Abstract
Targeting of the vascular endothelium compartment explains, in part, the therapeutic efficacy of the nonselective β-adrenergic antagonist propranolol against common endothelial tumors such as hemangiomas. In vitro, the antiangiogenic biological activity of propranolol was shown to inhibit human brain microvascular endothelial cell tubulogenesis. However, possible interference of propranolol with cell signaling associated with the tumoral compartment remains unexplored. We therefore assessed the potency of propranolol against a pediatric brain tumor- derived DAOY medulloblastoma cell model. Gene expression of β1-, β2-, and β3-adrenergic receptors was confirmed in DAOY cells by semiquantitative RT-PCR. We next found that propranolol dose-dependently inhibited induction of the key extracellular matrix-degrading and blood–brain barrier disrupting enzyme matrix metalloproteinase- 9 (MMP-9) by phorbol 12-myristate 13-acetate (PMA). Propranolol not only inhibited PMA- induced phosphorylation of the extracellular signal-regulated kinase (Erk), but also that of IkappaB (IκB), preventing the IκB phosphorylation which is a prerequisite for IκB degradation. Propranolol inhibition of IκB phosphorylation was shown to occur with optimal efficacy at 30 μM. Although propranolol, at up to 100 μM, did not affect cell viability, it potentiated PMA- mediated signaling that ultimately led to diminished phosphorylation of Akt. The anti-Erk and anti-Akt phosphorylation effects are both suggestive of antiproliferative and antisurvival signaling, respectively. Our data are therefore indicative of a pharmacological role for propranolol against β-adrenergic receptor signaling functions involving the nuclear factor-kappaB-mediated regulation of MMP-9.
Introduction
The expression of matrix metalloproteinase-9 (MMP-9) is significantly increased during tumor progression and is considered as a major contributor to the opening of the blood– brain barrier (BBB).Citation1 Although human brain microvascular endothelial cells (HBMEC) play an essential role as structural and functional components of the BBB, it is unclear whether MMP-9 that causes its disruption originates from the vascular or the tumoral compartment. Recent evidence from adenoviral-mediated MMP-9 downregulation demonstrated a key role for MMP-9 in endothelial cell network organization as human dermal microvascular endothelial cell migration and capillary-like tube formation were reduced in cell wounding and spheroid migration assays.Citation2 Aside from involvement in angiogenesis, MMP-9 is also known to be required for tumor vasculogenesis,Citation3 an alternative pathway for neovascularization that is increasingly being found in a variety of states characterized by vascular growth such as hemangioma.Citation4 In the latter, MMP-9 was among the increased hypoxia-induced mediators characterizing the stem/progenitor cells in children with hemangioma.Citation5
Any therapeutic strategies leading to specific targeting of MMP-9 is therefore likely to be of utility in treating common endothelial tumors such as hemangiomas of infancy. Accordingly, therapeutic targeting of β-adrenergic receptor functions with propranolol was found to efficiently inhibit neovascularization during the proliferative phase of infantile hemangioma.Citation6,Citation7 The exact mechanism and signaling pathways involved in this inhibition of MMP-9 expression still remain undefined, and it is believed that marrow-derived endothelial progenitor cells may be partly involved.Citation5 While recent studies delineated a unique brain endothelial phenotype in which MMP-9 secretion by HBMEC was increased upon treatment with the tumor-promoting agent phorbol 12-myristate 13-acetate,Citation8–Citation10 the effects of propranolol and the contribution of β-adrenergic receptor function to the regulation of MMP-9 secretion by the tumor compartment itself has received little attention. In fact, we have shown that MMP-9 is secreted by numerous cell types and that its presence is often indicative of an invasive phenotype during tumor development. Citation8,Citation11–Citation14 Leakiness of the vascular endothelium is among the best known of the deleterious brain tumor-associated effects.Citation15,Citation16 Whether any β-adrenergic receptor-mediated functions are involved in such events is unknown.
In this study, we used the pediatric brain tumor-derived DAOY cell line model to assess the potential contributions of β-adrenergic receptor functions regulating MMP-9 secretion. Propranolol’s pharmacological effects were tested and we provide molecular evidence showing that inhibition of nuclear factor-kappaB (NF-κB)-mediated brain tumor signaling specifically reduces the secretion of MMP-9.
Material and methods
Materials
Propranolol, sodium dodecylsulfate (SDS) and bovine serum albumin (BSA) were purchased from Sigma (Oakville, ON, Canada). Electrophoresis reagents were purchased from Bio-Rad (Mississauga, ON, Canada). The enhanced chemiluminescence (ECL) reagents were from Perkin Elmer (Waltham, MA, USA). Micro bicinchoninic acid protein assay reagents were from Pierce (Rockford, IL, USA). The polyclonal antibodies against phospho-ERK, Akt and phospho-Akt were purchased from Cell Signalling (Danvers, MA, USA), the polyclonal anti-ERK antibody was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The monoclonal antibody against GAPDH was from Advanced Immunochemical Inc. (Long Beach, CA). Horseradish peroxidase-conjugated donkey antirabbit and antimouse IgG secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA). All other reagents were from Sigma-Aldrich Canada.
Cell culture
The human DAOY medulloblastoma cell line was purchased from American Type Culture Collection and was maintained in Eagle’s Minimum Essential Medium containing 10% (v/v) calf serum (HyClone Laboratories, Logan, UT), 2 mM glutamine, 100 units/mL penicillin and 100 mg/mL streptomycin. Cells were incubated at 37°C, with 95% air and 5% CO2.
cDNA synthesis and real-time quantitative RT-PCR
Total RNA was extracted from cultured DAOY cells using TRIzol reagent. For cDNA synthesis, <1 μg total RNA was reverse-transcribed into cDNA using an oligo dT primer and the iScript reverse transcriptase cDNA synthesis kit (Bio-Rad, Mississauga, ON, Canada). cDNA was stored at −20°C for PCR (Applied Biosystems Inc, Foster City, CA). Human primers for β1-(QT00204309), β2-(QT00200011), and β3-(QT00200004) adrenergic receptors and for Peptidylprolyl isomerase A (PPIA, QT01866137) were from QIAGEN. Semi-quantitative RT-PCR analysis was performed starting with 1 μg cDNA, followed by specific gene product amplification with the One-Step RT-PCR Kit (Invitrogen, Burlington, ON, Canada). PCR conditions were optimized so that the gene products were examined at the exponential phase of their amplification and the products were resolved on 1.8% agarose gels containing 1 μg/mL ethidium bromide.
Gelatin zymography
Gelatin zymography was used to assess the extent of proMMP-2 and proMMP-9 activity as previously described.Citation10 Briefly, an aliquot (20 μL) of the culture medium was subjected to SDS– polyacrylamide gel electrophoresis (PAGE) in a gel containing 0.1 mg/mL gelatin. The gels were then incubated in 2.5% Triton X-100 and rinsed in nanopure distilled H2O. Gels were further incubated at 37°C for 20 hours in 20 mM NaCl, 5 mM CaCl2, 0.02% Brij-35, 50 mM Tris-HCl buffer, pH 7.6, then stained with 0.1% Coomassie Brilliant blue R-250 and destained in 10% acetic acid, 30% methanol in H2O. Gelatinolytic activity was detected as unstained bands on a blue background.
Immunoblotting procedures
Proteins from control and treated cells were separated by SDS–PAGE. After electrophoresis, proteins were electrotransferred to polyvinylidene difluoride membranes which were then blocked for 1 hour at room temperature with 5% nonfat dry milk in Tris-buffered saline (150 mM NaCl, 20 mM Tris–HCl, pH 7.5) containing 0.3% Tween-20 (TBST). Membranes were further washed in TBST and incubated with the primary antibodies (1/1000 dilution) in TBST containing 3% bovine serum albumin, followed by a 1-hour incubation with horseradish peroxidase-conjugated antirabbit or antimouse IgG (1/2,500 dilution) in TBST containing 5% nonfat dry milk. Immunoreactive material was visualized by enhanced chemiluminescence (Amersham Biosciences, Baie d’Urfé, QC, Canada).
Cytotoxicity and cell proliferation assays
To assess the effect of propranolol on DAOY cell viability, the release of lactate dehydrogenase (LDH) upon damage of the plasma membrane was analyzed in the same condition media that was used for gelatin zymography. LDH activity was measured at 30°C by a continuous optical test based on the extinction change of pyridine nucleotide at 340 nm as described by the manufacturer’s instructions (Promega). The cleavage of the tetrazolium salt WST-1 {4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulphonate} by mitochondrial dehydrogenases (Roche Diagnostics, Laval, QC, Canada) was also used to assess cell proliferation.
Results
Expression of β-adrenergic receptor transcripts in medulloblastoma-derived DAOY cells
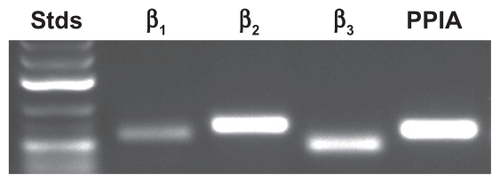
β1-, β2-, and β3-adrenergic receptor gene expression was first assessed for medulloblastoma-derived DAOY cells from which total RNA had been extracted. The design of primers enabled measurement of the expression levels for each of the individual human β-adrenergic genes and of the house keeping gene PPIA. This was validated by visualization of a single cDNA amplicon product obtained from total RNA by semi-quantitative RT-PCR on an agarose gel (). This confirms that β-adrenergic receptors are expressed in DAOY cells.
Figure 1 Gene expression analysis of β-adrenergic receptor expression in DAOY medulloblastoma cells. Total RNA was extracted from medulloblastoma-derived DAOY cells and semi-quantitative RT-PCR performed as described in the Methods section. cDNA amplicons were resolved on an agarose gel in order to confirm a single amplification product.
Propranolol inhibits phorbol 12-myristate 13-acetate (PMA)-induced secretion of MMP-9 in DAOY medulloblastoma cells
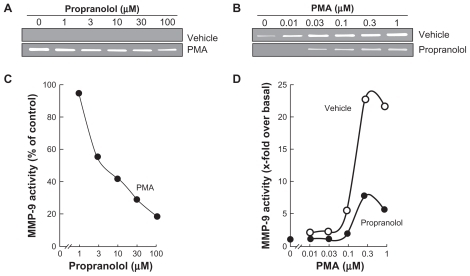
DAOY cells were serum-starved and treated for 18 hours with various doses of propranolol in the presence or absence of a fixed (1 μM) PMA concentration (); other cells were treated with various doses of PMA in the presence or absence of 30 μM propranolol (). The conditioned media were harvested to measure the levels of MMP-9 by gelatin zymography. While MMP-9 activity was undetectable under basal conditions (, upper panel), it was significantly increased in PMA-treated cells (, lower panel). When DAOY were treated with combined PMA and propranolol, MMP-9 was dose-dependently inhibited with an IC50 of <3.1 μM propranolol (). Maximal MMP-9 secretion was achieved with PMA at 1 μM (, upper panel). This induction was significantly inhibited in the presence of 30 μM propranolol (). Collectively, these results suggest that propranolol selectively inhibits MMP-9 in response to carcinogenic-promoting conditions.
Figure 2 Propranolol inhibits PMA-induced matrix metalloproteinase-9 (MMP-9) secretion in DAOY medulloblastoma cells. Medulloblastoma-derived DAOY cells were serum-starved in the presence of various concentrations of propranolol in combination with vehicle or 1 μM PMA for 18 hours (A), or in the presence of various concentrations of PMA in combination with vehicle or 30 μM propranolol for 18 hours (B). Scanning densitometry was used to quantify the extent of proMMP-9 gelatinolytic activity for each set of data (C, D). Data shown is representative of two independent experiments.
Propranolol reverses PMA-mediated IκB decrease
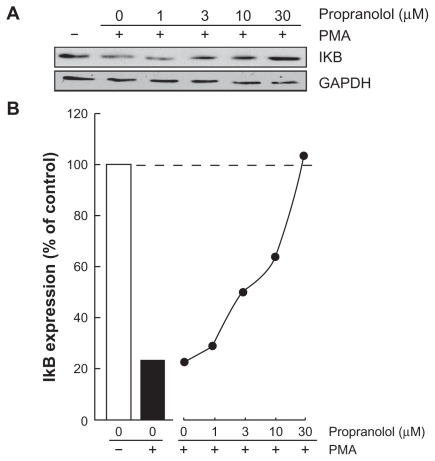
Among MMP-9 expression regulators, the nuclear factor-kappaB (NF-κB) signaling pathway has been demonstrated to link cancer to inflammatory diseases.Citation17 We therefore first assessed whether this signaling was activated upon PMA treatment and whether it was reflected in IkappaB (IκB) degradation. Cells were treated with 1 μM PMA for 18 hours, lysates were isolated and IκB expression was assessed through Western blotting. PMA signaling led to decreased IκB expression (, black bar). Increasing doses of propranolol were found to dose-dependently reverse the PMA-mediated decrease of IκB, suggesting possible signaling interference by propranolol of IκB, whose phosphorylation is essential for its degradation.Citation18
Figure 3 Propranolol reverses PMA-mediated IκB degradation. Medulloblastoma-derived DAOY cells were serum-starved in the presence of various concentrations of propranolol in combination with vehicle or1 μM PMA for 18 hours. A) Lysates were isolated, electrophoresed via sodium dodecylsulfate–polyacrylamide gel electrophoresis and immunodetection of IκB and GAPDH proteins was performed as described in the Methods section. B) Quantification was performed by scanning densitometry of the autoradiograms. Data were expressed as the percent (%) expression of untreated basal conditions.
Propranolol inhibits PMA-induced IκB phosphorylation that leads to IκB degradation
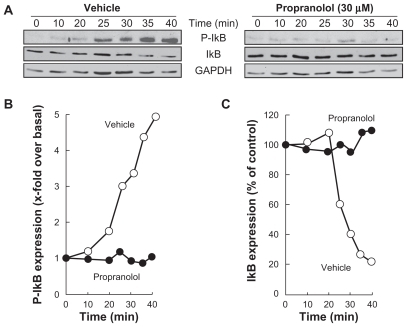
PMA-mediated phosphorylation of IκB was next assessed in order to show whether this explains the subsequent decrease in IκB expression. DAOY cells were treated for 45 minutes with 1 μM PMA following preincubation with either vehicle or 30 μM propranolol. Preincubation with vehicle followed by PMA treatment rapidly led to IκB phosphorylation and to a concomitant decrease in IκB (, left panel). When DAOY cells were preincubated with propranolol, PMA was unable to induce IκB phosphorylation and, consequently, IκB protein levels remained unchanged throughout the 45-minute treatment (, right panel). The corresponding levels of phosphorylated IκB () and of total IκB () expression were quantified by scanning densitometry.
Figure 4 Propranolol inhibits PMA-induced IκB phosphorylation that leads to IκB degradation. A) Medulloblastoma-derived DAOY cells were serum-starved for 30 minutes in the presence of vehicle or 30 μM propranolol. Cells were then incubated for the indicated time with vehicle or 1 μM PMA. Lysates were isolated, electrophoresed via sodium dodecylsulfate–polyacrylamide gel electrophoresis and immunodetection of phosphorylated IκB (P-IκB), IκB, and of GAPDH proteins was performed as described in the Methods section. B, C) Quantification was performed by scanning densitometry of the autoradiograms. Data were expressed as x-fold induction over basal untreated cells for P-IκB, and as the percent (%) expression of untreated basal conditions for IκB.
Propranolol inhibits PMA-induced phosphorylation of Erk, and potentiates PMA-mediated Akt dephosphorylation
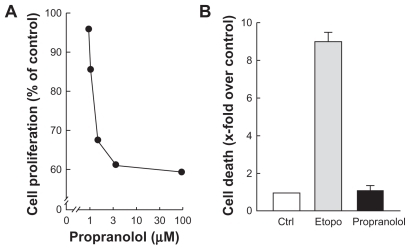
Alternative signaling pathways known to be triggered by PMA include the Erk pathway as well as the Akt pathway. Citation19,Citation20 Although Erk/Akt signaling cross talks are well documented, the former is involved in cell proliferationCitation21 while the latter regulates cell survival.Citation22 Pharmacological β-adrenergic blockade strategies specifically aimed at targeting these two signaling pathways may provide additional tools to reduce DAOY cell proliferation and/or survival. DAOY cells were therefore treated under conditions similar to those shown in (ie, stimulated for 45 minutes with 1 μM PMA following preincubation with either vehicle or 30 μM propranolol). We found that preincubation with vehicle followed by PMA treatment led to increased Erk phosphorylation with no effect on Akt phosphorylation status (, left panel). When DAOY cells were preincubated with propranolol, Erk phosphorylation by PMA was significantly reduced, while phosphorylation levels of Akt decreased as quickly as 10 minutes following PMA stimulation (, right panel). The corresponding ratios of phosphorylated Erk/total Erk () and of phosphorylated Akt/total Akt () were quantified by scanning densitometry. Cell proliferation assays confirmed the antiproliferative effect of propranolol (), while no cell death was induced at up to 100 μM propranolol (). Collectively, this experimental evidence suggests that β-adrenergic blockade rather exerts strong antiproliferative effects combining the converging signaling originating from Erk/Akt pathways.
Figure 5 Propranolol inhibits PMA-induced phosphorylation of Erk, and potentiates PMA-mediated Akt phosphorylation. A) Medulloblastoma-derived DAOY cells were serum-starved for 30 minutes in the presence of vehicle or 30 μM propranolol. Cells were then incubated for the indicated time with vehicle or 1 μM PMA. Lysates were isolated, electrophoresed via sodium dodecylsulfate–polyacrylamide gel electrophoresis and immunodetection of phosphorylated Erk (P-Erk), Erk, phosphorylated Akt (P-Akt), and of Akt proteins was performed as described in the Methods section. B, C) Quantification was performed by scanning densitometry of the autoradiograms. Data were expressed as x-fold induction over basal untreated cells for P-Erk/Erk, and as the percent (%) expression of untreated basal conditions for P-Akt/Akt.
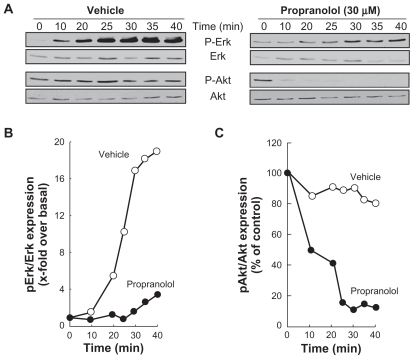
Figure 6 Propranolol inhibits PMA-induced cell proliferation but not cell survival. A) Medulloblastoma-derived DAOY cells were treated as described in and left to grow for 48 hours. Cell proliferation assay was performed as described in the Methods section. B) Cell death was assessed through the release of LDH into the conditioned media and assessed as described in the Methods section of serum-starved DAOY cells treated with vehicle (white bar), 50 μM etoposide (grey bar), or 100 μM propranolol (black bar).
Discussion
Propranolol is a nonselective β-adrenergic antagonist that crosses the BBB and which is widely used clinically for various conditions including hypertension, anxiety and excessive sympathetic responses that often characterize patients during the perioperative period.Citation23 Clinical benefits have been observed in combination with COX-2 inhibitors in postoperation cancer patients, in whom perioperative treatment resulted in improved immune competence and in reduced risk of tumor metastasis.Citation24 It was therefore inferred that blockade of β-adrenergic receptor functions would affect tumor development, an effect that was confirmed by the inhibition of experimentally induced pulmonary adenocarcinoma development.Citation25 The contribution of β-adrenergic receptor functions to tumorigenesis was also reflected by the suggested antiangiogenic effects of β-blockers on a tumor-associated endothelial cell model. As such, evidence for increased expression of β2-adrenergic receptors in the brain tumor-derived vascular compartment was demonstrated, and adrenergic blockade with propranolol resulted in the inhibition of HBMEC tubulogenesis.Citation26 Targeting brain tumor-associated endothelial cell functions with β-blockers, as part of cancer treatments, may therefore become an appealing prospect to be further investigated.
To date, no human clinical study has documented the specific chemotherapeutic effect of propranolol in anticancer therapy. In vivo clinical data will ultimately provide definitive proof as to the therapeutic efficacy of propranolol in anticancer treatments, and may benefit from our in vitro demonstration and elucidation of propranolol’s molecular mechanism of action. Among the strongest evidence, and perhaps the best in vivo study that supports our current data, is the demonstration that MMP-9 and the proangiogenic factor VEGF are both inhibited by propranolol in nasopharyngeal carcinoma tumor cells.Citation27 Several other in vivo approaches have also shed light on the chemopreventive actions of propranolol in reducing pancreatic ductal adenocarcinoma growth in animal modelsCitation28 and in reducing metastatic development of PC-3 prostate cancer in nude mice.Citation29 Such published data strongly suggest a potent anticancer action in line with those we infer in this study using a DAOY pediatric brain tumor-derived cellular model.
One major implication of our study relates to the documented relationship between inflammation and cancer. Increasing evidence suggests that the inflammatory microenvironment in and around tumors is an indispensable participant in the neoplastic process.Citation30 NF-κB plays an important role in the regulation of inflammatory responses and where NF-κB signaling can be activated by diverse stimuli including proinflammatory cytokines, infectious agents and cellular stresses.Citation31 It may therefore be appealing in both the prevention and treatment of brain cancers to target NF-κB signaling that regulates, in part, MMP-9 expression. Accordingly, targeting capacity of several pharmacological agents has led to inhibition of MMP-9; these agents include numerous nutraceutical molecules. Among these, sulforaphane, Citation8,Citation32 epigallocatechingallate, Citation14,Citation33,Citation34 curcumin,Citation35,Citation36 resveratrol,Citation37,Citation38 proanthocyanidinsCitation39 and lycopeneCitation40 have all been proved to inhibit MMP-9 expression/ secretion. More interestingly, all of the above-mentioned diet-derived molecules also abrogated the NF-κB signaling pathway which regulates MMP-9 expression.Citation41
PMA-induced IκB phosphorylation and subsequent degradation, which together results in the release of NF-κB p65 and p50 subunits followed by their nuclear translocation, subsequently regulates MMP-9 transcription.Citation42 We show that inhibition of IκB phosphorylation by propranolol accordingly results in diminished downstream expression of MMP-9 expression. Finally, our data also provide support to the Erk/Akt signaling crosstalk regulating cell proliferation. It is well documented that Erk activation is required for cell proliferation to proceed.Citation43 Furthermore, natural biological regulation of Erk nuclear localization has been demonstrated to regulate cell proliferation, while overactivation of Akt has been shown to prevent the nuclear translocation of Erk by stabilizing endogenous PEA15, resulting in cell proliferation restriction.Citation44 Inhibition of such signaling crosstalk, as we observe for the effect of propranolol, may therefore be viewed as a double check-point control since propranolol not only inhibits Erk phosphorylation status, but would also possibly prevent Akt-mediated Erk nuclear translocation, the overall effect of which will result in blocking cell proliferation, in agreement with .
We have previously reported that medulloblastoma-derived cancer stem cells possessed increased MMP-9 expression in neurosphere cultures,Citation13 and that members of the low-density lipoprotein receptor-related proteins, which also exhibit important functions in MMP-9 recycling,Citation45,Citation46 provided a differential molecular signature between parental and CD133+ DAOY medulloblastoma cells.Citation47 Increased MMP-9 expression was also associated with colospheres derived from colon cancer cultures.Citation48 Collectively, these data suggest that cancer stem cell targeted strategies involving MMP-9 expression may possibly be envisioned. Whether β-adrenergic blockade would be involved remains to be determined. In support of our current data with brain tumor cells, β2-adrenergic antagonists suppressed pancreatic cancer cell invasion by inhibiting NF-κB and MMP-9 expression, Citation49 while MMP-9 levels were decreased upon β1- and β2-adrenoceptor blockade.Citation50 In summary, our data are indicative of a role for propranolol against carcinogen-mediated signaling that leads to the secretion of the BBB disruptor enzyme MMP-9. Our results also illuminate the alternative roles that excessive MMP-9 expression may play in inflammatory diseases and in inflammation associated with tumor development.
Acknowledgment
BA holds a Canada Research Chair in Molecular and Metabolic Oncology from the Canadian Institutes of Health Research (CIHR). RB holds a Research Chair in Cancer Prevention and Treatment (UQAM), and the Claude Bertrand Chair in Neurosurgery (CHUM). This study was funded by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) and by the Claude Bertrand Chair to RB.
Disclosure
The authors declare they have no competing interests.
References
- ShigemoriYKatayamaYMoriTMaedaTKawamataTMatrix metalloproteinase-9 is associated with blood-brain barrier opening and brain edema formation after cortical contusion in ratsActa Neurochir Suppl20069613013316671440
- JadhavUChigurupatiSLakkaSSMohanamSInhibition of matrix metalloproteinase-9 reduces in vitro invasion and angiogenesis in human microvascular endothelial cellsInt J Oncol2004251407141415492832
- AhnGOBrownJMMatrix metalloproteinase-9 is required for tumour vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cellsCancer Cell20081319320518328424
- BoscoloEBischoffJVasculogenesis in infantile hemangiomaAngiogenesis20091219720719430954
- KleinmanMEGreivesMRChurginSSHypoxia-induced mediators of stem/progenitor cell traff icking are increased in children with hemangiomaArterioscler Thromb Vasc Biol2007272664267017872454
- Léauté-LabrèzeCDumas de la RoqueEHubicheTBoraleviFThamboJBTaïebAPropranolol for severe hemangiomas of infancyN Engl J Med20083582649265118550886
- SiegfriedECKeenanWJAl-JureidiniSMore on propranolol for hemangiomas of infancyN Engl J Med20083592846 author reply 2846–284719109584
- AnnabiBRojas-SutterlinSLarocheMLachambreMPMoumdjianRBéliveauRThe diet-derived sulforaphane inhibits matrix metalloproteinase- 9-activated human brain microvascular endothelial cell migration and tubulogenesisMol Nutr Food Res20085269270018435488
- RoomiMWMonterreyJCKalinovskyTRathMNiedzwieckiADistinct patterns of matrix metalloproteinase-2 and -9 expression in normal human cell linesOncol Rep20092182182619212645
- SinaALord-DufourSAnnabiBCell-based evidence for aminopeptidase N/CD13 inhibitor actinonin targeting of MT1-MMP-mediated proMMP-2 activationCancer Lett200927917117619264392
- AbécassisIOlofssonBSchmidMZalcmanGKarniguianARhoA induces MMP-9 expression at CD44 lamellipodial focal complexes and promotes HMEC-1 cell invasionExp Cell Res200329136337614644158
- DemeuleMRéginaAAnnabiBBertrandYBojanowskiMWBéliveauRBrain endothelial cells as pharmacological targets in brain tumoursMol Neurobiol20043015718315475625
- AnnabiBRojas-SutterlinSLaflammeCTumour environment dictates medulloblastoma cancer stem cell expression and invasive phenotypeMol Cancer Res2008690791618567795
- AnnabiBCurrieJCMoghrabiABéliveauRInhibition of HuR and MMP-9 expression in macrophage-differentiated HL-60 myeloid leukemia cells by green tea polyphenol EGCgLeuk Res2007311277128417081606
- HigashidaTKreipkeCWRafolsJAThe role of hypoxia-inducible factor-1alpha, aquaporin-4, and matrix metallo-proteinase-9 in blood-brain barrier disruption and brain edema after traumatic brain injuryJ Neurosurg2010
- TsugeMYasuiKIchiyawaTIncrease of tumour necrosis factor-alpha in the blood induces early activation of matrix metalloproteinase- 9 in the brainMicrobiol Immunol20105441742420618688
- DongJJimiEZeissCHaydenMSGhoshSConstitutively active NF-kappaB triggers systemic TNFalpha-dependent inflammation and localized TNFalpha-independent inflammatory diseaseGenes Dev2010241709171720713516
- SoltLAMayMJThe IkappaB kinase complex: master regulator of NF-kappaB signallingImmunol Res20084231818626576
- AgarwalRAgarwalCIchikawaHSinghRPAggarwalBBAnticancer potential of silymarin: from bench to bed sideAnticancer Res2006264457449817201169
- ShiodaNHanFFukunagaKRole of Akt and ERK signalling in the neurogenesis following brain ischemiaInt Rev Neurobiol20098537538719607982
- BurkhardKSmithSDeshmukhRMacKerellADJrShapiroPDevelopment of extracellular signal-regulated kinase inhibitorsCurr Top Med Chem2009967868919689374
- DillonRLMullerWJDistinct biological roles for the akt family in mammary tumour progressionCancer Res2010704260426420424120
- EmilienGMaloteauxJMCurrent therapeutic uses and potential of beta-adrenoceptor agonists and antagonistsEur J Clin Pharmacol1998533894049551698
- BenishMBartalIGoldfarbYPerioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumour metastasisAnn Surg Oncol2008152042205218398660
- ParkPGMerrymanJOrloffMSchullerHMBeta-adrenergic mitogenic signal transduction in peripheral lung adenocarcinoma: implications for individuals with preexisting chronic lung diseaseCancer Res199555350435087627955
- AnnabiBLachambreMPPlouffeKMoumdjianRBéliveauRPropranolol adrenergic blockade inhibits human brain endothelial cells tubulogenesis and matrix metalloproteinase-9 secretionPharmacol Res20096043844519467330
- YangEVSoodAKChenMNorepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumour cellsCancer Res200666103571036417079456
- Al-WadeiHAAl-WadeiMHSchullerHMPrevention of pancreatic cancer by the beta-blocker propranololAnticancer Drugs20092047748219387337
- PalmDLangKNiggemannBThe norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockersInt J Cancer20061182744274916381019
- WangHChoCHEffect of NF-κB signalling on apoptosis in chronic inflammation-associated carcinogenesisCurr Cancer Drug Targets201010593599
- TakPPFiresteinGSNF-kappaB: a key role in inflammatory diseasesJ Clin Invest200110771111134171
- MoonDOKimMOKangSHChoiYHKimGYSulforaphane suppresses TNF-alpha-mediated activation of NF-kappaB and induces apoptosis through activation of reactive oxygen species-dependent caspase-3Cancer Lett200927413214218952368
- SenTDuttaAChatterjeeAEpigallocatechin-3-gallate (EGCG) downregulates gelatinase-B (MMP-9) by involvement of FAK/ERK/ NFkappaB and AP-1 in the human breast cancer cell line MDA-MB-231Anticancer Drugs20102163264420527725
- FarabegoliFPapiAOrlandiM(−)Epigallocatechin-3-gallate downregulates EGFR, MMP-2, MMP-9 EMMPRIN and inhibits the invasion of MCF-7 tamoxifen resistant cellsBiosci Rep2010
- BangaruMLChenSWoodliffJKansraSCurcumin (diferuloylmethane) induces apoptosis and blocks migration of human medulloblastoma cellsAnticancer Res20103049950420332461
- YuYMLinHCCurcumin prevents human aortic smooth muscle cells migration by inhibiting of MMP-9 expressionNutr Metab Cardiovasc Dis20102012513219447587
- BedirliASalmanBPasaogluHOfluogluESakrakOEffects of Nuclear Factor-kappaB Inhibitors on Colon Anastomotic Healing in RatsJ Surg Res2010
- LiuPLTsaiJRCharlesALResveratrol inhibits human lung adenocarcinoma cell metastasis by suppressing heme oxygenase 1-mediated nuclear factor-kappaB pathway and subsequently down-regulating expression of matrix metalloproteinasesMol Nutr Food Res201054S196S20420461740
- DézielBAPatelKNetoCGottschall-PassKHurtaRAProantho-cyanidins from the american cranberry (vaccinium macrocarpon) inhibit matrix metalloproteinase-2 and matrix metalloproteinase-9 activity in human prostate cancer cells via alterations in multiple cellular signalling pathwaysJ Cell Biochem201011174275420626034
- HwangESLeeHJInhibitory effects of lycopene on the adhesion, invasion, and migration of SK-Hep1 human hepatoma cellsExp Biol Med (Maywood)200623132232716514180
- RalhanRPandeyMKAggarwalBBNuclear factor-kappa B links carcinogenic and chemopreventive agentsFront Biosci (Schol Ed)20091456019482682
- St-PierreYCouillardJvan ThemscheCRegulation of MMP-9 gene expression for the development of novel molecular targets against cancer and inflammatory diseasesExpert Opin Ther Targets2004847348915469396
- ChambardJCLeflochRPouysségurJLenormandPERK implication in cell cycle regulationBiochim Biophys Acta200717731299131017188374
- GervaisMDugourdCMullerLAkt down-regulates ERK1/2 nuclear localization and angiotensin II-induced cell proliferation through PEA-15Mol Biol Cell2006173940395116822839
- DesrosiersRRRivardMEGrundyPEAnnabiBDecrease in LDL receptor-related protein expression and function correlates with advanced stages of Wilms tumorsPediatr Blood Cancer200646404916106426
- JinRYangGLiGMolecular insights and therapeutic targets for blood-brain barrier disruption in ischemic stroke: critical role of matrix metalloproteinases and tissue-type plasminogen activatorNeurobiol Dis20103837638520302940
- AnnabiBDoumitJPlouffeKLaflammeCLord-DufourSBéliveauRMembers of the low-density lipoprotein receptor-related proteins provide a differential molecular signature between parental and CD133+ DAOY medulloblastoma cellsMol Carcinog20104971071720564348
- WeiswaldLBRichonSValidirePNewly characterised ex vivo colospheres as a three-dimensional colon cancer cell model of tumour aggressivenessBr J Cancer200910147348219603013
- ZhangDMaQYHuHTZhangMbeta2-adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NFkappaB and AP-1Cancer Biol Ther201010192920424515
- Romana-SouzaBSantosJSMonte-Alto-CostaAbeta-1 and beta-2, but not alpha-1 and alpha-2, adrenoceptor blockade delays rat cutaneous wound healingWound Repair Regen20091723023919320892