Abstract
Purpose
This study aimed to systematically evaluate the efficacy and safety of dendritic cells–cytokine-induced killer (DC–CIK) cells immunotherapy in treating pancreatic cancer (PC) patients.
Methods
Data were collected from published articles of clinical trials. Databases including Web of Science, EMBASE, PubMed, Cochrane Library, Wanfang, and CNKI were searched. The main outcome measures in this research included the overall response rate (ORR), disease control rate (DCR), overall survival (OS), patients’ quality of life (QoL), immune function, and adverse events. Comparative analysis was conducted between DC–CIK immunotherapy and chemotherapy (combined therapy) and chemotherapy alone.
Results
This analysis covered 14 trials with 1,088 PC patients involved. The combined therapy showed advantages over chemotherapy alone in ORR (odds ratio [OR] =1.69, 95% confidence interval [CI] =1.20–2.38, P=0.003), DCR (OR =2.33, 95% CI =1.63–3.33, P<0.00001), OS (1-year OS, OR =3.61, 95% CI =2.41–5.40, P<0.00001; 3-year OS, OR =2.65, 95% CI =1.56–4.50, P=0.0003) and patients’ QoL (P<0.01) with statistical significance. After immunotherapy, lymphocyte subsets’ percentages of CD3+ (P<0.00001), CD4+ (P=0.01), CD3+CD56+ (P<0.00001), and cytokine levels of IFN-γ (P<0.00001) were significantly increased, and the percentages of CD4+CD25+CD127low (P<0.00001) and levels of IL-4 (P<0.0001) were significantly decreased, whereas analysis on CD8+ (P=0.59) and CD4+/CD8+ ratio (P=0.64) did not show a significant difference.
Conclusion
The combination of DC–CIK immunotherapy and chemotherapy is effective for PC treatment, indicated by prolonging the PC patients’ survival time, which benefit from reconstructed immune function of patients.
Introduction
Pancreatic cancer (PC) is a fatal disease with high mortality and poor prognosis.Citation1 It is the twelfth most common cancer and is the seventh leading cause of cancer-related deaths in the world with 338,000 new cases per year.Citation2 In recent years, PC incidence has been significantly raised. The median overall survival (OS) of patients with advanced PC is 4–6 months,Citation3 and the 5-year OS rate is <10%.Citation4 Common therapeutic options for PC are surgery, radiotherapy, and chemotherapy,Citation3 but none of these strategies were able to thoroughly remove small residuals and metastatic cells, which is a main problem to be solved in tumor therapeutics. Therefore, effective therapeutic method should be developed.
Adoptive cellular immunotherapy has demonstrated efficacy for the treatment of various malignant tumors, especially dendritic cells–cytokine-induced killer (DC–CIK) cells mediate immunotherapy.Citation5,Citation6 Compared to immunotherapy using other types of cells, such as lymphokine-activated killer (LAK) cells,Citation7,Citation8 natural killer (NK) cells,Citation9,Citation10 and other immune cells,Citation11,Citation12 DC–CIK-mediated immunotherapy exhibited a stronger antitumor ability and broader antitumor spectrum.Citation13,Citation14 Cytokine-induced killer (CIK) cell is a heterogeneous subset of T lymphocytes, which primarily consist of CD3+CD56+ cells and is easy to be collected from human peripheral and umbilical cord blood, and subsequently induced by IFN-γ, anti-CD3 monoclonal antibodies (OKT-3), and IL-2 in vitro.Citation6 DC are the most potent antigen-presenting cells. DC have the capacity to enhance CIK’s cytotoxicity by coculture with CIK cells, which is indicated by increased proportion of CD3+CD56+ cells and improved levels of cytokines such as IFN-γ and IL-2.Citation5,Citation15
Clinical application of DC–CIK immunotherapy for PC has been reported in several clinical trials.Citation16–Citation19 In a meta-analysis comparing cellular immunotherapy combined with chemotherapy and chemotherapy alone, the former showed significantly prolonged OS,Citation3 while the discussed outcomes were not complete. Analysis considering overall response rate (ORR) and disease control rate (DCR), patients’ quality of life (QoL) and safety were not involved in this analysis. Moreover, the immunotherapy regimens among studies were different (including DC, NK, and LAK), which may influence the analysis of clinical therapy. Our study focused on PC patients treated by DC–CIK immunotherapy and chemotherapy combined therapy or chemotherapy alone, and we performed an up-to-date meta-analysis to provide reliable evidence on the efficacy and safety of DC–CIK immunotherapy in treating PC patients.
Methods
Search strategy and selection criteria
Data were collected from Web of Science, EMBASE, PubMed, Cochrane Library, Wanfang, and CNKI databases using the key terms of “dendritic cells”, “cytokine-induced killer cells” combined with “pancreatic cancer”. No language limits were applied. Literature published before May 2017 was involved in our analysis.
The main selection criteria are that PC patients in the experimental group underwent DC–CIK immunotherapy combined with chemotherapy and patients in the control group were treated by chemotherapy alone.
Data collection and quality assessment
Literature screening and data extraction were carried out by two independent reviewers (YZ and XZ), and disagreements were eliminated upon discussing with a third researcher (AZ). Extracted information included first author’s names, years of publication, study locations, tumor stages, number of cases, patient ages, therapeutic regimens, administration route, in vitro cell culture conditions, and dosages of utilized immune cells. The quality of the included trials was evaluated based on Cochrane Handbook.Citation20
Treatment efficacy
Treatment efficacy was assessed in terms of the complete response (CR) rates, partial response (PR) rates, stable disease (SD) rates, progressive disease (PD) rates, ORR, (ORR = CR + PR) and DCR (DCR = CR + PR + SD). Prognosis was estimated by OS, which was defined as the length of time from the start of treatment to the death of patient from any cause,Citation21 patients’ QoL, and adverse events. Immune function of PC patients before and after treatment was determined by lymphocyte subsets’ percentages (CD3+, CD4+, CD8+, CD3+CD56+, and CD4+CD25+CD127low) and cytokines secretion levels (IFN-γ and IL-4).
Statistical analysis
This meta-analysis was performed using RevMan 5.2 (version 5.2, Nordic Cochran Centre, Copenhagen, Denmark). P<0.05 indicates the statistical significance of the difference. Heterogeneity among studies was assessed to determine suitable analysis model.Citation5,Citation22 Cochran’s Q test was performed to evaluate the homogeneity, and funnel plots were used to assess the publication bias of included studies. I2<50% or P>0.1 indicated that the studies were homogenous. Odds ratio (OR) was the principal measurement for treatment efficacy and is presented with a 95% confidence interval (CI). Sensitivity analysis was conducted to evaluate the consistency of the results and evaluate the influence of single studies on overall risk estimate.Citation23
Results
Search results
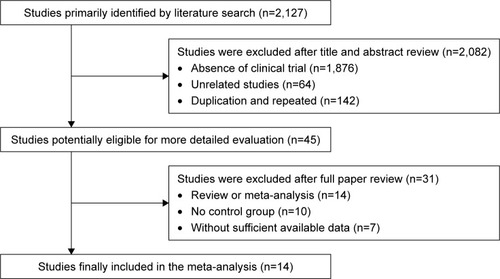
A total of 2,127 articles were identified upon initial retrieve; 2,082 articles were excluded because they lacked clinical trial (n=1,876), were unrelated studies (n=64), and were duplicated (n=142). After a detailed assessment of full texts, 14 reviews or meta-analyses, 10 articles without control group, and seven papers with insufficient data were excluded. Finally, 14 papers of clinical trials that included a total of 1,088 patients were eligible for inclusion in this meta-analysis ().Citation16,Citation17,Citation24–Citation35
Patient’s characteristics
All of the involved trials turn out to be conducted in China. In total, 513 PC patients in eight trials were treated by DC-CIK in combination with chemotherapy, while 575 patients in six trials were treatment by CIK alone. Detailed clinical information of the patients is presented in . DC and CIK cells were obtained from autologous peripheral blood, confirming free of bacterial and fungal contaminations before venous transfusion to the patients. Information of DC–CIK mediate immunotherapy is shown in .
Table 1 Clinical information from the eligible trials in the meta-analysis
Table 2 Information of DC–CIK immunotherapy
Quality assessment
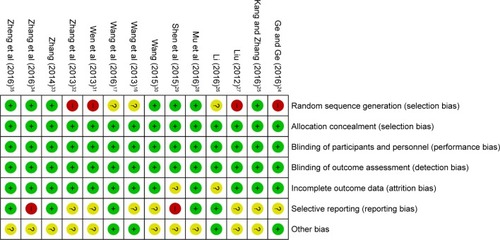
Bias risk assessment is shown in . Seven studies were determined as low risk, four researches were not truly randomized controlled trials, and the remaining three studies lacked clear description of randomization process. Risks of allocation, performance, and detection were low. Two studies absent of follow-up, seven trials with selective reporting were regarded as an unclear risk, and other two studies were considered as high risk for lacking primary outcome data.
Efficacy assessments
In our pooled analysis, patients treated by combined therapy showed higher PR (Figure S1A, OR =1.49, 95% CI =1.06–2.10, P=0.02), ORR (, OR =1.69, 95% CI =1.20–2.38, P=0.003), and DCR (, OR =2.33, 95% CI =1.63–3.33, P<0.00001) and lower PD rates (Figure S1B, OR =0.43, 95% CI =0.30–0.61, P<0.00001) with statistical significance, whereas CR and SD did not show obvious difference from chemo-alone group (Figure S1C and D, CR: OR =1.97, 95% CI =0.85–4.54, P=0.11; SD: OR =1.31, 95% CI =0.95–1.80, P=0.10). Fixed-effect models were used in this analysis because of low heterogeneity ().
Figure 3 Forest plots of the comparison of ORR (A) and DCR (B) between the experimental and control groups.
Abbreviations: CI, confidence interval; DC–CIK, dendritic cells–cytokine-induced killer; DCR, disease control rate; M–H, Mantel–Haenszel; ORR, overall response rate.
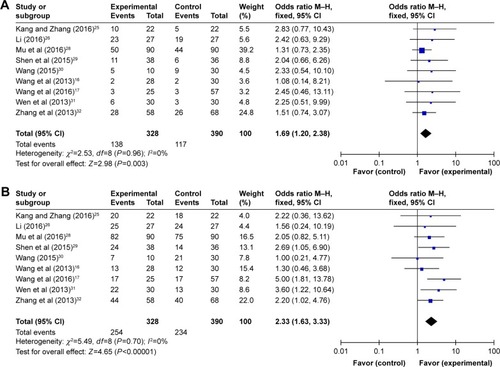
Table 3 Comparison of CR, PR, SD, PD, ORR, and DCR between the experimental and control groups
Prognosis evaluation
In the 14 studies, patients treated by combined therapy had higher OS than those treated by chemotherapy alone (, 1-year OS: OR =3.61, 95% CI =2.41–5.40, P<0.00001; 3-year OS: OR =2.65, 95% CI =1.56–4.50, P=0.0003). Considering slightly significant heterogeneity, fixed-effects model were applied in this analysis. Compared to control group, patients in experimental group showed significantly improved QoL (, OR =3.04, CI =1.58–5.88, P=0.0009) and Karnofsky Performance Score (Kps) (, OR =9.06, 95% CI =7.27–10.84, P<0.00001), which also indicates the performance status of patients.
Figure 4 Forest plot of the comparison of OS between the experimental and control groups.
Abbreviations: CI, confidence interval; DC–CIK, dendritic cells–cytokine-induced killer; M–H, Mantel–Haenszel; OS, overall survival.
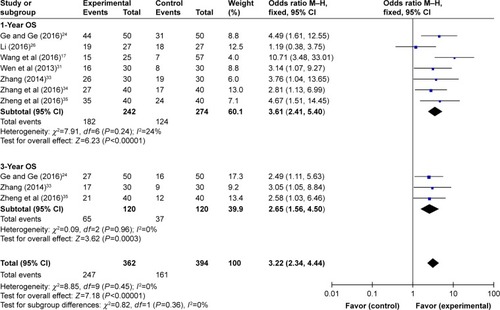
Figure 5 Forest plots of the comparison of QoL between the experimental and control groups.
Abbreviations: CI, confidence interval; DC–CIK, dendritic cells–cytokine-induced killer; Kps, Karnofsky Performance Score; M–H, Mantel–Haenszel; QoL, quality of life.
Immune function evaluation
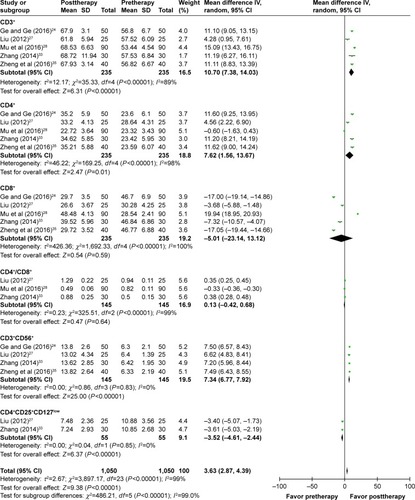
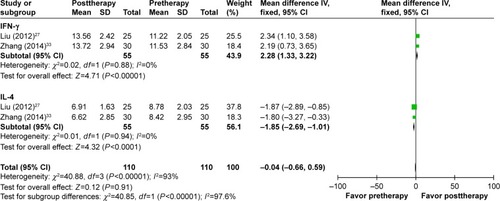
The immune status of patients was examined before and after treatment. As shown in , after DC–CIK treatment, percentages of CD3+, CD4+, and CD3+CD56+ were increased (CD3+: OR =10.70, 95% CI =7.38–14.03, P<0.00001; CD4+: OR =7.62, 95% CI =1.56–13.67, P=0.01; CD3+CD56+: OR =7.34, 95% CI =6.77–7.92, P<0.00001), and percentage of CD4+CD25+CD127low was decreased (OR =−3.52, 95% CI =−4.61 to −2.44, P<0.00001); the changes were statistically significant, whereas proportions of CD8+ and CD4+/CD8+ ratio were not apparently changed (CD8+: OR =−5.01, 95% CI =−23.14 to 13.12, P=0.59; CD4+/CD8+ ratio: OR =0.13, 95% CI =−0.42 to 0.68, P=0.64). In contrast, IFN-γ level was increased distinctly after DC–CIK immunotherapy (IFN-γ: OR =2.28, 95% CI =1.33 to 3.22, P<0.00001), while IL-4 level was dramatically decreased (OR =−1.85, 95% CI =−2.69 to −1.01, P<0.0001) ().
Adverse events’ assessment
In the involved clinical trials, no serious adverse events or death occurrence was reported in patients receiving DC–CIK immunotherapy. As shown in Figures S2 and 3 and , no significant difference was found on adverse events including fever, skin rash, leukopenia, thrombocytopenia, diarrhea, nausea and vomiting, gastrointestinal adverse reaction (AE), fatigue, neutropenia, and myelosuppression between the experimental and control groups (fever: OR =2.39, 95% CI =0.70–8.23, P=0.17 [Figure S3A, fever I + II: OR =4.34, 95% CI =1.35–13.89, P=0.01; fever III + IV: OR =3.11, 95% CI =0.12–79.87, P=0.49]; skin rash: OR =1.32, 95% CI =0.54–3.19, P=0.54 [Figure S3B, skin rash I + II: OR =2.21, 95% CI =0.52–9.36, P=0.28; skin rash III + IV: OR =3.33, 95% CI =0.13–85.11, P=0.47]; leukopenia: OR =0.56, 95% CI =0.22–1.47, P=0.24 [Figure S3C, leukopenia I + II: OR =0.86, 95% CI =0.36–2.06, P=0.73; leukopenia III + IV: OR =0.32, 95% CI =0.06–1.64, P=0.17]; thrombocytopenia: OR =0.54, 95% CI =0.18–1.64, P=0.27 [Figure S3D, thrombocytopenia I + II: OR =0.61, 95% CI =0.22–1.73, P=0.36; thrombocytopenia III + IV: OR =0.32, 95% CI =0.01–8.24, P=0.49]; diarrhea: OR =1.52, 95% CI =0.57–4.03, P=0.40 [Figure S3E, diarrhea I + II: OR =1.58, 95% CI =0.58–4.32, P=0.37; diarrhea III + IV: OR =1.08, 95% CI =0.14–8.21, P=0.94]; nausea and vomiting: OR =0.83, 95% CI =0.30–2.28, P=0.72 [Figure S3F, nausea and vomiting I + II: OR =0.95, 95% CI =0.35–2.60, P=0.92; nausea and vomiting III + IV: OR =0.35, 95% CI =0.01–8.83, P=0.52]; gastrointestinal AE: OR =0.65, 95% CI =0.23–1.90, P=0.43; fatigue: OR =0.66, 95% CI =0.08–5.80, P=0.71; neutropenia: OR =1.09, 95% CI =0.28–4.25, P=0.90; and myelosuppression: OR =0.48, 95% CI =0.19–1.26, P=0.14).
Table 4 Comparison of adverse events between the experimental and control groups
Sensitivity analysis
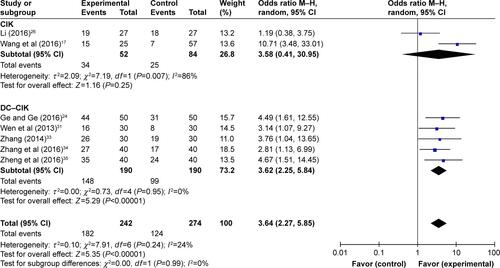
PC patients were treated by DC–CIK immunotherapy in eight trialsCitation24,Citation25,Citation27,Citation28,Citation31,Citation33–Citation35 and by CIK alone in the other six trials.Citation16,Citation17,Citation26,Citation29,Citation30,Citation32 Studies were grouped according to different immunotherapy strategies (CIK or DC–CIK), and pooled results were compared (). The comparison showed both CIK and DC–CIK were effective in treating PC, and no obvious difference between these two methods was observed in most pooled analyses including ORR (Figure S4A), DCR (Figure S4B), and 1-year OS (Figure S5).
Table 5 Meta-analysis of 1-year OS, ORR, and DCR in CIK and DC–CIK subgroups
Publication bias
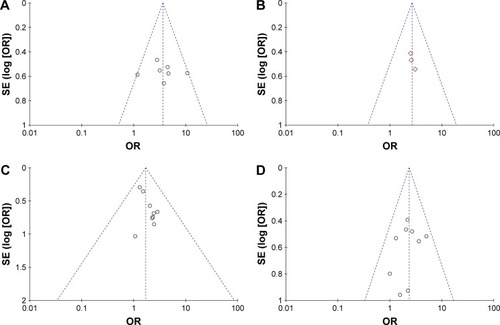
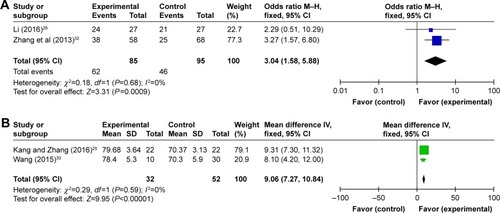
Funnel plots drawn for the studies on primary outcomes (1- and 3-year OS, ORR, and DCR) were symmetrical approximately, which indicated generally controlled publication bias and reliability of our primary conclusions (, 1-year OS; , 3-year OS; , ORR; , DCR).
Discussion
In recent years, immunotherapy using DC–CIK was found effective in PC treatment.Citation16–Citation18 Even though there was statistical analysis of published clinical trials, the exact therapeutic effects were not systematically evaluated and demonstrated because of sample sizes’ variability among these trials. In addition, the different applied protocols in different clinical trials may lead to different clinical response. In this research, we performed an extensive online search followed by rigorous contrasting and combining data analysis in categorization, by which to provide clear and systematical conclusion.
Our analysis showed that DC–CIK immunotherapy enhanced the curative effect of chemotherapy for PC, which was supported by markedly increased ORR (P=0.0003) and DCR (P<0.00001) in PC patients treated by combined therapy. With the addition of DC–CIK immunotherapy, prognosis of PC patients was also improved, according to the significantly prolonged survival time (1-year OS, P<0.00001; 3-year OS, P<0.00001) and QoL (P=0.0009).
Previous study has reported the immunosuppressed status in cancer patient, and several researchers found that adjuvant immunotherapy of DC–CIK was able to enhance the efficacy of chemotherapy for various malignant tumors by reconstructing cancer patient’s immune function.Citation5,Citation6 Our analysis showed that DC–CIK treatment can significantly improve the percentages of CD3+, CD4+, and CD3+CD56+ T cells in PC patients. Moreover, CD4+CD25+CD127low regulatory T cells negatively regulate the antitumor activity of DC–CIK immune cellsCitation36 and our analysis showed result that CD4+CD25+CD127low regulatory T-cell subset proportion decreased after DC–CIK immunotherapy. These results indicated that immune function of chemotherapy-treated PC patients was improved after DC–CIK immunotherapy. However, no significant difference was found in CD8+ T cells’ proportion and CD4+/CD8+ ratio between with and without immunotherapy, which may be caused by various choices of treatment opportunity and DC–CIK transfusion dosages in different clinical trials.Citation6 The balance between Th1 and Th2 cells is crucial in immunotherapy.Citation5 Our analysis showed that after DC–CIK immunotherapy, IFN-γ (Th1 cytokine) level in PC patients was significantly increased, whereas IL-4 (Th2 cytokine) level was obviously decreased, indicating a passably more important role of IFN-γ and IL-4 during the DC–CIK immunotherapy.
Safety is a crucial criterion for the popularization of clinical application of DC–CIK immunotherapy. Based on published literature up to May 2017, our meta-analysis shows that DC–CIK immunotherapy is a safe therapeutic strategy for PC, as no significant difference in adverse events was observed between with and without immunotherapy. Most side effects of DC–CIK immunotherapy were well tolerated by PC patients, and no serious adverse events or death occurred during DC–CIK therapy.
In this analysis, PC patients were treated by DC–CIK immunotherapy in eight trials and CIK alone in the other six trials. To provide evidence for making the choice of using CIK or DC–CIK, difference between their therapeutic effects was evaluated by sensitivity analysis, which showed that both CIK and DC–CIK were effective in treating PC without statistical difference. These results are inconsistent with in vitro study in which DC–CIK represented higher antitumor activity than CIK alone and need to be further explored. Moreover, we conducted publication bias to verify the reliability of our result and no obvious bias exists in our primary conclusions.
Limitations
A total of 14 included trials, which met our selection criteria, turned out to be conducted on Chinese population. One trial conducted in Korea was included in our research originally but was then excluded because it lacked insufficient data. Besides, data analyzed in this research were collected from published papers rather than original patient records, which may lead to overestimation of curative effects.
Conclusion
Our meta-analysis shows that the combination of DC–CIK immunotherapy and chemotherapy is a promising immune treatment for PC patients. It markedly prolongs PC patients’ survival time passably by reconstructing patients’ immune function.
Author contributions
All authors contributed toward data analysis and drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This study was supported by National Science Foundation of China (Nos 81201549 and 81602374), the Fundamental Research Funds for the Central Universities (No 2016qngz05), and the Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University, China (No XJTU1AF-CRF-2015-011).
Supplementary materials
Figure S1 Forest plots of the comparison of PR (A), PD (B), CR (C), and SD (D) rates between the experimental and control groups.
Notes: Control group, chemotherapy alone group; experimental group, chemotherapy with DC–CIK immunotherapy. The fixed-effects meta-analysis model (M–H method) was used.
Abbreviations: CI, confidence interval; CR, complete response; CIK, cytokine-induced killer; DC–CIK, dendritic cells–CIK; M–H, Mantel–Haenszel; PD, progressive disease; PR, partial response; SD, stable disease.
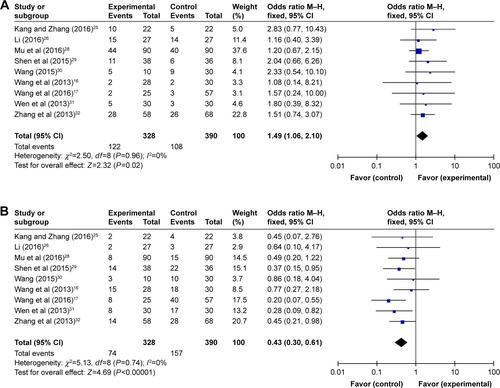
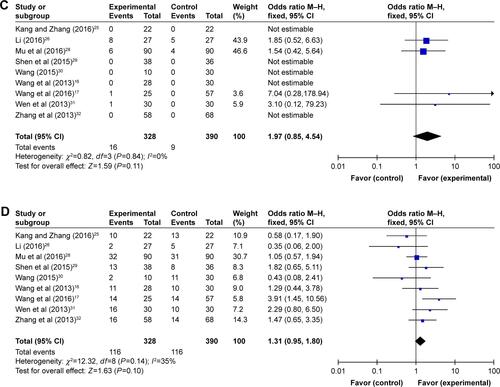
Figure S2 Forest plot of the comparison of adverse effects between the experimental and control groups.
Notes: Control group, chemotherapy alone group; experimental group, chemotherapy with DC–CIK immunotherapy. The random effects meta-analysis model (M–H method) was used.
Abbreviations: CI, confidence interval; DC–CIK, dendritic cells–cytokine-induced killer; M–H, Mantel–Haenszel.
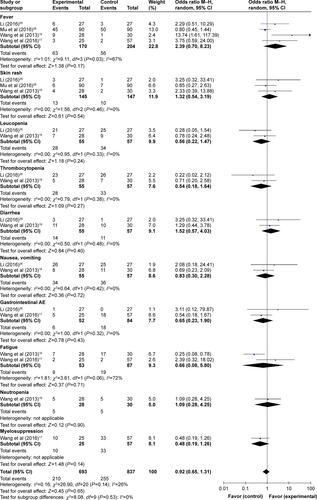
Figure S3 Forest plots of the comparison of all-grade adverse effects including fever (A), skin rash (B), leukopenia (C), thrombocytopenia (D), diarrhea (E), and nausea and vomiting (F).
Notes: Control group, chemotherapy alone group; experimental group, chemotherapy with DC–CIK immunotherapy. The fixed-effects meta-analysis model (M–H method) was used.
Abbreviations: CI, confidence interval; DC–CIK, dendritic cells–cytokine-induced killer; M–H, Mantel–Haenszel.
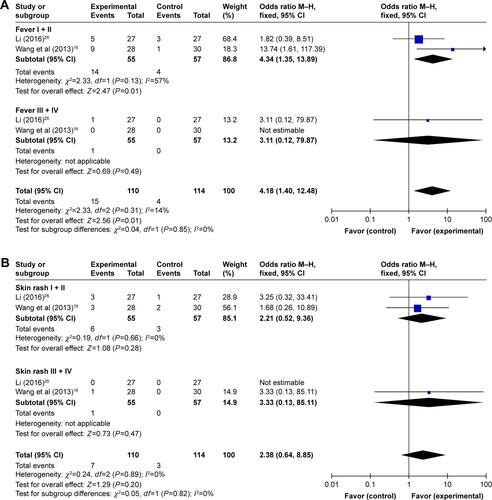
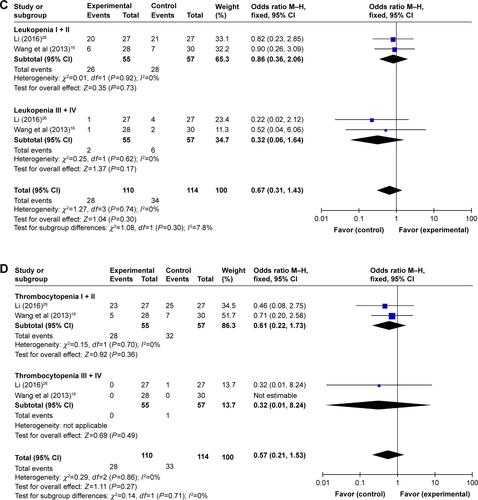
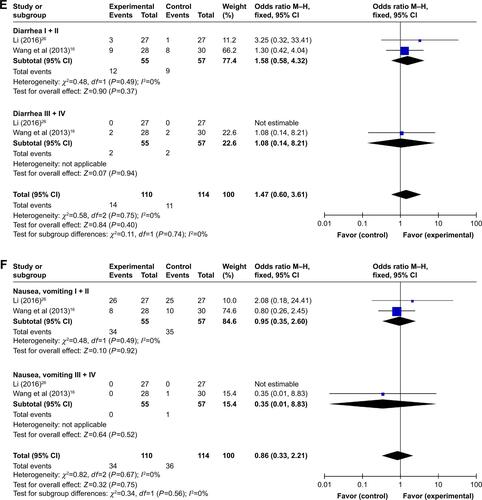
Figure S4 Forest plots of the comparison of ORR (A) and DCR (B) in CIK and DC–CIK subgroups.
Notes: Control group, chemotherapy alone group; experimental group, chemotherapy with DC–CIK immunotherapy. The fixed-effects meta-analysis model (M–H method) was used.
Abbreviations: CI, confidence interval; CIK, cytokine-induced killer; DC–CIK, dendritic cells–CIK; DCR, disease control rate; M–H, Mantel–Haenszel; ORR, overall response rate.
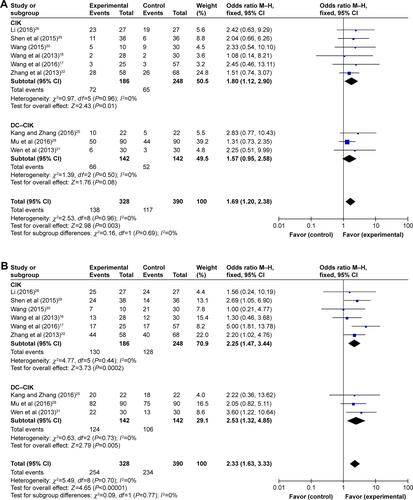
Figure S5 Forest plot of the comparison of 1-year OS in CIK and DC–CIK subgroups.
Notes: Control group, chemotherapy alone group; experimental group, chemotherapy with DC–CIK immunotherapy. The random effects meta-analysis model (M–H method) was used.
Abbreviations: CI, confidence interval; CIK, cytokine-induced killer; DC–CIK, dendritic cells–CIK; M–H, Mantel–Haenszel; OS, overall survival.
Disclosure
The authors report no conflicts of interest in this work.
References
- ChenJGuoXZQiXSClinical outcomes of specific immunotherapy in advanced pancreatic cancer: a systematic review and meta-analysisJ Immunol Res20172017116
- ThindKPadrnosLJRamanathanRKBoradMJImmunotherapy in pancreatic cancer treatment: a new frontierTherap Adv Gastroenterol2017101168194
- ChenLZhangXPrimary analysis for clinical efficacy of immunotherapy in patients with pancreatic cancerImmunotherapy20168222323426565954
- ChangJHJiangYPillarisettyVGRole of immune cells in pancreatic cancer from bench to clinical application: an updated reviewMedicine (Baltimore)20169549e554127930550
- ZhangLMuYZhangACytokine-induced killer cells/dendritic cells–cytokine induced killer cells immunotherapy combined with chemotherapy for treatment of colorectal cancer in China: a meta-analysis of 29 trials involving 2,610 patientsOncotarget2017828451644517728404886
- ZhouCLiuDLiJChemotherapy plus dendritic cells co-cultured with cytokine-induced killer cells versus chemotherapy alone to treat advanced non-small-cell lung cancer: a meta-analysisOncotarget2016752865008651027863436
- DillmanRODumaCMEllisRAIntralesional lymphokine-activated killer cells as adjuvant therapy for primary glioblastomaJ Immunother200932991491919816190
- YamaguchiYHiharaJHironakaKPostoperative immunosuppression cascade and immunotherapy using lymphokine-activated killer cells for patients with esophageal cancer: possible application for compensatory anti-inflammatory response syndromeOncol Rep200615489590116525677
- YangYLimOKimTMPhase I study of random healthy donor-derived allogeneic natural killer cell therapy in patients with malignant lymphoma or advanced solid tumorsCancer Immunol Res20164321522426787822
- ZhaoZLiaoHJuYEffect of compound Kushen injection on T-cell subgroups and natural killer cells in patients with locally advanced non-small-cell lung cancer treated with concomitant radiochemotherapyJ Tradit Chin Med2016361141826946613
- DudleyMEGrossCASomervilleRPRandomized selection design trial evaluating CD8+-enriched versus unselected tumor-infiltrating lymphocytes for adoptive cell therapy for patients with melanomaJ Clin Oncol201331172152215923650429
- KageyamaSIkedaHMiyaharaYAdoptive transfer of MAGE-A4 T-cell receptor gene-transduced lymphocytes in patients with recurrent esophageal cancerClin Cancer Res201521102268227725855804
- MuYZhouCHChenSFEffectiveness and safety of chemotherapy combined with cytokine-induced killer cell/dendritic cell-cytokine-induced killer cell therapy for treatment of gastric cancer in China: a systematic review and meta-analysisCytotherapy20161891162117727421742
- WangZXCaoJXWangMAdoptive cellular immunotherapy for the treatment of patients with breast cancer: a meta-analysisCytotherapy201416793494524794183
- LiuYMuYZhangACytokine-induced killer cells/dendritic cells and cytokine-induced killer cells immunotherapy for the treatment of esophageal cancer in China: a meta-analysisOnco Targets Ther2017101897190828408841
- WangMShiSBQiJLTangXYTianJS-1 plus CIK as second-line treatment for advanced pancreatic cancerMed Oncol201330474724122257
- WangZLiuYLiRAutologous cytokine-induced killer cell transfusion increases overall survival in advanced pancreatic cancerJ Hematol Oncol201691626842696
- ChungMJParkJYBangSParkSWSongSYPhase II clinical trial of ex vivo-expanded cytokine-induced killer cells therapy in advanced pancreatic cancerCancer Immunol Immunother201463993994624916038
- LiWWangYKellnerDBXuLMaiLEfficacy of cytokine-induced killer cells in the treatment of elderly patients with metastatic pancreatic adenocarcinomaCent Eur J Immunol201540218819326557033
- ZengXZhangYKwongJSThe methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic reviewJ Evid Based Med20158121025594108
- HanRXLiuXPanPJiaYJYuJCEffectiveness and safety of chemotherapy combined with dendritic cells co-cultured with cytokine-induced killer cells in the treatment of advanced non-small-cell lung cancer: a systematic review and meta-analysisPLoS One201499e10895825268709
- JacksonDWhiteIRRileyRDQuantifying the impact of between-study heterogeneity in multivariate meta-analysesStat Med201231293805382022763950
- CaiXRLiXLinJXAutologous transplantation of cytokine-induced killer cells as an adjuvant therapy for hepatocellular carcinoma in Asia: an update meta-analysis and systematic reviewOncotarget2017819313183132828412743
- GeJNGeCLEffect of DC–CIK cells on immune function in patients with pancreatic cancer after chemotherapyJ Community Med201614172627
- KangJZhangHYClinical analysis of gemcitabine chemotherapy coast CIK cell treatment of pancreatic cancerChina Contin Med Educ201685141142
- LiXDClinical observation of three dimensional conformal radiotherapy concurrent with chemotherapy combined with autologous cytokine induced killer cell immunotherapy for treatment of locally advanced pancreatic cancerChina Clin Prac Med2016726466
- LiuAHInfluence of chemotherapy combined with DC–CIK cell on the immune function in patients with pancreatic cancerChin J Clin201262063016305
- MuLZhongXPLiuHMClinical efficacy evaluation of DC–CIK in the treatment of advanced pancreatic cancer after chemotherapyGuizhou Med J2016405532535
- ShenDLiuTLinQFClinical evaluation of cytokine-induced killer cells combination with chemotherapy in patients with advanced pancreatic cancerJ Prac Med2015311321482151
- WangJEffects of gemcitabine chemotherapy combined with CIK cells therapy in the treatment of pancreatic cancerMed Pharm Yunnan2015362183185
- WenQXZhuYXuBWangMFThe study of clinical application of DC–CIK combined with chemotherapy on advanced pancreatic cancerMod J Integr Trad Chin West Med2013223640654066
- ZhangYHWangMHTianYXuFClinical research of patients with advanced pancreatic cancer treated with CIK cells combined with chemotherapyJ Basic Clin Oncol2013264317319
- ZhangYLStudy of the influence on immunologic balance and clinical effect by chemotherapy combined with DC–CIK cell to patients with pancreatic cancerChina J Mod Med20142494245
- ZhangZQWangZYZhengSWangXZHeLJEffect of DC–CIK cell therapy on immune function and clinical efficacy analysis of DC–CIK for treatment of pancreatic cancer after chemotherapyChina Prac Med2016117183184
- ZhengCYinYLJinHFLiuHQEffect of chemotherapy combined with DC–CIK cell therapy on immune function in patients with pancreatic cancerBao Jian Wen Hui20166153
- LiuHLiJWangFComparative study of different procedures for the separation of peripheral blood mononuclear cells in cytokine-induced killer cell immunotherapy for hepatocarcinomaTumour Biol20153642299230725417201