Abstract
Cabozantinib is an oral tyrosine-kinase inhibitor whose targets include VEGFR, MET, and AXL. Cabozantinib is approved for the treatment of patients with advanced clear-cell renal-cell carcinoma (RCC) who have received prior antiangiogenic therapy. In the pivotal Phase III trial of second-line RCC, cabozantinib was associated with a significant improvement in overall survival, progression-free survival, and antitumor response compared with everolimus. Adverse events (AEs) were common for patients receiving cabozantinib, but were effectively managed with supportive care and dose modifications, as discontinuations of cabozantinib due to an AE were infrequent. This article reviews the management of the more common AEs associated with cabozantinib based on findings from the pivotal study, clinical practice guidelines, and the authors’ real-world clinical experience, with support from published literature. We focus on hypertension, palmar–plantar erythrodysesthesia, diarrhea, nausea, vomiting, decreased appetite, fatigue, and stomatitis. Effective management of these AEs involves a multimodal strategy that includes patient education, prophylactic and supportive care, and dose modifications. Effective AE management can allow patients to maintain antitumor activity with cabozantinib while mitigating the impact on quality of life.
Introduction
In the US, an estimated 64,000 new cases of kidney cancer will be diagnosed in 2017, primarily composed of clear-cell renal-cell carcinoma (RCC).Citation1 Until as recently as 2006, treatments for advanced RCC were limited to cytokines, including IFNα and IL2. These therapies were associated with considerable toxicity and showed limited efficacy.Citation2 This led to the development of therapies, including tyrosine-kinase inhibitors (TKIs), that target the VEGF pathway, which drives angiogenesis and tumor growth.Citation3–Citation5 Clinical studies have demonstrated that sequential use of VEGF-pathway inhibitors is a feasible and effective approach to treatment,Citation6–Citation8 and is supported in treatment guidelines.Citation9–Citation11
In 2016, the TKI cabozantinib was approved by the US Food and Drug Administration and the European Medicines Agency for the treatment of patients with advanced clear-cell RCC who have received prior antiangiogenic therapy, and became a preferred second-line option for RCC in US and European treatment guidelines.Citation9–Citation13 Cabozantinib maintains VEGF-pathway inhibition but also targets MET and AXL, receptor tyrosine kinases that are overexpressed in RCCCitation14–Citation16 and have been shown to promote tumor-cell survival and resistance to VEGF-pathway inhibition.Citation17–Citation20 Approval of cabozantinib in RCC was based on efficacy and safety results from the Phase III METEOR study, where it improved progression-free survival (PFS), objective response rate (ORR), and overall survival (OS) compared with everolimus in patients who had received prior antiangiogenic therapy.Citation7,Citation8 The safety and tolerability profile of cabozantinib was manageable: the majority of patients required dose modifications, but treatment discontinuations due to adverse events (AEs) were infrequent.
Given the recent approval of cabozantinib for RCC, it is timely to discuss strategies to manage the more common AEs associated with cabozantinib. In this article, we review the clinical outcomes from the pivotal Phase III METEOR trialCitation7,Citation8 and provide practical guidance to prepare patients for cabozantinib therapy. We propose management approaches for the more common AEs based on guidelines from cancer organizations, the literature on managing AEs associated with TKIs, and our own clinical experience.
Cabozantinib for the treatment of advanced RCC
Cabozantinib (Cabometyx™; Exelixis Inc., South San Francisco, CA, USA), is approved for patients with advanced clear-cell RCC who have received prior treatment with an antiangiogenic therapy, and is formulated as tablets with a starting dose of 60 mg daily.Citation12,Citation13 Three tablet strengths (20 mg, 40 mg, and 60 mg) provide clinicians with the flexibility to modify doses. Cabozantinib is also approved for progressive, metastatic medullary thyroid cancer, but is formulated as a capsule (Cometriq®, Exelixis Inc.) with a starting dose of 140 mg once daily.Citation21 Importantly, the tablet and capsule formulations are not bioequivalent and not interchangeable.Citation22
METEOR, a randomized Phase III open-label trial in advanced RCC
In the pivotal Phase III, open-label METEOR study, 658 patients with advanced clear-cell RCC and at least one prior VEGFR-targeted therapy were randomized to receive 60 mg of cabozantinib or 10 mg of everolimus daily, an approved second-line therapy for advanced RCC.Citation7,Citation8 Cabozantinib was associated with significant improvement in all three key efficacy parameters: the primary end point of PFS and the key secondary end points of ORR and OS. In the overall study population, median PFS was 7.4 months for cabozantinib versus 3.9 months for everolimus (HR 0.51, 95% CI 0.41–0.62; P<0.0001), and ORR by independent radiological review was 17% versus 3% (P<0.0001). Median OS was 21.4 months for cabozantinib versus 16.5 months for everolimus (HR 0.66, 95% CI 0.53–0.83; P=0.00026). PFS and OS benefits with cabozantinib were observed across all studied subgroups, including those defined by prior therapy, metastatic site, and tumor burden.
During METEOR, there were no unexpected types or rates of treatment-emergent AEs in the cabozantinib arm, and safety data were comparable between the initial data cutoff for PFS (May 22, 2015) and the later cutoff for OS (December 31, 2015).Citation7,Citation8 For consistency in this review, we discuss results from the May 22, 2015 cutoff in the overall study population, as the majority of safety analyses reported were with this data set, including the product label.Citation12 The most frequent AEs of any grade in the cabozantinib arm were diarrhea (74%), fatigue (56%), nausea (50%), decreased appetite (46%), palmar–plantar erythrodysesthesia syndrome (PPES) (42%), hypertension (37%), vomiting (32%), decreased weight (31%), constipation (25%), dysgeusia (24%), and stomatitis (22%).Citation7 Generally, these AEs were grade 1 or 2 in severity and occurred early on during treatment.Citation7,Citation8,Citation23 Median time to onset for hypertension, PPES, and diarrhea of any grade was less than 5 weeks from the start of treatment ().Citation23
Figure 1 Median time to first occurrence for AEs of clinical interest in patients receiving cabozantinib during the METEOR study.
Abbreviations: AEs, adverse events; GI, gastrointestinal; IQR, interquartile range: PPES, palmar–plantar erythrodysesthesia syndrome; TE, thromboembolism; TKIs, tyrosine-kinase inhibitors.
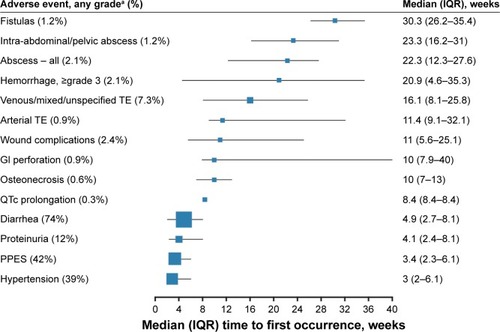
During the METEOR study, dose interruptions and reductions were common with cabozantinib, but effective. Dose reductions were reported for 60% of patients receiving cabozantinib, resulting in a median average daily dose of 44 mg, but only 9% of patients discontinued cabozantinib due to an AE.Citation7,Citation13 The most common AEs leading to cabozantinib dose reductions were diarrhea (16%), PPES (11%), fatigue (10%), and hypertension (8%).Citation23 Dose modifications of cabozantinib were implemented early on, with median time to first dose interruption of 38 days and median time to first dose reduction of 55 days.Citation13
Although this review focuses on managing more common AEs, health-care providers should be knowledgeable of cabozantinib’s overall safety profile, including rare but serious AEs. These include hemorrhage, gastrointestinal (GI) perforations, fistulas, thrombotic events, and reversible posterior leukoencephalopathy syndrome.Citation12 During METEOR, rates for grade ≥3 events were 3.6% for venous and mixed/unspecified thrombotic events, 2.1% for hemorrhage, and <1% for GI perforations and fistulas.Citation23 There were no reversible posterior leukoencephalopathy-syndrome events.
Overall, the safety and tolerability profile of cabozantinib was consistent with that of other TKIs approved for RCC.Citation7,Citation8,Citation24–Citation26 Dose reductions are not uncommon among TKIs, because of interpatient pharmacokinetic variability.Citation22,Citation27–Citation29 The cabozantinib dose should be tailored to the maximum tolerable level for the individual patient, along with prophylactic and supportive-care measures.
Cabozantinib dose modifications
The cabozantinib label provides a dosing algorithm to resolve AEs based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) grading scale ().Citation12,Citation13,Citation30,Citation31 Cabozantinib should be withheld for grade 4 events, and can be restarted after resolution to grade ≤1, with daily dose reduced by 20 mg. This approach is also recommended for grade 3 or intolerable grade 2 events that cannot be managed with supportive care or dose reduction. If the patient previously received 20 mg, then the dose should be resumed at 20 mg, as tolerated, or if necessary discontinued.
Figure 2 Cabozantinib-dosing algorithm.
Abbreviations: AEs, adverse events; GI, gastrointestinal; RPLS, reversible posterior leukoencephalopathy syndrome.
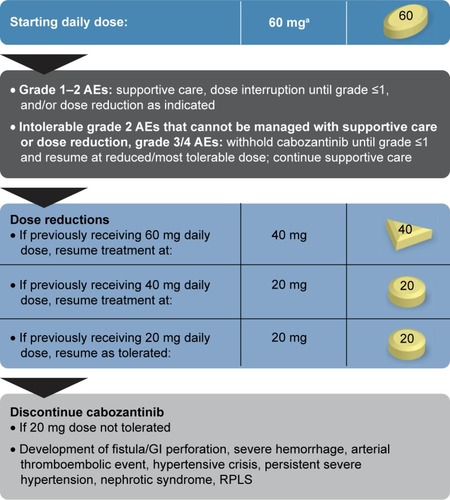
Cabozantinib is metabolized mainly by CYP3A4, so CYP3A4 inhibitors and inducers should be avoided if possible.Citation12,Citation13,Citation30 Strong CYP3A4 inhibitors include clarithromycin, telithromycin, conivaptan, grapefruit juice, protease inhibitors, antifungal azoles, and nefazodone. Strong CYP3A4 inducers include rifampin, phenytoin, carbamazepine, phenobarbital, rifabutin, rifapentine, and St John’s Wort. If a patient requires concomitant treatment with a strong CYP3A4 inhibitor, the cabozantinib dose should be reduced by 20 mg. If the CYP3A4 inhibitor is discontinued, the prior dose should be resumed after an appropriate washout period. Conversely, the cabozantinib dose should be increased by 20 mg if a patient requires concomitant treatment with a strong CYP3A4 inducer. The daily cabozantinib dose should not exceed 80 mg, and if a CYP3A4 inducer is discontinued, the prior cabozantinib dose should be resumed after an appropriate washout period.
AE-management strategies
Before cabozantinib treatment begins, it is important to evaluate the overall health of a patient and provide appropriate education related to therapy, including risk of AEs.Citation9,Citation32 Although previously treated patients may be familiar with AEs associated with TKIs, some are relatively unique to specific TKIs, and individual patients can experience different AEs with different TKIs.Citation24,Citation25,Citation33,Citation34 With each new treatment, it is important to educate and assess a patient’s understanding and implementation of AE management.
It is important to obtain a detailed medical history, including comorbidities, concomitant therapies, and baseline vital and laboratory assessments, including baseline blood pressure (BP) and liver-function tests.Citation9,Citation32 It is helpful for patients routinely to record vital signs and dietary intake and notify their health-care providers of any changes. Also, patients should be instructed to avoid eating for at least 2 hours before and at least 1 hour after taking cabozantinib, as food can affect its bioavailability.Citation12,Citation13,Citation35
In subsequent sections, we discuss the more common AEs experienced by patients receiving cabozantinib during the METEOR trial and practical prophylactic and supportive-care measures, including those provided on the label, in the published literature, and our own clinical experience. These include hypertension, PPES, diarrhea, nausea and vomiting, decreased appetite, fatigue, and stomatitis. Many of the recommendations are derived from guidelines of oncology societies, including the American Cancer Society, National Comprehensive Cancer Network, Oncology Nursing Society, and European Society of Medical Oncology.
Hypertension
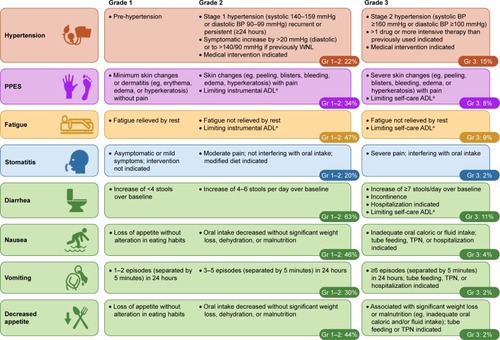
Hypertension is a common AE associated with therapies targeting the VEGF pathway.Citation37 Elevation in BP may be caused by increasing vascular tone secondary to reduced nitric oxide levels, as well as vascular resistance from endothelial-cell dysfunction and damage. In METEOR, 22% of patients experienced grade 1–2 hypertension and 15% experienced grade 3; no grade 4 events were reported ().Citation7 The median time to onset of hypertension was 3 weeks.Citation23
Figure 3 Common AEs experienced by patients in the cabozantinib arm of the METEOR study (safety-analysis set, n=331).Citation7
Abbreviations: ADL, activities of daily living; AE, adverse event; BP, blood pressure; Gr, grade; TPN, total parenteral nutrition; PPES, palmar–plantar erythrodysesthesia syndrome; WNL, within normal limits.
Because hypertension has an early onset, patients and/or their caretakers should be encouraged to keep a record with home BP monitoring, taking BP measurements prior to starting cabozantinib and routinely during treatment.Citation12,Citation24,Citation38,Citation39 Providers should confirm that patients and caretakers are able to operate home BP-monitoring systems correctly and help them plan for measuring and recording BP on a daily basis.Citation39 A meta-analysis of adults with hypertension found that self-measured monitoring lowered BP compared with in-clinic monitoring, and that additional support (eg, educational materials, Internet resources) enhanced this effect.Citation38
For medical management of hypertension, current guidelines recommend initial antihypertensive treatment with a thiazide diuretic, calcium-channel blocker (CCB), ACE inhibitor, or angiotensin-receptor blocker ().Citation40 The BP goal is <150/90 mmHg for patients older than 60 years and <140/90 mmHg for younger patients. If target BP is not reached, the dose of antihypertensive medication can be increased or a second medication added, although ACE inhibitors and angiotensin-receptor blockers should not be combined.Citation40 Renin–angiotensin inhibitors or CCBs may be preferred over thiazide diuretics to reduce electrolyte loss and risk of QT prolongation.Citation37,Citation41,Citation42 However, caution should be taken when selecting a CCB, as some agents can be potent CYP3A4 inhibitors;Citation43 as detailed earlier, the cabozantinib dose should be reduced by 20 mg when used concomitantly with a strong CYP3A4 inhibitor. Studies indicate that early (rather than delayed) antihypertensive treatment is more effective, and that early combination of antihypertensive treatments is more effective than dose escalation of a single agent.Citation44
Table 1 Summary of prophylactic and supportive-care strategies for patients treated with cabozantinib
For persistent hypertension despite optimized anti-hypertensive therapy, the cabozantinib dose should be withheld and/or reduced as indicated.Citation12,Citation13 Cabozantinib should be discontinued for hypertensive crisis or if severe hypertension (≥160/100 mmHg) persists despite supportive interventions.
Palmar–plantar erythrodysesthesia syndrome
During METEOR, 34% of patients experienced grade 1–2 PPES, and 8% experienced grade 3 ().Citation7 Median onset to the first PPES event was 3.4 weeks ().Citation23 Manifestations of PPES with cabozantinib include tingling, numbness, slight redness, mild hyperkeratosis, pain, and symmetrical red and swollen areas on palms and soles.Citation45–Citation49 Although PPES is not life-threatening and patients may present with mild–moderate skin changes, it can develop into a painful condition that limits activities of daily living and significantly impacts quality of life.Citation47
Examples of typical PPES events associated with cabozantinib are shown in . Although there is overlap among the TKIs in the presentation of PPES, the location and appearances of cutaneous abnormalities with cabozantinib can manifest differently from other TKIs in our clinical experience. For instance, the lateral sides of fingers or periungual zones may be affected in patients receiving cabozantinib. In a patient who was a professional writer, the callused areas from the knuckles of his writing hand developed PPES. Studies of cabozantinib treatment in solid tumors have described other dermatologic events to be monitored, including generalized pigment dilution, hair depigmentation, xerosis, scrotal erythema/ulceration, and subungual nail-splinter hemorrhages.Citation45,Citation48,Citation49 Given the potential for dermatologic AEs, a full skin and nail assessment should be conducted before starting cabozantinib, with routine evaluation during treatment.
Figure 4 Examples of palmar–plantar erythrodysesthesia syndrome resulting from treatment with cabozantinib in patients with solid tumors.
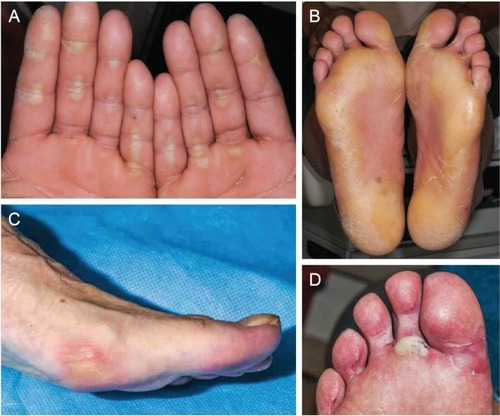
Prophylactic strategies to prevent dermatologic AEs are aimed at treating existing skin abnormalities (eg, calluses, skin disorders), protecting the skin, reducing friction to hands and feet, and maintaining skin moisture ().Citation50–Citation52 If needed, supportive treatments include topical interventions (corticosteroids, keratolytics, moisturizers, and analgesics). For the aforementioned patient who was a writer, PPES resolved with the use of cotton gloves and 20% urea cream. Dose reduction usually ameliorates dermatologic AEs that cannot be managed with supportive care.Citation52
Diarrhea
Although TKIs are associated with diarrhea, the underlying pathogenesis is not well understood.Citation53 During METEOR, 63% of patients in the cabozantinib arm experienced grade 1–2 diarrhea and 11% experienced grade 3; there were no grade 4 events ().Citation7 The median onset of diarrhea was 4.9 weeks, and diarrhea was the commonest reason for dose reductions with cabozantinib (16%).Citation23
To decrease the risk of developing diarrhea, patients should be encouraged to maintain hydration, consume small and frequent meals, and avoid lactose-containing products, high-fat foods, and alcohol.Citation24,Citation54 Patients should log their dietary intake to help identify and remedy potential nontreatment causes of diarrhea.Citation50,Citation51 Uncomplicated diarrhea often can be managed by replacement of fluids and electrolytes, self-administration of antidiarrheal medications (eg, loper-amide and octreotide), and diet modifications ().Citation24,Citation50,Citation52 Patients should be advised to clean and dry the anal area with mild soap and water, use disposable wipes, and apply a moisture-barrier ointment.Citation50,Citation55,Citation56 Patients experiencing intolerable diarrhea of grade 2 despite supportive measures or grade 3 diarrhea should have their cabozantinib dose withheld until improvement to grade ≤1, and the dose should be reduced upon resuming treatment.Citation12 However, providers should ensure that patients understand and are implementing antidiarrheal strategies before any dose modification.
Nausea and vomiting
Grade 1–2 nausea (46%) and vomiting (30%) were common among patients in the cabozantinib arm of METEOR, but grade 3 events were infrequent (4% and 2%, respectively; ).Citation7 Antiemetics can be provided as prophylaxis or at the first sign of nausea and vomiting. 5-HT3-receptor antagonists, such as ondansetron, are recommended over the use of NK1-receptor antagonists and dexamethasone, because the latter agents can modulate CYP3A4 ().Citation12,Citation13,Citation30,Citation57–Citation59 The cannabinoid nabilone is a weak inhibitor of CYP3A4.Citation60 We typically evaluate and treat for gastroesophageal reflux disease when a patient complains of nausea. Guidelines for gastroesophageal reflux disease recommend lifestyle and dietary modifications and the use of proton-pump inhibitors.Citation61 Histamine H2-receptor antagonists may also provide relief as a maintenance option or to those with refractory symptoms.Citation59,Citation61 Head-of-bed elevation and refraining from eating 2–3 hours prior to bedtime may also provide overnight relief.Citation61 In terms of diet, simple foods (eg, rice, noodles, skinless chicken or turkey) may be better tolerated by patients, and chocolate, caffeine, alcohol, and nicotine should be avoided.Citation54,Citation55,Citation61,Citation62 If vomiting does develop, patients should rinse their mouth and delay food intake until the nausea subsides.Citation55
Decreased appetite
Patients treated with cabozantinib may experience decreased appetite. Decreased appetite of grade 1–2 affected 44% of cabozantinib-treated patients during METEOR, and 2% experienced grade 3 ().Citation7 In our experience, decreased appetite can occur with or without GI-related AEs. Although decreased appetite may appear benign, it is important for patients to continue high-calorie, high-protein nutrition during therapy to maintain lean body mass in light of a potential correlation with prognosis.Citation63 To overcome the challenge of consuming food in the absence of appetite, we encourage patients to partake in high-calorie, appetizing foods (eg, ice cream or nonfat frozen yogurt) that do not result in GI complications.Citation54 Snacks could include nutrition bars and supplemental shakes.Citation54,Citation55 Appetite-stimulating medication, such as dronabinol, can be provided prophylactically and as supportive care for appropriate patients ().Citation52,Citation64 We recommend starting appetite-stimulating medication at the lowest dose and titrating to effect while monitoring for AEs.
Fatigue
Fatigue is one of the more frequent AEs experienced by patients with RCC receiving TKI therapy, and may be severe enough to require dose reduction or even discontinuation.Citation65 It is important to recognize and appropriately manage this common, yet often underdiagnosed AE, and to note that fatigue can have multiple causes that should be considered part of the differential diagnosis, including disease-specific morbidities, hypothyroidism, anemia, deconditioning, sleep disturbances, alcohol/substance abuse, medications, emotional distress, and suboptimal diet and fluid intake.Citation34,Citation55,Citation65–Citation67 During METEOR, 47% of patients receiving cabozantinib experienced grade 1–2 fatigue, 9% experienced grade 3 (),Citation7 and 10% of patients required dose reductions to manage fatigue.Citation23 Hypothyroidism was reported in 20% of patients receiving cabozantinib with no grade 3/4 events, and 17% of patients experienced anemia of any grade with a grade 3/4 rate of 5%.Citation7
There are few evidence-based interventions for ameliorating fatigue, so it is vital to establish baseline fatigue levels, educate patients for early recognition of symptom worsening, and routinely reevaluate patients.Citation34,Citation52,Citation67 During treatment, patients should be encouraged to conduct relaxing activities and rest often.Citation52,Citation67,Citation68 We advise daily light exercises (20–30 minutes), such as resistance training, walking, or yoga.Citation52 Aerobic activity can ameliorate fatigue in some patients,Citation34,Citation52,Citation67,Citation68 and resistance training may attenuate cancer-related skeletal muscle wasting.Citation69
For patients with persistent fatigue without an etiology, pharmacologic management with psychostimulants, such as methylphenidate, may be appropriate.Citation67,Citation70 Modafinil should be avoided, because of its potential to reduce cabozantinib exposure ().Citation71 Dose interruptions can be considered for grade 3 fatigue, particularly if it is part of a constellation of symptoms, and dose reduction should be considered if pharmacologic interventions do not reduce symptoms.Citation12,Citation13,Citation30
Stomatitis
Symptoms of stomatitis or oral mucositis secondary to TKI treatment often include dry mouth, oral sensitivity or soreness, and taste changes.Citation24,Citation34 There may not be obvious morphologic changes characteristic of chemotherapy-induced mucositis. Among cabozantinib-treated patients in METEOR, 20% experienced grade 1–2 stomatitis and 2% experienced grade 3 ().Citation7 Oral AEs have been shown to emerge early on with cabozantinib treatment, usually within the first few months.Citation72
Preventive measures are strongly recommended. A comprehensive dental exam and treatment prior to initiation of cabozantinib, along with replacement or removal of ill-fitting dentures, should be conducted to reduce the risk of developing stomatitis.Citation50 During treatment, patients should routinely practice gentle oral-hygiene strategies, preferably after every meal, and adapt their diet to avoid irritating (eg, spicy or sticky) foods ().Citation50,Citation54,Citation55,Citation73
In our clinical experience, grade 3 stomatitis results in dose interruption and usually dose reduction, whereas grade 1–2 levels can be managed without dose modification. Oral-care interventions aim to promote cleansing, moisture, and comfort,Citation50 and can include “magic mouthwash” (equal parts 2% viscous lidocaine, diphenhydramine, bismuth subsalicylate, or aluminum/magnesium hydroxide) and swish-and-spit rinses, such as Biotène® Dry Mouth Oral Rinse, salt water, or sodium bicarbonate-containing mouthwash.Citation34,Citation50,Citation52,Citation72,Citation73 Oral rinses with alcohol should be avoided, as these can exacerbate dry mouth.Citation50,Citation55,Citation73
Special considerations
Besides dose modifications for AE management, specific patient populations may also require dose adjustments of cabozantinib. Although no dose adjustment is recommended for patients 65 years of age or older,Citation12,Citation13,Citation30 a lower cabozantinib starting dose may be appropriate for some elderly patients, particularly those who are frail with poor performance status and likely to require dose modification given the safety profile of cabozantinib. Patients with mild or moderate hepatic impairment should start with 40 mg once daily.Citation12,Citation13,Citation30 Cabozantinib is not recommended for use in patients with severe hepatic impairment. The METEOR study protocol advised that patients with drug-related grade 2 elevated alanine transaminase, aspartate transaminase, or bilirubin lasting longer than 1 week have their cabozantinib dose reduced by 20 mg, and patients with grade ≥3 elevations have doses withheld until returning to grade ≤1 and then restarting cabozantinib at a reduced dose.Citation36 Patients with mild–moderate renal impairment do not require dose modifications.Citation12,Citation13,Citation30 Cabozantinib has not been studied in patients with severe renal impairment.
Patients with preexisting conditions that put them at greater risk of experiencing serious AEs should be monitored carefully. For example, patients with metastases located in the pulmonary or GI regions should be examined for risk of hemorrhages, perforations, and fistulas, particularly those with invasive metastases, concomitant use of anticoagulants, prior surgery, or a history of GI disorders (eg, diverticulitis, inflammatory bowel disease).Citation12,Citation13,Citation30,Citation74–Citation76
Conclusion
Cabozantinib has shown significant clinical benefit in the treatment of advanced clear-cell RCC, and has become a preferred standard of care for second-line treatment.Citation7–Citation11 Similarly to other targeted therapies, there is a need to anticipate and manage AEs to optimize treatment outcomes.Citation24,Citation25,Citation28,Citation33 Patients receiving cabozantinib should be educated on common AEs, prophylactic and symptomatic measures, and be routinely followed to provide timely and appropriate dose modifications and supportive care and monitor for rare, asymptomatic events. Effective AE management should help to mitigate the impact of AEs and allow patients to maintain extended treatment with cabozantinib. Further evidence, from both clinical experience and prospective trials, is needed to drive practice consensus and define optimal approaches for cabozantinib and other TKI therapies.
Author contributions
Both authors contributed to study conception/design, provision of study material/patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing, and final approval of the manuscript.
Acknowledgments
Medical writing and editorial assistance were provided by Christin Chong, PhD and Michael Raffin of Fishawack Communications (Conshohocken, PA). Funding for this work was provided by Exelixis Inc (South San Francisco, CA).
Disclosure
BG has received honoraria from Exelixis, and PAC has received honoraria from Pfizer and Exelixis. The authors report no other conflicts of interest in this work.
References
- American Cancer SocietyCancer Facts & Figures 2017AtlantaACS2017
- LiPWongYNArmstrongKSurvival among patients with advanced renal cell carcinoma in the pretargeted versus targeted therapy erasCancer Med20165216918126645975
- RiniBISmallEJBiology and clinical development of vascular endothelial growth factor-targeted therapy in renal cell carcinomaJ Clin Oncol20052351028104315534359
- ClarkPEThe role of VHL in clear-cell renal cell carcinoma and its relation to targeted therapyKidney Int200976993994519657325
- NiuGChenXVascular endothelial growth factor as an anti-angiogenic target for cancer therapyCurr Drug Targets20101181000101720426765
- RiniBIEscudierBTomczakPComparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trialLancet201137898071931193922056247
- ChoueiriTKEscudierBPowlesTCabozantinib versus everolimus in advanced renal-cell carcinomaN Engl J Med2015373191814182326406150
- ChoueiriTKEscudierBPowlesTCabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trialLancet Oncol201617791792727279544
- MotzerRJonaschEAgarwalNNCCN guidelines: kidney cancer – version 22017 Available from: https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdfAccessed February 6, 2017
- LjungbergBBensalahKBexAEuropean Association of Urology guidelines: renal cell carcinoma2016 http://uroweb.org/guideline/renal-cell-carcinomaAccessed February 20, 2017
- EscudierBPortaCSchmidingerMRenal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-upAnn Oncol201627Suppl 5v58v6827664262
- Cabometyx® (cabozantinib) [prescribing information]South San FranciscoExelixis, Inc2016
- Cabometyx® (cabozantinib) [summary of product characteristics] Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004163/WC500214071.pdfAccessed March 3, 2017
- KoochekpourSJeffersMWangPHThe von Hippel-Lindau tumor suppressor gene inhibits hepatocyte growth factor/scatter factor-induced invasion and branching morphogenesis in renal carcinoma cellsMol Cell Biol19991995902591210454537
- NakaigawaNYaoMBabaMInactivation of von Hippel-Lindau gene induces constitutive phosphorylation of MET protein in clear cell renal carcinomaCancer Res20066673699370516585196
- RankinEBFuhKCCastelliniLDirect regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and METProc Natl Acad Sci U S A201411137133731337825187556
- YuHLiuRMaBAxl receptor tyrosine kinase is a potential therapeutic target in renal cell carcinomaBr J Cancer2015113461662526180925
- BenvenutiSComoglioPMThe MET receptor tyrosine kinase in invasion and metastasisJ Cell Physiol2007213231632517607709
- YakesFMChenJTanJCabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growthMol Cancer Ther201110122298230821926191
- ZhouLLiuXDSunMTargeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinomaOncogene201635212687269726364599
- Cometriq® (cabozantinib) [prescribing information]South San FranciscoExelixis, Inc2012
- LacySAMilesDRNguyenLTClinical pharmacokinetics and pharmacodynamics of cabozantinibClin Pharmacokinet201756547749127734291
- European Medicines AgencyCHMP assessment report: Cabometyx2016 Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/004163/WC500214070.pdfAccessed January 20, 2017
- EisenTSternbergCNRobertCTargeted therapies for renal cell carcinoma: review of adverse event management strategiesJ Natl Cancer Inst201210429311322235142
- CohenRBOudardSAntiangiogenic therapy for advanced renal cell carcinoma: management of treatment-related toxicitiesInvest New Drugs20123052066207922327313
- RavaudAEditorial comment to Therapy management of cardiovascular adverse events in the context of targeted therapy for metastatic renal cell carcinomaInt J Urol201219980522651229
- TeradaTNodaSInuiKManagement of dose variability and side effects for individualized cancer pharmacotherapy with tyrosine kinase inhibitorsPharmacol Ther201515212513425976912
- RuizJNBelumVRCreelPCohnAEwerMLacoutureMECurrent practices in the management of adverse events associated with targeted therapies for advanced renal cell carcinoma: a national survey of oncologistsClin Genitourin Cancer201412534134725035283
- MilesDJumbeNLLacySNguyenLPopulation pharmacokinetic model of cabozantinib in patients with medullary thyroid carcinoma and its application to an exposure-response analysisClin Pharmacokinet20165519310526149244
- ExelixisCABOMETYX dosing and administration guide2016 Available from: https://hcp.cabometyx.com/downloads/CABOMETYXDosingandAdministrationGuide.pdfAccessed February 16, 2017
- National Cancer InstituteCommon Terminology Criteria for Adverse Events (CTCAE). Version 4.03WashingtonUS Department of Health and Human Services2009
- HurriaADotanEBaumgartnerJNCCN guidelines: older adult oncology – version 22016 Available from: https://www.nccn.org/professionals/physician_gls/pdf/senior.pdfAccessed February 22, 2017
- RavaudATreatment-associated adverse event management in the advanced renal cell carcinoma patient treated with targeted therapiesOncologist201116Suppl 2324421346038
- KollmannsbergerCBjarnasonGBurnettPSunitinib in metastatic renal cell carcinoma: recommendations for management of noncardiovascular toxicitiesOncologist201116554355321490127
- NguyenLHollandJMamelokREvaluation of the effect of food and gastric pH on the single-dose pharmacokinetics of cabozantinib in healthy adult subjectsJ Clin Pharmacol201555111293130225907407
- ChoueiriTKEscudierBPowlesTCabozantinib versus everolimus in advanced renal-cell carcinomaN Engl J Med2015373191814182326406150
- LenihanDJKoweyPROverview and management of cardiac adverse events associated with tyrosine kinase inhibitorsOncologist201318890090823918069
- UhligKPatelKIpSKitsiosGDBalkEMSelf-measured blood pressure monitoring in the management of hypertension: a systematic review and meta-analysisAnn Intern Med2013159318519423922064
- PickeringTGWhiteWBGilesTDWhen and how to use self (home) and ambulatory blood pressure monitoringJ Am Soc Hypertens201042566120400049
- JamesPAOparilSCarterBL2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8)JAMA2014311550752024352797
- MaitlandMLBakrisGLBlackHRInitial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitorsJ Natl Cancer Inst2010102959660420351338
- LarochellePKollmannsbergerCFeldmanRDHypertension management in patients with renal cell cancer treated with anti-angiogenic agentsCurr Oncol201219420220822876146
- ZhouSFXueCCYuXQLiCWangGClinically important drug interactions potentially involving mechanism-based inhibition of cytochrome P450 3A4 and the role of therapeutic drug monitoringTher Drug Monit200729668771018043468
- IvanyiPBeutelGDrewesNTherapy of treatment-related hypertension in metastatic renal-cell cancer patients receiving sunitinibClin Genitourin Cancer201715228029027863831
- ChoYTChanCCCabozantinib-induced hand-foot skin reaction with subungual splinter hemorrhages and hypertension: a possible association with inhibition of the vascular endothelial growth factor signaling pathwayEur J Dermatol201323227427523518371
- EsfandiariNHHesseltineEAVisual vignette: cabozantinib-induced hand-foot syndromeEndocr Pract2013196107124014004
- BelumVRSerna-TamayoCWuSLacoutureMEIncidence and risk of hand-foot skin reaction with cabozantinib, a novel multikinase inhibitor: a meta-analysisClin Exp Dermatol201641181526009777
- ZuoRCApoloABDiGiovannaJJCutaneous adverse effects associated with the tyrosine-kinase inhibitor cabozantinibJAMA Dermatol2015151217017725427282
- JayaramAZafeiriouZde BonoJSCabozantinib: getting under the skin of cutaneous toxicityJAMA Oncol20151453553626181263
- PolovichPOLeFebvreKBSide effects of cancer therapyPolovichPOOlsenMLeFebvreKBChemotherapy and Biotherapy Guidelines and Recommendations for Practice4th edPittsburghOncology Nursing Society2016171435
- EdmondsKHullDSpencer-ShawAStrategies for assessing and managing the adverse events of sorafenib and other targeted therapies in the treatment of renal cell and hepatocellular carcinoma: recommendations from a European nursing task groupEur J Oncol Nurs201216217218421641280
- Oncology Nursing SocietyPEP rating system overview2016 Available from: https://www.ons.org/practice-resources/pepAccessed February 16, 2017
- BowenJMMechanisms of TKI-induced diarrhea in cancer patientsCurr Opin Support Palliat Care20137216216723399616
- American Cancer SocietyNutrition for the person with cancer during treatment2017 Available from: https://www.cancer.org/treatment/survivorship-during-and-after-treatment/staying-active/nutrition/nutrition-during-treatment.htmlAccessed February 16, 2017
- RyanKPSymptom control and side effectsWhen Tumor is the Rumor and Cancer is the AnswerBloomington (IN)AuthorHouse2013181209
- Cancer Research UKTips on coping with diarrhoea Available from: http://www.cancerresearchuk.org/about-cancer/coping/physically/bowel-problems/types/diarrhoea/tips-coping-diarrhoeaAccessed August 24, 2017
- McCreaJBMajumdarAKGoldbergMREffects of the neurokinin1 receptor antagonist aprepitant on the pharmacokinetics of dexamethasone and methylprednisoloneClin Pharmacol Ther2003741172412844131
- HeskethPJGrunbergSMGrallaRJThe oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin: the Aprepitant Protocol 052 Study GroupJ Clin Oncol200321224112411914559886
- EttingerDBergerMAstonJNCCN guidelines: antiemesis – version 22016 Available from: https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdfAccessed February 16, 2017
- Cesamet® (nabilone) [prescribing information]Somerset (NJ)Meda Pharmaceuticals Inc2013
- KatzPOGersonLBVelaMFGuidelines for the diagnosis and management of gastroesophageal reflux diseaseAm J Gastroenterol2013108330832923419381
- CreelPAOptimizing patient adherence to targeted therapies in renal cell carcinomaClin J Oncol Nurs201418669470025427704
- TsaiSImportance of lean body mass in the oncologic patientNutr Clin Pract201227559359822898746
- LevyMSmithTAlvarez-PerezANCCN guidelines: palliative care – version 12016 Available from: https://www.nccn.org/professionals/physician_gls/pdf/palliative.pdfAccessed February 17, 2017
- AnandDEscalanteCPOngoing screening and treatment to potentially reduce tyrosine kinase inhibitor-related fatigue in renal cell carcinomaJ Pain Symptom Manage201550110811725701692
- GeorgeGCIwuanyanwuECAndersonKOSleep quality and its association with fatigue, symptom burden, and mood in patients with advanced cancer in a clinic for early-phase oncology clinical trialsCancer2016122213401340927412379
- BergerAMooneyKBanerjeeCNCCN guidelines: cancer-related fatigue – version 12016 Available from: https://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdfAccessed February 17, 2017
- MitchellSAHoffmanAJClarkJCPutting evidence into practice: an update of evidence-based interventions for cancer-related fatigue during and following treatmentClin J Oncol Nurs201418Suppl385825427608
- Al-MajidSWatersHThe biological mechanisms of cancer-related skeletal muscle wasting: the role of progressive resistance exerciseBiol Res Nurs200810172018705151
- MintonORichardsonASharpeMHotopfMStonePDrug therapy for the management of cancer-related fatigueCochrane Database Syst Rev20107CD00670420614448
- Provigil® (modafinil) [prescribing information]Frazer (PA)Cephalon, Inc1998
- YuanAKurtzSLBarysauskasCMPilotteAPWagnerAJTreisterNSOral adverse events in cancer patients treated with VEGFR-directed multitargeted tyrosine kinase inhibitorsOral Oncol201551111026103326403941
- PetersonDEBoers-DoetsCBBensadounRJHerrstedtJManagement of oral and gastrointestinal mucosal injury: ESMO clinical practice guidelines for diagnosis, treatment, and follow-upAnn Oncol201526Suppl 5v139v15126142468
- BlevinsDPDaduRHuMAerodigestive fistula formation as a rare side effect of antiangiogenic tyrosine kinase inhibitor therapy for thyroid cancerThyroid201424591892224635127
- BurgerRABradyMFBookmanMARisk factors for GI adverse events in a phase III randomized trial of bevacizumab in first-line therapy of advanced ovarian cancer: a Gynecologic Oncology Group studyJ Clin Oncol201432121210121724637999
- ShinagareABHowardSAKrajewskiKMZukotynskiKAJagannathanJPRamaiyaNHPneumatosis intestinalis and bowel perforation associated with molecular targeted therapy: an emerging problem and the role of radiologists in its managementAJR Am J Roentgenol201219961259126523169717
- WeitzmanSPCabanillasMEThe treatment landscape in thyroid cancer: a focus on cabozantinibCancer Manag Res2015726527826316818
- McLellanBCiardielloFLacoutureMESegaertSvan CutsemERegorafenib-associated hand-foot skin reaction: practical advice on diagnosis, prevention, and managementAnn Oncol201526102017202626034039
- AlexandrescuDTVaillantJGDasanuCAEffect of treatment with a colloidal oatmeal lotion on the acneform eruption induced by epidermal growth factor receptor and multiple tyrosine-kinase inhibitorsClin Exp Dermatol2007321717417034418
