Abstract
Lung cancer is a major cause of cancer mortality. Thus, novel therapies are urgently needed. Repositioning of old drugs is gaining great interest in cancer treatment. Astemizole is an antihistamine proposed to be repositioned for cancer therapy. This drug targets several molecules involved in cancer including histamine receptors, ABC transporters and the potassium channels Eag1 and HERG. Astemizole inhibits the proliferation of different cancer cells including those from cervix, breast, leukemia and liver. Gefitinib is widely used to treat lung cancer; however, no response or drug resistance occurs in many cases. Here, we studied the combined effect of astemizole and gefitinib on the proliferation, survival, apoptosis and gene and protein expression of Eag1 channels in the human lung cancer cell lines A549 and NCI-H1975. Cell proliferation and survival were studied by the MTT method and the colony formation assay, respectively; apoptosis was investigated by flow cytometry. Gene expression was assessed by real-time polymerase chain reaction (RT-PCR), and protein expression was studied by Western blot analysis and immunocytochemistry. We obtained the inhibitory concentrations 20 and 50 (IC20 and IC50, respectively) values for each drug from the cell proliferation experiments. Drug combination at their IC20 had a superior effect by reducing cell proliferation and survival in up to 80% and 100%, respectively. The drugs alone did not affect apoptosis of H1975 cells, but the drug combination at their IC20 increased apoptosis roughly four times in comparison to the effect of the drugs alone. Eag1 mRNA levels and protein expression were decreased by the drug combination in A549 cells, and astemizole induced subcellular localization changes of the channel protein in these cells. Our in vitro studies strongly suggest that the combination astemizole–gefitinib may be a novel and promising therapy for lung cancer patients.
Introduction
Lung cancer is the major cause of cancer-related deaths worldwide.Citation1,Citation2 Most of the primary lung cancers are non-small-cell lung cancer (NSCLC).Citation3,Citation4 The most important oncogene drivers in NSCLC patients are mutations in the epidermal growth factor receptor (EGFR) gene; actually, activating mutations are a prime therapeutic target.Citation5,Citation6 The most common “sensitizing mutations” in EGFR (exon 19 deletions and exon 21 L858R mis-sense substitutions) result in constitutive activation of the receptor without ligand binding. In accordance, some of the EGFR tyrosine kinase inhibitors (TKIs) target mutant EGFRs.Citation5,Citation7–Citation9 The first-generation EGFR inhibitors such as gefitinib were the first EGFR-targeted therapies to be registered and later approved by the US Food and Drug Administration (FDA) as a treatment for lung cancer.Citation6,Citation10 Unfortunately, although many patients initially respond to EGFR-targeted therapies, most of them eventually developed resistance and relapsed.Citation11 One of the mechanisms of drug resistance involves the extrusion of TKIs by ATP-binding cassette (ABC) multidrug efflux pumps.Citation12,Citation13 ABCG2 (breast cancer resistance protein) transports gefitinib which also interacts with ABCB1 and ABCC1.Citation14 Because drug resistance decreases the efficacy of the drug, it is necessary to find alternative therapeutic strategies for lung cancer patients.
Drug repositioning involves the identification of novel indications for already existing, well-characterized and well-tolerated drugs reducing costs and bypassing safety concerns; this reposition strategy has emerged as an attractive alternative for cancer treatment.Citation15–Citation18 Astemizole is an anti-histamine that has gained enormous interest to be repositioned for cancer treatment because it targets several molecules involved in cancer including H1 histamine receptors, P-glycoproteins and members of the potassium channel family ether à-go-go.Citation19–Citation28 Human ether à go-go-1 (Eag1, Kcnh1 and Kv10.1) is a voltage-gated channel that displays oncogenic properties and has gained great interest in cancer research.Citation25,Citation26,Citation29,Citation30 The distribution of Eag1 is very restricted in normal tissues. It is mainly expressed in the brain, but low amounts can be detected in placenta, testes and adrenal glands and transiently in myoblasts.Citation29,Citation31,Citation32 Conversely, Eag1 is overexpressed in most human tumors, including liver, cervical, lung, breast, colon and prostate cancer.Citation28,Citation32–Citation35 Astemizole decreases tumor cell proliferation in vitro in breast, liver, cervical and lung cancer cell lines as well as in in vivo breast and liver cancer models.Citation16,Citation24,Citation28,Citation36 In addition, astemizole potentiates the effect of other anticancer drugs in leukemia and breast cancer cells and downregulates Eag1 mRNA expression in breast cancer cells; in addition, astemizole binds to chromatine.Citation36–Citation39 Interestingly, the use of astemizole and loratadine was associated with reduced mortality from different cancers, and astemizole induced sensitization to chemotherapy and reversion of mul-tidrug resistance in NSCLC cells.Citation16
Therefore, because of the high mortality-to-incidence ratio of lung cancer and the potential use of anti-histamines in cancer treatment as well as due to the multitarget properties of astemizole and its synergism with anticancer drugs, here we investigated whether the combination astemizole–gefitinib may be a potential anticancer treatment for lung cancer cells.
Materials and methods
Cells lines and reagents
The human NSCLC cell lines A549 and NCI-H1975 were obtained from the American Type Culture Collection (Manassas, VA, USA) and cultured according to the manufacturer’s instructions. Astemizole and DMSO were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Gefitinib (Iressa®, ZD1839) was kindly provided by AstraZeneca plc (Cambridge, UK). The anti-Eag1 antibody was purchased from Novus International (Littleton, CO, USA) and the anti-actin antibody from Sigma-Aldrich Co.
Metabolic activity
Cell proliferation (assessed by metabolic activity) was assayed by a colorimetric method with 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide (MTT) as described previously.Citation28 Briefly, 3×103 cells per well were seeded in 96-well plates and incubated for 72 hours in culture medium either alone or in the presence of astemizole, gefitinib or DMSO as vehicle. MTT (0.5 mg/mL) was added 4 hours before completing the whole incubation time. Absorbance data were obtained with a microplate photometer (Sunrise Touchscreen).
Colony formation assay
Cell survival was studied with the colony formation assay. Briefly, 2×102 A549 and 5×102 NCI-H1975 cells were cultured in 60 mm Petri dishes to allow the growth of colonies from single separated cells. Twenty-four hours after plating, the cells were incubated for 72 hours in culture medium alone or in the presence of DMSO or the drugs. Afterward, A549 and NCI-H1975 cells were left to grow for 6 and 11 days more, respectively, in the absence of the drugs. Then, cells were fixed in ethanol (absolute grade) for 15 minutes, stained with crystal violet (1%) for 15 minutes and then rinsed four times with water, observed with a microscope and counted.
Apoptosis
Apoptosis was studied by flow cytometry as described previously.Citation28 Briefly, 4×104 cells were seeded in culture plates and incubated during 72 hours in culture medium alone or in the presence of astemizole, gefitinib or vehicle (DMSO). Camptothecin (apoptosis inductor) and methanol (necrosis inductor) were used as controls. Apoptosis was determined with the Annexin V-FITC kit (Thermo Fisher Scientific, Waltham, MA, USA) binding to phosphatidylserine and DNA staining by propidium iodide (PI). Experiments were performed by flow cytometry (CYAN ADP; Dako, Glostrup, Denmark). Percentages of viable (FITC-negative and PI-negative), apoptotic (FITC-positive and PI-negative) and late apoptotic (FITC-positive and PI-positive) cells were obtained by quadrant analysis using the Summit 4.3 software.
Immunocytochemistry
Cell lines were grown on charged glass slides and boiled for antigen retrieval, then blocked with endogenous peroxidase blocker (Bio SB, Santa Barbara, CA, USA) for 10 minutes and then incubated in the presence of 1:500 anti-Eag1 antibody overnight at 4°C. The slides were then incubated with secondary biotin antibody (Bio SB) for 15 minutes and then incubated with streptavidin polymer (Bio SB) for 15 minutes. The specific staining reaction was completed by incubating the slides in the presence of diaminobenzidine in buffer reaction solution (Bio SB) and observed as a brown staining. Sections were counterstained with hematoxylin (Dako). The slides were observed in an Olympus IX51 microscope, Olympus DP70 camera (Tokyo, Japan).
Real-time polymerase chain reaction (RT-PCR)
Total RNA was extracted from cell cultures with TRI-zol reagent. Five micrograms of total RNA was reverse transcribed using the Moloney Murine Leukemia Virus Reverse transcriptase (M-MuLV) (New England BioLabs Inc., Ipswich, MA, USA). RT-PCR was performed with 1 μL of cDNA using the TaqMan™ detection system (Thermo Fisher Scientific) and the Universal PCR Master Mix reagents kit (Thermo Fisher Scientific). Probes previously developed from TaqMan were used to study Eag1 (ID: Hs00924320_m1) and Gusb (ID: Hs00939627_m1, as a constitutive gene) expression. The PCR reaction protocol was 95°C for 15 seconds and 60°C for 1 minute (40 cycles). Data were analyzed with the 2−ΔΔCt method.
Western blot
Cells were washed, scrapped and centrifuged, and the obtained pellet was resuspended in lysis buffer supplemented with protease inhibitors. Lysis was completed with the freezing–thawing process; the lysate was centrifuged and the supernatant collected. Forty micrograms of protein was separated on sodium dodecyl sulfate polyacrylamide gel electrophoresis (10%), transferred to a nitrocellulose membrane and incubated with either the anti-Eag1 antibody (1:750) or the anti-actin antibody (1:100,000). The relative protein quantification was performed with the ImageJ software (NIH, Bethesda, MD, USA).
Statistical analysis
Statistical analysis was performed with analysis of variance (ANOVA) followed by the Tukey–Kramer test using Graph-Pad Prism software version 5.0 (La Jolla, CA, USA). The differences between the drug combination groups and either the drug-alone groups at the corresponding concentration or the control or the vehicle are shown. The analysis of Western blot data was performed with the Student’s t-test. P-values <0.05 were considered to be statistically significant.
Results
Concentration-dependent effect of astemizole and gefitinib on the proliferation of lung cancer cells
We first investigated the effect of astemizole and gefitinib alone on the proliferation of A549 and NCI-H1975 lung cancer cells, assessed by the metabolic activity. The effect of gefitinib is well known in both cell lines,Citation40,Citation41 and the effect of astemizole has been previously reported in A549 cells.Citation16 As expected, both drugs decreased the metabolic activity in a concentration-dependent manner in both cell lines (). From these experiments, we obtained the IC20 and IC50 values for each drug in each cell line ().
Figure 1 Effect of astemizole or gefitinib on the metabolic activity of lung cancer cells.
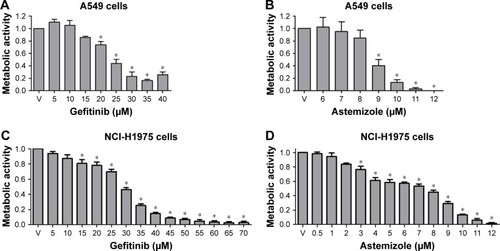
Table 1 Inhibitory concentrations (ICs) of astemizole or gefitinib in the proliferation of human lung cancer cells
Enhanced effect of the combination astemizole–gefitinib on the proliferation, survival and apoptosis of lung cancer cells
Cell proliferation experiments combining the drugs at their IC20 and IC50 concentrations were performed. The combination of the drugs had superior anti-proliferative effects in both cell lines. Astemizole-IC20 in combination with gefitinib-IC20 decreased metabolic activity by 80%, whereas the combination of astemizole-IC20 and gefitinib-IC50 decreased metabolic activity by 95% in A549 cells (). In the NCI-H1975 cells, the combinations of astemizol-IC20 plus gefitinib-IC20 and astemizole-IC20 plus gefitinib-IC50 showed a decrease of metabolic activity by 65% and 90%, respectively (). Based on these results, for the rest of the experiments, we decided to focus on the drug combination at the IC20 found in the cell proliferation assays ().
Figure 2 Enhanced effect of the combination astemizole–gefitinib on the metabolic activity, survival and apoptosis of lung cancer cells.
Abbreviations: A, astemizole; G, gefitinib; FACS, fluorescence-activated cell sorting.
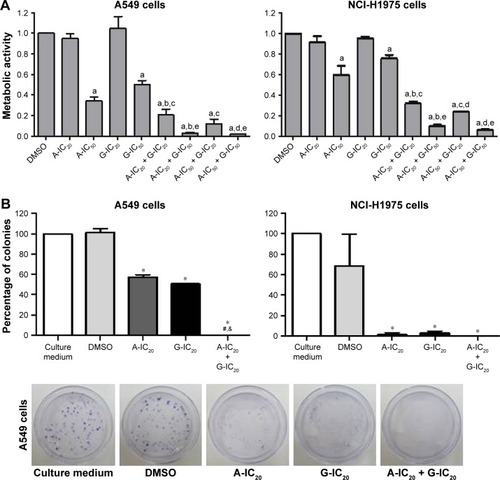
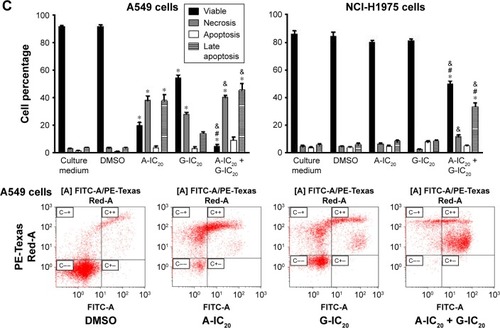
Cell survival (assessed by the colony formation assay) was decreased in a very pronounced manner by the drug combination in comparison with the drugs alone in both cell lines (). In this assay, the combination decreased cell survival completely in both cell lines. Some representative images are shown in . Then, we studied apoptosis by flow cytometry. The drug combination decreased the percentage of viable cells more than the drugs alone in A549 cells, but the effect of the combination on apoptosis was not higher than that produced by astemizole alone (). However, while the drugs alone did not increase apoptosis in comparison with control experiments (in the absence of the drugs) in NCI-H1975 cells, the drug combination increased apoptosis almost four times in comparison with the effect of any of the drugs alone ().
The combination astemizole–gefitinib regulates Eag1 channel expression
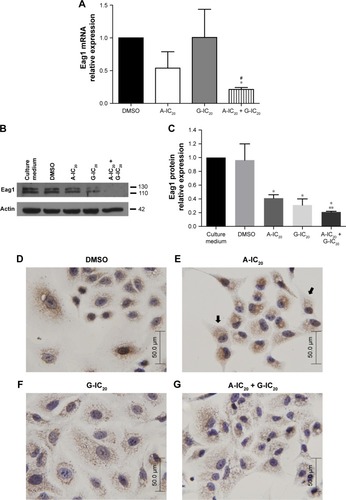
Because Eag1 channel is one of the potential targets of astemizole, and channel expression is regulated by this drug in breast cancer cells,Citation36 we wondered if the drug treatment might regulate Eag1 expression in both cell lines. The drug treatment did not modify Eag1 mRNA levels or protein expression in NCI-H1975 cells (data not shown). However, while the drugs alone did not affect Eag1 mRNA expression in A549 cells, the drug combination decreased Eag1 mRNA levels by 75% in comparison with the expression in either control experiments or the presence of any drug alone (). Western blot analysis also revealed that the drug treatment significantly decreased Eag1 protein expression in A549 cells (). Immunocytochemistry studies showed that control cells treated with either the vehicle or astemizole displayed strong brown immunostaining (). Despite that immunostaining was not that strong in the gefitinib-treated cells (), the weakest signal was observed in the cells treated with the drug combination (). Interestingly, astemizole induced subcellular accumulation of the channel in some parts of the cell adjacent to the nucleus (). This subcellular rearrangement of Eag1 after treatment was exhibited only in A549 cells.
Figure 3 The combination astemizole–gefitinib downregulates Eag1 expression in A549 cells.
Abbreviations: A, astemizole; G, gefitinib.
Discussion
Lung cancer is the main cause of cancer-related deaths worldwide, and thus, identifying new therapeutic strategies is urgently needed.Citation1,Citation2 Unfortunately, most of the patients either do not respond or develop resistance to one of the most common treatments for lung cancer, namely, gefitinib.Citation11 Recently, the anti-histamine astemizole has gained great interest by its anticancer effects, either aloneCitation20,Citation22,Citation23,Citation25,Citation28,Citation42 or in combination.Citation16,Citation20,Citation36,Citation37 In addition, epidemiological studies associated the use of astemizole and loratadine with reduced mortality from different cancers.Citation16
The effect of gefitinib is well known in the A549 and NCI-H1975 lung cancer cells studied,Citation40,Citation41 whereas the effect of astemizole has been previously reported in A549 cells.Citation16
Because astemizole has several targets involved in cancer,Citation19–Citation28,Citation36,Citation37,Citation39 the possible mechanisms explaining the anti-proliferative effects here observed include blockage of oncogenic Eag1 potassium channels, decrease of Eag1 mRNA expression, inhibition of ABC multidrug transporters (which generate resistance to gefitinib)Citation14 and antagonism of the histamine receptor H1. On the other hand, gefitinib may decrease cell proliferation by at least two possible mechanisms. A potential mechanism is the well-known EGFR-dependent pathway inhibition.Citation6,Citation10 The other is EGFR-independent by blocking H2-histamine and H4-histamine receptors, since gefitinib is able to antagonize these receptors and induce cytostasis and differentiation in leukemia cells.Citation43
Here, we also observed that the combination astemizole–gefitinib at low concentrations had superior effects on the metabolic activity, survival and apoptosis of human lung cancer cells. The combination of the drugs at their IC20 decreased cell proliferation in up to 80%, whereas the combination of astemizole-IC20 and gefitinib-IC50 almost completely abolished proliferation in A549 cells. The drug combination also inhibited completely the survival of both cell lines. On the other hand, increased apoptosis was exclusively observed in NCI-H1975 cells only in the presence of the drug combination but not with the drugs alone. Some of the plausible mechanisms explaining the enhanced effects of the drug combination are the convergence on the histamine pathways (both can antagonize different histamine receptors), the increase in gefitinib concentration due to the blockage of ABC transporters by astemizole and/or the decrease in the expression of oncogenic Eag1 channels. Further studies are needed to elucidate the precise mechanism of the enhanced effects of the combination like testing the effect of other antihistamines, silencing or overexpressing the drug targets including histamine receptors, Eag1 potassium channels and ABC transporters, as well as testing the effect of other EGFR inhibitors.
In addition, we found that astemizole induced subcellular accumulation of Eag1 channels. More studies are needed to elucidate in which subcellular compartments this channel relocalization may be taking place and if this rearrangement may be associated with the anti-proliferative mechanism of astemizole. The cell lines displayed some differences in the drug responses. Despite that both cell lines were derived from biopsies of patients with NSCLC, the differences may reflect the heterogeneity of lung cancer cells observed in patients. However, the superior effect of the drug combination was maintained in both cell lines in several experimental approaches.
Astemizole is a nonsedating second-generation antihistamine that does not cross the blood–brain barrier.Citation44 This molecule was withdrawn from the market in several countries especially because severe cardiac side effects including prolongation of the Q-T segment and Torsade de Pointes were observed in cases of overdose.Citation20,Citation45–Citation47 Our results show very strong effects when low concentrations of astemizole and gefitinib were combined. Then, astemizole may be safely administered at proper dose, and especially in combination with gefitinib, the dose may be even lowered. Despite that several studies explaining the precise mechanism of the combination effect are needed, these results suggest the combination astemizole–gefitinib as a novel therapeutic strategy for lung cancer that may help to decrease mortality from this disease.
Acknowledgments
This work was partially supported by AstraZeneca, Mexico (Project 07-1057 to JC). A similar abstract of this paper was presented at the 12th World Cancer Conference 2016 as a conference talk with interim findings. The abstract was published in “Scientific Tracks Abstracts” in Journal of Cancer Science & Therapy: https://www.omicsonline.org/proceedings/the-combination-astemizolegefitinib-as-a-novel-and-promising-therapy-for-human-lung-cancer-in-vitro-studies-54573.html. DOI:10.4172/1948-5956.C1.084.
Disclosure
CAC-A and MV-Y received financial support for undergraduate studies from Universidad de las Fuerzas Armadas, ESPE, Ecuador. The authors report no other conflicts of interest in this work.
References
- FerlayJSoerjomataramIErvikMCancer Incidence and Mortality Worldwide: IARC CancerBase No 11Lyon, FranceInternational Agency for Research on Cancer2013 GLOBOCAN 2012 v1
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- EspositoLContiDAilavajhalaRKhalilNGiordanoALung cancer: are we up to the challenge?Curr Genomics201011751351821532835
- de GrootPMundenRFLung cancer epidemiology, risk factors, and preventionRadiol Clin North Am201250586387622974775
- ChanBAHughesBGTargeted therapy for non-small cell lung cancer: current standards and the promise of the futureTrans Lung Cancer Res20154136
- LiuXWangPZhangCMaZEpidermal growth factor receptor (EGFR): a rising star in the era of precision medicine of lung cancerOncotarget2017830502095022028430586
- ZhuQZhangSDingXHeBZhangHDriver genes in non-small cell lung cancer: characteristics, detection methods, and targeted therapiesOncotarget2017834576805769228915704
- JackmanDMYeapBYSequistLVExon 19 deletion mutations of epidermal growth factor receptor are associated with prolonged survival in non–small cell lung cancer patients treated with gefitinib or erlotinibClin Cancer Res200612133908391416818686
- RosellRMoranTQueraltCScreening for epidermal growth factor receptor mutations in lung cancerN Engl J Med20093611095896719692684
- ZhangHThree generations of epidermal growth factor receptor tyrosine kinase inhibitors developed to revolutionize the therapy of lung cancerDrug Des Devel Ther2016103867
- KrisMGNataleRBHerbstRSEfficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trialJAMA2003290162149215814570950
- Hopper-BorgeEANastoRERatushnyVWeinerLMGolemisEAAstsaturovIMechanisms of tumor resistance to EGFR-targeted therapiesExpert Opin Ther Targets200913333936219236156
- Özvegy-LaczkaCCserepesJElkindNBSarkadiBTyrosine kinase inhibitor resistance in cancer: role of ABC multidrug transportersDrug Resist Updat200581152615939339
- SharomFJABC multidrug transporters: structure, function and role in chemoresistancePharmacogenomics20089110512718154452
- LeeHKangSKimWDrug repositioning for cancer therapy based on large-scale drug-induced transcriptional signaturesPLoS One2016113e015046026954019
- EllegaardA-MDehlendorffCVindACRepurposing cationic amphiphilic antihistamines for cancer treatmentEBioMedicine2016913013927333030
- AshburnTTThorKBDrug repositioning: identifying and developing new uses for existing drugsNat Rev Drug Discov20043867368315286734
- ChengFLiuCJiangJPrediction of drug-target interactions and drug repositioning via network-based inferencePLoS Comput Biol201285e100250322589709
- ParsonsMEGanellinCRHistamine and its receptorsBr J Pharmacol2006147S1S127S13516402096
- García-QuirozJCamachoJAstemizole: an old anti-histamine as a new promising anti-cancer drugAnticancer Agents Med Chem201111330731421443504
- ReynoldsJAkhterJMorrisDIn vitro effect of histamine and histamine H1 and H2 receptor antagonists on cellular proliferation of human malignant melanoma cell linesMelanoma Res199662951008791266
- García-FerreiroREKerschensteinerDMajorFMonjeFStühmerWPardoLAMechanism of block of hEag1 K+ channels by imipramine and astemizoleJ Gen Physiol2004124430131715365094
- DiazLCeja-OchoaIRestrepo-AnguloIEstrogens and human papilloma virus oncogenes regulate human ether-a-go-go-1 potassium channel expressionCancer Res20096983300330719351862
- DownieBRSánchezAKnötgenHEag1 expression interferes with hypoxia homeostasis and induces angiogenesis in tumorsJ Biol Chem200828352362343624018927085
- PardoLAStühmerWEag1: an emerging oncological targetCancer Res20086861611161318339837
- WulffHCastleNAPardoLAVoltage-gated potassium channels as therapeutic targetsNat Rev Drug Discov2009812982100119949402
- AvilaEGarcia-BecerraRRodríguez-RasgadoJACalcitriol down-regulates human ether a go-go 1 potassium channel expression in cervical cancer cellsAnticancer Res20103072667267220682996
- de Guadalupe Chávez-LópezMPérez-CarreónJIZuñiga-GarcíaVAstemizole-based anticancer therapy for hepatocellular carcinoma (HCC), and Eag1 channels as potential early-stage markers of HCCTumor Biol201536861496158
- PardoLAdel CaminoDSanchezAOncogenic potential of EAG K(+) channelsEMBO J199918205540554710523298
- Rodriguez-RasgadoJAAcuna-MaciasICamachoJEag1 channels as potential cancer biomarkersSensors20121255986599522778627
- OcchiodoroTBernheimLLiuJ-HCloning of a human ether-à-go-go potassium channel expressed in myoblasts at the onset of fusionFEBS Lett19984341–21771829738473
- HemmerleinBWeselohRMde QueirozFMOverexpression of Eag1 potassium channels in clinical tumoursMol Cancer2006514117022810
- FariasLMBOcañaDBDíazLEther a go-go potassium channels as human cervical cancer markersCancer Res200464196996700115466192
- OusingsawatJSpitznerMPuntheeranurakSExpression of voltage-gated potassium channels in human and mouse colonic carcinomaClin Cancer Res200713382483117289873
- OrtizCSMontante-MontesDSaqui-SalcesMEag1 potassium channels as markers of cervical dysplasiaOncol Rep20112661377138321887469
- García-QuirozJGarcía-BecerraRBarreraDAstemizole synergizes calcitriol antiproliferative activity by inhibiting CYP24A1 and upregulating VDR: a novel approach for breast cancer therapyPLoS One201279e4506322984610
- IshikawaMFujitaRTakayanagiMTakayanagiYSasakiKReversal of acquired resistance to doxorubicin in K562 human leukemia cells by astemizoleBiol Pharm Bull200023111211510706423
- García-BecerraRDíazLCamachoJCalcitriol inhibits Ether-a go-go potassium channel expression and cell proliferation in human breast cancer cellsExp Cell Res2010316343344219932096
- KongXChenLJiaoLAstemizole arrests the proliferation of cancer cells by disrupting the EZH2-EED interaction of polycomb repressive complex 2J Med Chem201457229512952125369470
- XuRShenHGuoRSunJGaoWShuYCombine therapy of gefitinib and fulvestrant enhances antitumor effects on NSCLC cell lines with acquired resistance to gefitinibBiomed Pharmacother201266538438922560634
- StabileLPLykerJSGubishCTZhangWGrandisJRSiegfriedJMCombined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effectsCancer Res20056541459147015735034
- de Guadalupe Chávez-LópezMHernández-GallegosEVázquez-SánchezAYGariglioPCamachoJAntiproliferative and proapoptotic effects of astemizole on cervical cancer cellsInt J Gynecol Cancer201424582482824819656
- YadavMSinghAKKumarHEpidermal growth factor receptor inhibitor cancer drug gefitinib modulates cell growth and differentiation of acute myeloid leukemia cells via histamine receptorsBiochim Biophys Acta20161860102178219027180173
- NolenTMSedative effects of antihistamines: safety, performance, learning, and quality of lifeClin Ther1997193955
- KingswoodJCRoutledgePALazarisJHA report of overdose with astemizoleHum Toxicol1986543443081429
- BishopROGaudryPLProlongated Q-T interval following astemizole overdoseArch Emerg Med1989663652565725
- CraftTMTorsade de pointes after astemizole overdoseBr Med J1986292660
