Abstract
Background
Colon cancer is the third most common cancer in the world, and its metastasis and drug resistance are challenging for its effective treatment. The PI3K/Akt/mTOR pathway plays a crucial role in the pathogenesis of colon cancer. The aim of this study was to investigate the targeting of PI3K in colon cancer cells HT-29 and HCT-116 in vitro.
Methods
In HT-29 and HCT-116 cells, BEZ235, a dual inhibitor of PI3K/mTOR, and shRNAtarget to PI3KCA were used to inhibit PI3K/Akt/mTOR pathway. The inhibition efficiency of PI3K/Akt/mTOR pathway was detected by RT-PCR and Western blot. Cell proliferation, migration, invasion, and apoptosis were evaluated by Cell Counting Kit-8, Transwell, and flow cytometry assays. The expression of apoptosis-related proteins (cleavage caspase 3, Bcl-2, Bax, and Bim) were also detected.
Results
We found that in HT-29 and HCT-116 cells, the treatment of BEZ235 (1 μM) and PI3KCA knockdown inhibited the activation of PI3K/Akt/mTOR pathway and significantly suppressed cell proliferation, migration, and invasion of HT-29 and HCT-116 cells. In addition, we confirmed that knockdown of BEZ235 and PI3KCA induced cell apoptosis through the upregulated levels of cleavage caspase 3 and Bax and downregulated expression of Bcl-2 and Bim.
Conclusion
Our results indicated that targeted inhibition of the PI3K/Akt/mTOR pathway impaired cell proliferation, survival, and invasion in human colon cancer.
Introduction
Colon cancer is one of the most common cancers in the world, and the mortality rate is rising every year. In the case of metastasis, the 5-year survival rate for colon cancer patients is only 10%.Citation1 With the continuous study of colon cancer, its occurrence and development are found to be multi-stage, multi-step processes involving numerous signal pathways.
The targets of the PI3K/protein kinase B (Akt)/mammalian rapamycin (mTOR) are usually associated with the development of cancer, including colon cancer.Citation2–Citation5 This pathway is upregulated in cancers and is associated with increased proliferation, decreased apoptosis, and promoted cancer pathogenesis.Citation6 Moreover, the activation of PI3K/Akt/mTOR pathway is closely related to the drug resistance of colon cancer, thus reducing the success rate of treatment.Citation7,Citation8 In addition, the activation of PI3K regulates various downstream proteins involved in tumor progression, such as p70S6K, cyclin D1, β-catenin, E-cad, Bcl-2/Bax, and so on. Among them, p70S6K is a substrate for mTOR that is regulated by PI3K/Akt/mTOR and related to cell proliferation, survival, and epithelial–mesenchymal transition (EMT).Citation9,Citation10 Therefore, inhibiting the PI3K/Akt/mTOR pathway may have the potential for cancer treatment and may enhance the sensitivity of chemotherapy and radiotherapy.
BEZ235 has dual inhibitory effects on PI3K and m-TOR, blocking the PI3K activity by simultaneously inhibiting mTOR. In tumor research and clinical trials, BEZ235 has been used because of its effectiveness and low side effects.Citation11–Citation14 A previous study showed that, even in PIK3CA mutation status, the cell proliferation of colorectal cancer cell lines (HCT116, DLD-1, and SW480) was effectively inhibited by BEZ235.Citation15 Chen et al found that BEZ235 suppressed colon cancer cell HCT-116 proliferation, leading to cell apoptosis.Citation16 Therefore, BEZ235 was selected as an inhibitor to investigate the effects of targeting the PI3K/Akt/mTOR pathway in colon cancer cells. shRNA transfection is a simple and effective way to knock down PI3K and PI3KCA, which is used in various cancer studies including HT-29 and HCT-116 cells.Citation17–Citation19 In the present study, we determined to explore the effects of targeted inhibition of PI3K on proliferation, migration, invasion, and apoptosis of colon cancer cells.
Methods and materials
Cell culture
The colon cancer HT-29 and HCT-116 cell lines were purchased from China Cell Bank (Shanghai, China) and cultured at 37°C with 5% CO2 in DMEM medium (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum (FBS) (Thermo Fisher Scientific), 100 U/mL penicillin, and 100 mg/mL streptomycin (Thermo Fisher Scientific). The cells in the logarithmic phase with the sound condition were used for further detection. When the cells were in the total growth phase, they were divided into three groups and treated under three culture conditions: BEZ235, shRNA transfection, and dimethyl sulfoxide (DMSO) as negative control.
Inhibition of PI3K/Akt/mTOR by BEZ235
Different concentrations of BEZ235 (Cell Signaling Technology, Beverly, MA, USA; dissolved in DMSO), the specific inhibitor of PI3K/Akt pathway, were used to inhibit the PI3K/Akt/mTOR pathway based on the manufacturer’s instruction. Approximately 5×106 HT-29/HCT-116 cells were seeded in a 6-cm diameter dish with DMEM and cultured at 37°C with 5% CO2 for 24 hours. Then, the cells treated with BEZ235 at 0, 1 nM, 10 nM, 100 nM, and 1 μM were incubated shortly thereafter for 24 hours. The appropriate concentrations of BEZ235 which achieved the maximum inhibition effect were selected for further experiments. DMSO was used as a control reagent. This experiment was repeated independently at least two times.
Knockdown of PI3KCA by shRNA transfection
We designed and synthetized the PI3KCA-shRNA as follows: shRNA (GCATTAGAATTTACAGCAAGA). The target vector pLVX-shRNA2 (Clontech Co. CA, US) was digested by BamHI and EcoRI. According to the study of Dang et al,Citation20 the shRNA vector was constructed and transfected into HT-29 and HCT-116 cells by Liposomes 2000 (Thermo Fisher Scientific) according to the manufacturer’s instructions. The transfection efficiency for HT-29 and HCT-116 cells was 81% and 85%, respectively. Forty-eight hours after transfection, the cells were collected for RT-PCR, Western blotting analysis, and other assays.
Real-time quantitative RT-PCR analysis
The mRNA level of PI3KCA was quantitatively estimated by real-time quantitative RT-PCR, to determine a definitive measurement of PI3KCA knockdown efficiency. Total RNA was isolated by RNeasy kit (Sigma, St Louis, MO, USA). Reverse transcription reactions were performed by using the PrimeScript® 1st strand cDNA synthesis kit (TaKaRa, Beijing, China). SYBR Green qPCR Master Mix (2×) kit (Thermo Fisher Scientific) was performed to analyze real-time RT-PCR with the following program: 95°C 10 minutes, 95°C 15 seconds, 60°C 60 seconds for annealing and extension, with a repetition of 40 cycles. Real-time RT-PCR data were analyzed by comparative Ct (ΔΔct) method. The expression level of PI3KCA mRNA was normalized to an endogenous control, GAPDH. Primer sequences were as follows: PI3KCA: forward primer 5′-CAATCGGTGACTGTGTGGGA-3′, reverse primer 5′-ACAGGTCAATGGCTGCATCA-3′; GAPDH: forward primer 5′-CATGAGAAGTATGACAACAGCCT-3′, reverse primer 5′-AGTCCTTCCACGATA CCAAA GT-3′.
Western blotting
The expression of PI3K/Akt/mTOR-related proteins and proteins associated with apoptosis was determined by Western blotting method. HT-29 and HCT-116 cells were treated with BEZ235 at different concentrations (0, 1 nM, 10 nM, 100 nM, and 1 μM) for 24 hours to detect inhibition efficiency. HT-29 and HCT-116 cells were then treated with 1 μM BEZ235 or transfected with PI3KCA shRNA transfection, respectively. Both the treatments lasted for 24 hours. The cells were then collected to extract proteins, which were quantified using a BCA Protein Assay Kit. Proteins were then separated with 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. After blocking with 5% nonfat dry milk in Tris-buffered saline, 0.1% Tween 20 (TBST) for 1 hour, membranes were incubated with the primary antibody (1:1,000) at 4°C overnight. After washing, secondary antibodies were applied to membranes and incubated at room temperature for 1 hour. Labeled protein bands were exposed using electro-chemiluminescence (ECL) solution. For quantification analyses, Western blotting was repeated for three times. The band intensity was measured by using ImageJ. The relative expression levels of proteins were calculated by standardized the band intensity with the amount of β-tubulin from the same sample.
Cell proliferation assay
According to the manufacturer’s instructions, Cell Counting Kit-8 (CCK8; Dojindo, Japan) was adopted to quantify the cell proliferation after treatment with BEZ235 and PI3KCA knockdown. HT-29 and HCT-116 cells were treated with 1 μM BEZ235 and shRNA transfection for 24 hours, respectively. Then, the cells were seeded in 96-well plates at a density of 1,000 cells/well and were constantly cultured with BEZ235 in normal medium. The supernatant was replaced with 100 mL of medium containing 10 mL of CCK8, enabling detection of optical density (OD) values at 450 nm every 24 hours.
For colony formation assays, 200 HT-29 and HCT-116 cells of three groups with 24-hour treatment of BEZ235, PI3KCA shRNA transfection, and respective NC were seeded in a disposable dish and cultured in a medium containing 10% FBS for 1 week. Colonies were fixed with methanol, stained with 0.1% crystal violet, and photographed and counted with an inverted microscope. Each treatment was performed three times.
Cell migration and invasion assay
Transwell chamber model was performed to measure cell migration and invasion after treatment with BEZ235 and PI3KCA shRNA transfection.
Migration
HT-29 and HCT-116 cells with 24-hour treatment of 1 μM BEZ235 and PI3KCA shRNA transfection were suspended at a concentration of 3×104 cells/mL. 100 μL of the suspension was added into the upper chamber of Transwell apparatus (EMD Millipore, Billerica, MA, USA; 0.8 μm). After incubation for 24 hours, we removed the cells that remained in the top chamber.
Invasion
Matrigel (BD Biosciences, San Jose, CA, USA) was used to coat the upper chambers, and the cells with the BEZ235 or PI3KCA shRNA transfection treatment were added to the upper chamber at a density of 5×104 cells/chamber (100 μL). 100 μL of medium with 10% FBS was added into the lower chamber. After incubation for 24 hours, cells that attached to the lower chamber were fixed in 4% paraformaldehyde and stained with 0.1% crystal violet to quantify cell migration. The Transwell assays were repeated three times.
Cell apoptosis assay
HT-29 and HCT-116 cells were seeded in six well plates and treated with BEZ235 and PI3KCA shRNA transfection at the logarithmic growth phase. After 48 hours of incubation, cells were collected at the concentration of 105/mL in binding buffer and stained with 5 μL Annexin V fluorescein isothiocyanate (FITC) and 10 μL propidium iodide (PI) (BD Biosciences). Flow cytometry was then performed to assess apoptosis. The experiment was repeated three times.
Statistical analysis
The quantitative experimental data were presented as mean ± SD and analyzed by SPSS 16.0 statistical software with one-way analysis of variance and unpaired t-test to analyze the differences between groups. P<0.05 for the difference was statistically significant.
Results
BEZ235 effectively inhibited PI3K/Akt/mTOR activation in HT-29 and HCT-116 cells
To establish a suitable dose of BEZ235 on PI3K/Akt/mTOR pathway, HT-29 and HCT-116 cells were treated with BEZ235 at 0, 1 nM, 10 nM, 100 nM, or 1 μM. Indeed, we found that BEZ235 efficiently decreased p-Akt levels in a dose-dependent manner. As 1 μM of BEZ235 had the greatest inhibitory effect, this concentration was used in the subsequent experiments (). The levels of p-Akt and p-mTOR in HT-29 and HCT-116 cells decreased when compared with the control groups ().
Figure 1 BEZ235 inhibited cell proliferation of HT-29 and HCT-116 cells.
Abbreviations: OD450, optical density 450 nm; NC, negative control.
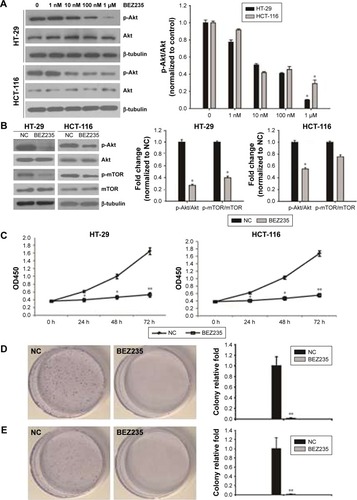
Inhibition of PI3K/Akt/mTOR by BEZ235 resulted in a decline in HT-29 and HCT-116 cell proliferation
In order to describe the BEZ235 effect on proliferative activity of HT-29 and HCT-116, after treating with 1 μM of BEZ235, the cell viability was detected with CCK8 and colony formation assay. The proliferation of HT-29 and HCT-116 cells was significantly inhibited by BEZ235 in 24 hours (). Compared with the control group, the proliferation ability of cells increased significantly during the treatment period. Similar results were observed in the analysis of colony formation. BEZ235 inhibition of the PI3K/Akt/mTOR pathway led to a significant reduction in the number of cell colonies of HT-29 and HCT-116 cells, compared with the control group ().
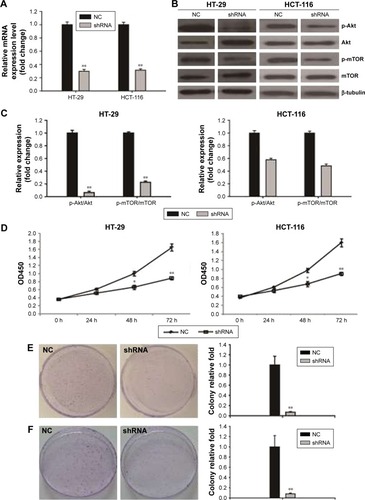
PI3KCA knockdown reduced HT-29 and HCT-116 cell proliferation
To confirm the inhibitory effect of the PI3K/Akt/mTOR pathway on colon cancer cells, PI3KCA shRNA transfection was used to inhibit PI3KCA expression. In knockdown HT-29 cells, the expression of PI3KCA mRNA was reduced by 70%, while the knockdown of HCT-116 cells decreased by 68%. The cells were detected by RT-PCR and compared with control cells of 48 hours past-transfection (). In knockdown HT-29 and HCT-116 cells, the PI3K/Akt/mTOR pathway activation was also significantly suppressed (). PI3KCA knockdown resulted in a significant decrease in the proliferation of HT-29 and HCT-116 cells at the time of 48 and 72 hours, respectively (), compared to the control group. These results were also confirmed by colony formation assays. Compared with the control group, the number of cells in the PI3KCA-knockdown HT-29 cell group decreased by >93% (), while the PI3KCA-knockdown HCT-116 cells () decreased by 92%.
Figure 2 PI3KCA knockdown inhibited proliferation of HT-29 and HCT-116 cells.
Abbreviations: OD450, optical density 450 nm; NC, negative control.
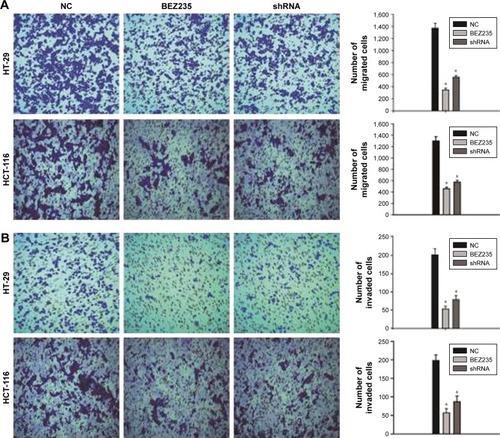
Inhibition of PI3K/Akt/mTOR suppressed migration and invasion of HT-29 and HCT-116 cells
In order to investigate the effect of inhibiting PI3K/Akt/mTOR pathway on HT-29 and HCT-116 cell migration and invasion, a Transwell assay was carried out. PI3K/Akt/mTOR inhibition by BEZ235 and PI3KCA knockdown both resulted in a migration rate through an 8 μm polycarbonate membrane filter dropped by 73% and 58% on HT-29 cell and HCT-116 cell migration rates were reduced to 68% and 55%, respectively (). The Transwell invasion assay showed that BEZ235 and PI3KCA knockdown both significantly decreased cell invasion in HT-29 (75%, 63%) and HCT-116 (72%, 60%) cells ().
Figure 3 Inhibition of PI3K/Akt/mTOR pathway by BEZ235 and PI3KCA knockdown resulted in decreased cell migration and invasion.
Abbreviation: NC, negative control.
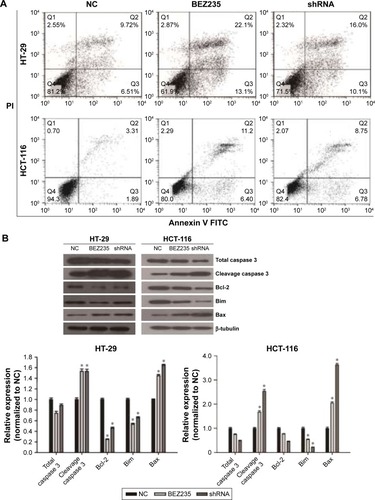
Inhibition of PI3K/Akt/mTOR induced HT-29 and HCT-116 cell apoptosis in vitro
To further evaluate the inhibitory effects of PI3K/Akt/mTOR by BEZ235 and PI3KCA knockdown on cell apoptosis, flow cytometry assay and Western blotting were performed. As shown in , 16% of apoptotic cells were found in the negative control group, but the proportions of apoptotic cells in HT-29 cells were significantly increased to 35% and 26% with treatment of BEZ235 and PI3KCA knockdown, respectively. In HCT-116 cells, BEZ235 treatment and PI3K knockdown also promoted apoptosis (). The expression levels of proteins associated with apoptosis changed, with the expression of cleavage caspase 3 and Bax increasing while Bcl-2 and Bim decreasing significantly ().
Figure 4 Inhibition of PI3K/Akt/mTOR pathway by BEZ235 and PI3KCA knockdown induced cell apoptosis.
Abbreviations: FITC, fluorescein isothiocyanate; NC, negative control; PI, propidium iodide.
Discussion
Colon cancer is the third most common cancer in the world and has not yet been adequately treated.Citation21 Current clinical routine treatment for colon cancer includes surgical resection of primary tumor, chemotherapy, radiotherapy, and immunotherapy, with limitations and side effects. Drug resistance also affects the treatment efficacy.Citation22,Citation23 The PI3K/Akt/mTOR pathway is closely related to the pathogenesis and process of cancer and is involved in drug resistance.Citation3–Citation8 Therefore, inhibition of PI3K/Akt/mTOR signaling pathway may be of great significance to the treatment of cancer.
Activated Akt will promote cell growth and survival by inhibiting the pro-apoptotic proteins of Bcl-2 family.Citation24 mTOR is a serine/threonine kinase that can be induced by the activity of activated Akt.Citation25 Activated mTOR is involved in gene transcription and protein translation. It has been proven that Akt and mTOR have strong expression in colon cancer tissue and HT-29 cells.Citation26 The PI3K/Akt/mTOR pathway is a crucial regulator in cell growth, including proliferation, growth, differentiation, and survival. Therefore, abnormal activation of PI3K/Akt/mTOR signaling pathway is commonly involved in the development and progression of various tumors.Citation27–Citation31 PI3K/Akt/mTOR signaling pathway is upregulated and plays an important role in colon cancer.Citation2–Citation4,Citation32,Citation33 In addition, a large number of studies have shown that this pathway is also related to drug resistance.Citation2,Citation5–Citation7 Thus, targeting the PI3K/Akt/mTOR pathway may be a promising technique for treating colon cancer.
BEZ235 is a novel dual inhibitor of both PI3K and mTOR and has been used in many research studies.Citation29 Several previous reports in these studies suggested that, in the form of apoptosis or autophagy in tumors, the treatment of BEZ235 can inhibit cell proliferation and induce cell death autophagy.Citation11–Citation14,Citation34 It has been proven that BEZ235 suppresses HCT-116 cell proliferation.Citation16 Hong confirmed that by inducing apoptosis, BEZ235 has inhibitory effects on HT-29 and HCT-116.Citation35 In our studies, we found that BEZ235 can effectively inhibit the activation of PI3K/Akt/mTOR pathway, cell proliferation, migration, invasion, and induced apoptosis in HT-29 and HCT-116 cells (, , and ). Inhibition of PI3K/Akt/mTOR pathway by PI3KCA knockdown can also reduce cell proliferation, migration, invasion, and induced apoptosis (–). Therefore, inhibition of PI3K/Akt/mTOR pathway may significantly inhibit cell progression in colon cancer.
Caspase 3 is a key member of the cysteine-aspartic acid protease family, an important protease that causes apoptosis.Citation36 Bcl-2 and Bax are important regulators of apoptosis, involved in the regulation of cell death.Citation37 Bcl-2 is an anti-apoptosis protein that is localized in mitochondria, while Bax is a pro-apoptotic protein that mainly resides in the cytoplasm. The ratio of Bcl-2/Bax determines cell survival, as a reduction in the ratio promotes cell apoptosis.Citation38,Citation39 Bim (cell death mediated by Bcl-2) is another important member of the Bcl-2 family and has a similar role to Bax in promoting cell apoptosis.Citation40 The present study shows that through a downregulation process of Bcl-2 and Bim expressions, inhibition of PI3K/Akt/mTOR pathway by BEZ235 and PI3KCA knockdown induced HT-29 and HCT-116 cells apoptosis, at the same time keeping an upregulation in cleavage caspase 3 and Bax ().
In summary, this study reveals that targeted inhibiting PI3K/Akt/mTOR pathway by BEZ235 and PI3KCA knockdown could inhibit cell proliferation, survival, and invasion in human colon cancer. Therefore, in addition to surgery, chemotherapy, and radiotherapy, targeted inhibiting PI3K/Akt/mTOR pathway may be a valuable technique for human colon cancer therapy.
Acknowledgments
This work was supported by the Science and Technology Plan of Shandong (Grant No 2014GSF118008).
Disclosure
The authors report no conflicts of interest in this work.
References
- DaviesJMGoldbergRMTreatment of metastatic colorectal cancerSemin Oncol20113855256021810514
- ChenJPotential value and limitation of dual inhibitors of PI3K and mTOR in the treatment of cancerCurr Cancer Drug Targets20131311712023215718
- BuZJiJTherapeutic implications of mTOR inhibitors in the treatment of gastric cancerCurr Cancer Drug Targets20131312112523215721
- ChoDCMierJWDual inhibition of PI3-kinase and mTOR in renal cell carcinomaCurr Cancer Drug Targets20131312614223259857
- ElfikyAAJiangZThe PI3 kinase signaling pathway in prostate cancerCurr Cancer Drug Targets20131315716423215719
- YuYYuXMaJTongYYaoJEffects of NVP-BEZ235 on the proliferation, migration, apoptosis and autophagy in HT-29 human colorectal adenocarcinoma cellsInt J Oncol20164928529327176231
- ChenJIversonDEstrogen in obesity-associated colon cancer: friend or foe? Protecting postmenopausal women but promoting late-stage colon cancerCancer Causes Control2012231767177323011535
- WangHZhangLYangXPUMA mediates the combinational therapy of 5-FU and NVP-BEZ235 in colon cancerOncotarget20156143851439825965911
- WuDChengJSunGp70S6K promotes IL-6-induced epithelial-mesenchymal transition and metastasis of head and neck squamous cell carcinomaOncotarget20167365393655027174914
- SegattoIBertonSSonegoMp70S6 kinase mediates breast cancer cell survival in response to surgical wound fluid stimulationMol Oncol2014876678024661902
- JiYDiWYangQLuZCaiWWuJInhibition of autophagy increases proliferation inhibition and apoptosis induced by the PI3K/mTOR inhibitor NVP-BEZ235 in breast cancer cellsClin Lab2015611043105126427150
- LiCXinPXiaoHYanZHuangYZhuXThe dual PI3K/mTOR inhibitor NVP-BEZ235 inhibits proliferation and induces apoptosis of burkitt lymphoma cellsCancer Cell Int2015156526130968
- MairaSMStaufferFBrueggenJIdentification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activityMol Cancer Ther200871851186318606717
- WangWLongLYangNNVP-BEZ235, a novel dual PI3K/mTOR inhibitor, enhances the radiosensitivity of human glioma stem cells in vitroActa Pharmacol Sin20133468169023603977
- RoperJRichardsonMPWeiVWThe dual PI3K/mTOR inhibitor NVP-BEZ235 induces tumor regression in a genetically engineered mouse model for sporadic colorectal cancerGastroenterology20116e25132
- ChenJShaoRLiFPI3K/Akt/mTOR pathway dual inhibitor BEZ235 suppresses the stemness of colon cancer stem cellsClin Exp Pharmacol Physiol20164213171326
- YanFShaoLJHuXYKnockdown of UHRF1 by lentivirus-mediated shRNA inhibits ovarian cancer cell growthAsian Pac J Cancer Prev2015161343134825743796
- HabibRAkhtarJTaqiMYuCZhangCLentiviral vector-mediated survivin shRNA delivery in gastric cancer cell lines significantly inhibits cell proliferation and tumor growthOncol Rep20153485926043753
- GayatriDVBadanaATherapeutic Potentials of CD151 shRNA in Targeting Metastasis of Triple Negative Breast Cancer Cell Line MDA-MB-231J Cancer Sci Ther20168104112
- DangNPangSSongHAnLMaXInactivation of mitogen-activated protein kinase signaling pathway reduces caspase-14 expression in impaired keratinocytesIran J Basic Med Sci201619283327096061
- CatalanoVDiFSIovinoFDieliFStassiGTodaroMCD133 as a target for colon cancerExpert Opin Ther Targets20121625926722385077
- ChenJHuangXFQiaoLKatsifisAInsulin caused drug resistance to oxaliplatin in colon cancer cell HT29J Gastrointest Oncol20112273322811824
- DallasNALingXFanFChemoresistant colorectal cancer cells, the cancer stem cell phenotype and increased sensitivity to insulin-like growth factor receptor-1 inhibitionCancer Res2009691951195719244128
- DuronioVThe life of a cell: apoptosis regulation by the PI3K/PKB pathwayBiochem J200841533334418842113
- SabatiniDMSarbassovDDPhosphorylation and regulation of Akt/PKB by the rictor-mTOR complexAm Assoc Advanc Sci201110981101
- RoyHKOlusolaBFClemensDLAKT proto-oncogene overexpression is an early event during sporadic colon carcinogenesisCarcinogenesis20022320111756242
- CourtneyKDCorcoranRBEngelmanJAThe PI3K pathway as drug target in human cancerJ Clin Oncol2010281075108320085938
- FumarolaCBonelliMAPetroniniPGAlfieriRRTargeting PI3K/AKT/mTOR pathway in non small cell lung cancerBiochem Pharmacol20149019720724863259
- Moon DuGLeeSEOHMMNVP-BEZ235, a dual PI3K/mTOR inhibitor synergistically potentiates the antitumor effects of cisplatin in bladder cancer cellsInt J Oncol2014451027103524969552
- GarciaJADanielpourDMammalian target of rapamycin inhibition as a therapeutic strategy in the management of urologic malignanciesMol Cancer Ther200871347135418566209
- CebullaJHuuseEMPettersenKMRI reveals the in vivo cellular and vascular response to BEZ235 in ovarian cancer xenografts with different PI3-kinase pathway activityBr J Cancer201511250451325535727
- DanielsenSAEidePWNesbakkenAGurenTLeitheELotheRAPortrait of the PI3K/AKT pathway in colorectal cancerBiochim Biophys Acta2015185510412125450577
- Papadatos-PastosDRabbieRRossPSarkerDThe role of the PI3K pathway in colorectal cancerCrit Rev Oncol Hematol201594183025591826
- HongSWShinJSMoonJHNVP-BEZ235, a dual PI3K/mTOR inhibitor, induces cell death through alternate routes in prostate cancer cells depending on the PTEN genotypeApoptosis20141989524652480
- HongZLiLCarcedoIGZhiPXMonteiroMGuWSynergistic inhibition of colon cancer cell growth with nanoemulsion-loaded paclitaxel and PI3K/mTOR dual inhibitor BEZ235 through apoptosisInt J Nanomedicine2016111947195827226714
- CakirEYilmazADemiragFPrognostic significance of micropapillary pattern in lung adenocarcinoma and expression of apoptosis-related markers: caspase-3, bcl-2, and p53APMIS201111957458021851414
- MallaRRGopinathSAlapatiKGorantlaBGondiCSRaoJSuPAR and cathepsin B inhibition enhanced radiation-induced apoptosis in glioma initiating cellsNeuro Oncol20121474576022573309
- SoaresNDCPTeodoroAJOliveiraFLLycopene induce apoptosis in human prostate cells and alters the expression of Bax and Bcl-2 genesLWT Food Sci Technol20145912901297
- LiuGWangTWangTSongJZhouZEffects of apoptosis-related proteins caspase-3, Bax and Bcl-2 on cerebral ischemia ratsBiomed Rep2013186186724649043
- O’ConnorLHuangDCO’ReillyLAStrasserAApoptosis and cell division: commentaryCurr Opin Cell Biol20001225726310819542
