotherapy and radiotherapy regimen for squamous cell head and neck cancer: a systematic review of the published literature with subgroup analysisHead Neck200123757958911400247
- ZhangZCFuSWangFWangHYZengYXShaoJYOncogene mutational profile in nasopharyngeal carcinomaOnco Targets Ther2014745746724672248
- MaBBPoonTCToKFPrognostic significance of tumor angiogenesis, Ki 67, p53 oncoprotein, epidermal growth factor receptor and HER2 receptor protein expression in undifferentiated nasopharyngeal carcinoma – a prospective studyHead Neck2003251086487212966511
- ChuaDTNichollsJMShamJSAuGKPrognostic value of epidermal growth factor receptor expression in patients with advanced stage nasopharyngeal carcinoma treated with induction chemotherapy and radiotherapyInt J Radiat Oncol Biol Phys20045911120
- VallathSHyndsRESucconyLJanesSMGiangrecoATargeting EGFR signalling in chronic lung disease: therapeutic challenges and opportunitiesEur Respir J201444251352224435005
- NicholasMKLukasRVChmuraSYaminiBLesniakMPytelPMolecular heterogeneity in glioblastoma: therapeutic opportunities and challengesSemin Oncol201138224325321421114
- WellsGASheaBO’ConnellDThe Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.aspAccessed December 10, 2015
- HigginsJPAltmanDGGøtzschePCCochrane Bias Methods GroupCochrane Statistical Methods GroupThe Cochrane Collaboration’s tool for assessing risk of bias in randomized trialsBMJ2011343d592822008217
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ2003327741455756012958120
- SongFGilbodySBias in meta-analysis detected by a simple, graphical test. Increase in studies of publication bias coincided with increasing use of meta-analysisBMJ19983167129471
- BeggCBMazumdarMOperating characteristics of a rank correlation test for publication biasBiometrics1994504108811017786990
- LiZZhouQLiYFuJHuangXShenLGrowth hormone replacement therapy reduces risk of cancer in adult with growth hormone deficiency: a meta-analysisOncotarget2016749818628186927835910
- LiZZhouQLiYMean cerebral blood volume is an effective diagnostic index of recurrent and radiation injury in glioma patients: a meta-analysis of diagnostic testOncotarget201789156421565028152505
- ZhuangWJLiuCHSituHRClinical observation and evaluation of the combination of nimotuzumab with radiotherapy and cisplatin + 5FU in the treatment of elderly patients with nasopharyngeal carcinomaElderly Med Health Care2016225309312
- ZhouTZLiaoZWClinical study of nimotuzumab and three dimensional conformal radiotherapy in the treatment of recurrent nasopharyngeal carcinomaInter Med Health Guidance News2013192132573260
- YuHWZhuagYJYuFLiaoZWZhouTCClinical study of nimotuzumab combined with cisplatin and three-dimensional conformal radiotherapy in treatment of nasopharyngeal carcinomaGuangxi Med J2014367882884
- TangWBYangWCaoYEffect of nimotuzumab combined with radiotherapy and chemotherapy for locally advanced nasopharyngeal carcinomaGuangdong Med2012331116581662
- ShaoJFLiSPLianYNMaoJXMoYMClinical study of nimotuzumab plus nadaplatin combined with concurrent chemoradiotherapy for locally advanced nasopharyngeal carcinomaModern Med J Chin2014412326
- LuHNiFCLiuLYWangQSiXYNimotuzumab concurrent radiotherapy and cisplatin chemotherapy in advanced nasopharyngeal carcinomaAntitumor Pharmacy201415053
- LouFYangPZhangRComparative study of nimotuzumab with radio-chemotherapy and radio-chemotherapy alone in the treatment of locally advanced nasopharyngeal carcinomaJ Clin Res2016335933935
- LiYLEffects of nimotuzumab with cisplatin and chemoradiotherapy for advanced nasopharyngeal carcinomaJ Med Forum2016364155156
- LiHMLiPQianYJA retrospective paired study: efficacy and toxicity of nimotuzumab versus cisplatin concurrent with radiotherapy in nasopharyngeal carcinomaBMC Cancer201616194627955638
- LiDBaiYXieKAoREfficacy and safety of nimotuzumab combined with nedaplatin and three-dimensional conformal concurrent radiotherapy for nasopharyngeal cancer chemotherapy and the impact of TGF-β1, S100A8 and S100A9J Med Res20154411136139
- HuJXGuWGHeZRLuoHTXuMShort-term effect of nimotuzumab combined with chemoradiotherapy for advanced nasopharyngeal carcinomaGuangdong Med J2013341320942096
- HeNSiYFSunYJEffect of nimotuzumab combined with concurrent chemoradiotherapy for locally advanced nasopharyngeal carcinomaGuangxi Med J2013357834837
- ChengJXunXLiGHClinical utility of nimotuzumab with cisplatin and chemoradiotherapy in advanced nasopharyngeal carcinomaHebei Med J2016222213215
- ChenYJQuanFWangLJLiHHClinical effect of nimotuzumab combined with cisplatin concurrent chemoradiotherapy in the treatment of advanced nasopharyngeal carcinomaChina Med Herald2016133118121
- WHOCancer country profiles 2014 Available from: http://who.int/cancer/country-profiles/en/Accessed March 7, 2017
- GondhowiardjoSMuthalibAKhotimahSRachmanANimotuzumab combined with radiotherapy reduces primary tumor and nodal volume in advanced undifferentiated nasopharyngeal carcinomaAsia Pac J Clin Oncol200953175180
- KameswaranMSamuelGDev SarmaHShindeSNDashAVenkateshM(131)I-Nimotuzumab – a potential radioimmunotherapeutic agent in treatment of tumors expressing EGFRAppl Radiat Isot20151029810226002276
- NabidAGangulyPVenkatesanVA phase I dose escalation study of a humanized monoclonal antibody to EGFR (hR3) in patients with locally advanced squamous cell cancer of the head and neck (SCCHN) treated with radiotherapyInt J Radiat Oncol Biol Phys2002542 Suppl288
- CrombetTNeningerECatalaMEpidermal growth factor based cancer vaccine for non-small cell lung cancer therapy: report from a phase I scale up trialEur J Cancer200238Suppl 7S137
- HuangXDYiJLGaoLMulti-center phase II clinical trial of humanized anti-epidermal factor receptor monoclonal antibody h-R3 combined with radiotherapy for locoregionally advanced nasopharyngeal carcinomaZhonghua Zhong Liu Za Zhi2007293197201 Chinese17649636