Abstract
A novel molecular classification of gastric cancer by the Asian Cancer Research Group (ACRG) is a potential advance in diagnosis and treatment, and it helps to determine prognosis. The use of immunohistochemistry (IHC) rather than gene expression analysis to determine tumor subtypes was evaluated with the aim of determining the feasibility of using the ACRG molecular classification. A total of 69 esophagogastric junction (EGJ) carcinomas were classified as microsatellite instable (MSI, 17.40%, 12 of 69), microsatellite stable with markers of epithelial-to-mesenchymal transition (MSS/EMT, 18.84%, 13 of 69), microsatellite stable with active tumor protein 53 (MSS/TP53+, 27.53%, 19 of 69), and microsatellite stable with inactive TP53 (MSS/TP53−, 36.23%, 25 of 69). The molecular classification did not significantly correlate with anyone of the clinicopathological characteristics of the EGJ carcinoma patients, including age, gender, depth of tumor invasion, the presence of lymph node metastasis, histologic grade, and p-TNM stage of the American Joint Committee on Cancer (P>0.05). Kaplan–Meier survival analysis and log rank tests showed that molecular classification, histologic grade, p-TNM stage, and postoperative adjuvant chemotherapy were significantly associated with overall survival (OS; P<0.05). MSI tumors had the best overall prognosis followed by MSS/TP53− and MSS/TP53+. MSS/EMT tumors had the worst overall prognosis. Multivariate analysis revealed that histologic grade (hazard ratio [HR] =2.216, 95% CI =1.202–4.086), p-TNM stage (HR =2.216, 95% CI =1.202–4.086), and molecular subtype (HR =2.216, 95% CI =1.202–4.086) were independently associated with OS. The preliminary results suggested that the ACRG molecular classification may be a valuable independent prognostic marker for EGJ carcinoma patients and could be performed by IHC analysis.
Introduction
Gastric cancer (GC) is the fourth most common malignancy and the second leading cause of cancer-related deaths worldwide.Citation1 Despite declines in incidence and mortality, GC remains a major contributor to the global cancer burden and cancer-related disability-adjusted life-years. In Western countries, ~70% of GC patients die within 5 years of diagnosis.Citation2,Citation3 There are two anatomical forms of GC, noncardia or distal GC and cardia GC, also known as esophagogastric junction (EGJ) carcinoma.Citation4 The incidence of EGJ carcinoma has been increasing, particularly in Western countries,Citation5,Citation6 where it has become a public health concern. The etiology and clinicopathological features of EGJ carcinoma differ from those of distal GC. It is usually diagnosed at a more advanced stage and has a worse prognosis,Citation7,Citation8 primarily because of a limited understanding of its molecular characteristics.
The existence of various subtypes, based on histopathology and anatomic site,Citation9 gene expression,Citation10–Citation13 gene amplification,Citation10,Citation11,Citation14 DNA methylation,Citation10,Citation11 and numerous cancer-related aberrations,Citation10,Citation11,Citation13,Citation15 reveals that GC is a heterogeneous, complex disease. An integrative genomic analysis of GC performed by the Asian Cancer Research Group (ACRG) provides a molecular classification that can be used to guide the development of targeted agents.Citation10 The ACGR classification is based on a principal component analysis of expression data and a small predefined set of gene expression signatures considered relevant to GC biology.Citation16–Citation18 The classification includes four subtypes characterized by microsatellite-instable (MSI), microsatellite stable with markers of epithelial-to-mesenchymal transition (MSS/EMT), microsatellite stable with active tumor protein 53 (MSS/TP53+), and microsatellite stable with inactive TP53 (MSS/TP53−). To reduce the costs encountered in clinical practice, the ACRG recommends the use of immunohistochemistry (IHC) and RNA in situ hybridization for tumor classification rather than gene expression assays. The MSI group can be identified by MutL protein homolog 1 (MLH1) assay, the MSS/EMT group can be identified by assay of E-cadherin (CDH1) expression, and the MSS/TP53 tumors can be identified by assays of mouse double minute 2 homolog (MDM2) and cyclin-dependent kinase inhibitor 1A (CDKN1A, alternatively P21) expression.Citation10
The four subtypes are linked to distinct patterns of molecular differences, disease progression, and prognosis. This novel molecular classification of GC may spur translational research to improve diagnosis and treatment approaching precision medicineCitation19 and help to determine prognosis and to customize treatment. In this study, we assayed MLH1, E-cadherin, MDM2, and P21 in 69 EGJ carcinoma patients by IHC and classified their carcinomas following the ACRG molecular criteria. The relationships of the molecular subtypes, clinicopathological characteristics, and prognosis of the EGJ patients were evaluated.
Materials and methods
Collection of clinical samples
The patients who underwent surgical resection and were pathologically diagnosed with EGJ carcinoma at the First Affiliated Hospital of Xi’an Jiaotong University (Xi’an, People’s Republic of China) between December 2010 and December 2012 were enrolled in this study. Patients with noncardia or distal GC and other organ primary malignant tumor were excluded from the study. Those whose information was incomplete were not in the range of analysis. The tumors were classified following the p-TNM staging system of the American Joint Committee on Cancer (AJCC).Citation20 Survival was defined as the interval from diagnosis to the end of follow-up, and patients were followed up until death or study completion in March 2017. The study was approved by the Ethics Committees of the First Affiliated Hospital of Xi’an Jiaotong University. All the patients selected for our study were fully informed about our experiment protocols and signed an informed consent to participate in this study.
Immunohistochemical staining
Formalin-fixed, paraffin-embedded EGJ carcinoma tissue was cut into 4-μm serial sections. For antigen retrieval, the sections were heated in 10 mM pH 6.0 citrate buffer in a microwave at high power for 8 minutes, followed by heating at mid-low power for 13 minutes. Immunohistochemical staining was performed using a streptavidin–biotin peroxidase kit (SP-9001/9002; Zhongshan Golden Bridge Biotechnology, Beijing, People’s Republic of China). Then, the sections were treated with 3% hydrogen peroxide in methanol for 10 minutes at room temperature followed by incubation with reagent A for 15 minutes at room temperature. They were then incubated overnight with primary antibodies against MLH1 (clone EPR3894, 1:100, ab92312), MDM2 (clone 2A10, 1:40, ab16895), P21 (clone EPR362, 1:100, ab109520), and E-cadherin (polyclonal, 1:50, 20874-1-AP) at 4°C. After washing with PBS, the sections were incubated with reagents B and C for 15 minutes each at 37°C. Diaminobenzidine (ZLI-9018; Zhongshan Golden Bridge Biotechnology) was added according to the kit manufacturer’s instructions. The sections were rinsed with tap water, counterstained with Harris’ hematoxylin, and coverslipped. Then, they were observed and independently scored by two pathologists.
Scoring methods and molecular classification
All the sections were observed and independently scored by two pathologists in a double-blind manner. Using a high-power microscope, ten fields of vision were randomly selected from each slice, with 100 cells counted in each field. In cases in which there was disagreement between the two pathologists that impacted the categorization of a case as having positive or negative staining, the case was reviewed jointly until consensus was achieved. The Sinicrope scoring methodCitation21 was used to evaluate both the IHC staining intensity and the proportion of stained epithelial cells in each field. The scores were 0 for ≤5% stained cells, 1 for 6%–25% stained cells, 2 for 26%–50% stained cells, 3 for 51%–75% stained cells, and 4 for >75% stained cells. Intensity was 0 for negative staining, 1 for weak staining, 2 for moderate staining, and 3 for strong staining. The overall immunostaining score for each tumor specimen was calculated by multiplying the percentage score by the intensity score. For MLH1 expression, a score of 0 indicated negative expression (ie, loss of MLHI).Citation22,Citation23 For MDM2, p21, and E-cadherin expression, a final score of 0–2 indicated negative expression and 3–12 indicated positive expression. Tumors with loss of MLHI expression were classified as the MSI subtype, and the remaining specimens were classified as MSS. Among the MSS specimens, tumors with negative E-cadherin expression were classified as MSS/EMT, and those with negative MDM2 expression and positive P21 expression were classified as the MSS/TP53+. Tumors with positive MDM2 expression and negative P21 expression were classified as MSS/TP53−.Citation10
Statistical analysis
Statistical analysis was performed using SPSS software, version 21.0 (SPPS, Inc., Chicago, IL, USA). Bivariate associations of categorical variables were evaluated using Pearson’s χ2 test or Fisher’s exact test as appropriate. Kaplan–Meier plots and log rank tests were used for survival analysis. Multivariate analyses were based on Cox proportional hazards regression models. All statistical tests were two-sided, and statistical significances were defined as P<0.05.
Results
Patient baseline characteristics
A cohort of 69 patients, 56 men (81%) and 13 women (19%), with a median of 62 and a range of 38–81 years of age were included. All patients enrolled in the study received surgical treatment and were pathologically diagnosed with EGJ carcinoma; 57 patients were treated by radical resection of gastric cardia cancer, ten by total gastrectomy, one by radical resection of GC, and one by omentectomy. All patients did not receive postoperative adjuvant radiotherapy and neoadjuvant therapy; 41 patients received postoperative adjuvant chemotherapy. There were no perioperative mortalities. According to the eighth edition of the AJCC Cancer Staging Manual,Citation20 15 (21.7%) patients were in Stage I/II, and 54 (of 69; 78.3%) patients were in Stage III/IV; 36 (52.2%) patients were diagnosed with moderately differentiated (histologic grade 2) tumors, and 33 (47.8%) patients were diagnosed with poorly differentiated (histologic grade 3) tumors.
Molecular EGJ carcinoma subtypes and their association with clinicopathological characteristics
IHC staining revealed that MLH1, MDM2, and P21 were located in the nucleus, and E-cadherin was located on cell membrane or in cytoplasm of the EGJ cancer cells (). The molecular subtypes and baseline patient clinicopathological characteristics are shown in . Of the 69 EGJ carcinomas, 12 (17.4%) were MSI tumors, 13 (18.8%) were MSS/EMT tumors, 25 (36.2%) were MSS/TP53− tumors, and 19 (27.5%) were MSS/TP53+ tumors. There were no significant differences in age, gender, depth of tumor invasion, the presence of lymph node metastasis, histologic grade, p-TNM stages as well as whether these patients received adjuvant therapy in the four molecular grade groups (P>0.05; ).
Figure 1 Representative images of multiple markers in EGJ carcinoma.
Abbreviations: CDKN1A, cyclin-dependent kinase inhibitor 1A; EGJ, esophagogastric junction; MDM2, mouse double minute 2 homolog; MLH1, MutL protein homolog 1.
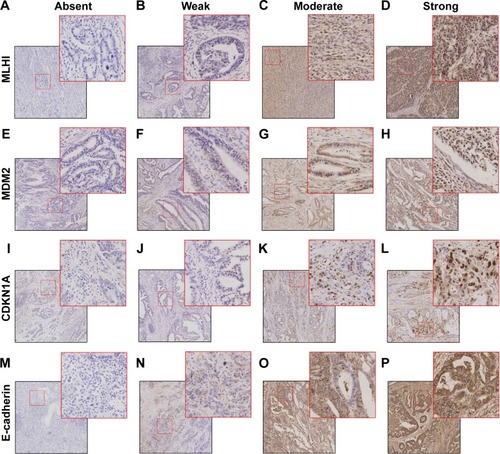
Table 1 Four molecular subtypes and characteristics of 69 EGJ carcinoma patients
Molecular subtypes of EGJ carcinoma associated with overall survival (OS)
The median follow-up was 36.3 (range 1.0–75.0) months, and the median OS was 36.3 months. The median and mean OS for each subgroup by histologic grade, p-TNM stage, postoperative adjuvant chemotherapy, and molecular subtype are shown in the Kaplan–Meier survival graphs, and the results of log-rank tests are shown in . Kaplan–Meier survival analysis showed that the patients with poor histologic grade, advanced AJCC p-TNM stage, and MSS/EMT molecular subtype and those who did not accept postoperative adjuvant chemotherapy had a worse prognosis (). Further post hoc analysis showed that only patients with MSI subtype vs those with MSS/EMT subtype performed a significant different prognosis (P=0.039). MSI tumors had the best overall prognosis, followed by MSS/TP53− and MSS/TP53+. Multivariate analysis () revealed that histologic grade (hazard ratio [HR] =2.216, 95% CI =1.202–4.086), p-TNM stage (HR =2.216, 95% CI =1.202–4.086), and molecular subtype (HR =2.216, 95% CI =1.202–4.086) were independently associated with the OS of EGJ carcinoma patients. The results revealed that molecular classification was an independent prognostic marker for EGJ carcinoma patients and that the ACRG subtype classification could be performed by IHC.
Figure 2 Kaplan–Meier survival curves for 69 EGJ carcinoma patients, grouped according to histologic grade (A), p-TNM stage (B), molecular subtypes (C), and postoperative adjuvant chemotherapya (D). HR and 95% CI were calculated by multivariable analysis after adjusting for several covariates (histologic grade, p-TNM stage, molecular subtypes, and postoperative adjuvant chemotherapy).
Abbreviations: EGJ, esophagogastric junction; HR, hazard ratio; MSI, microsatellite instable; MSS/EMT, microsatellite stable with markers of epithelial-to-mesenchymal transition; MSS/TP53+, microsatellite stable with active tumor protein 53, and MSS/TP53−, microsatellite stable with inactive TP53.
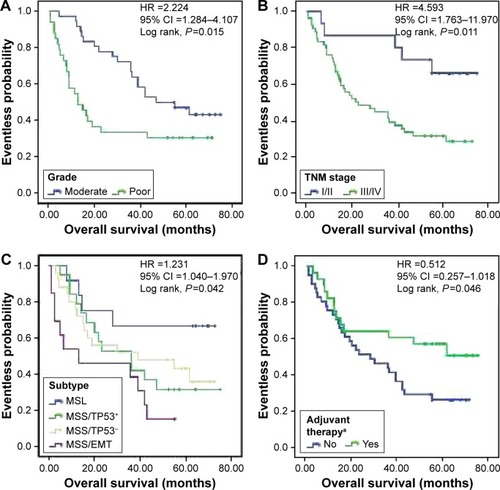
Table 2 Univariate analysis of prognosis for 69 EGJ carcinoma patients
Table 3 Multivariate analysis of prognosis for 69 EGJ carcinoma patients
Discussion
GC is a highly heterogeneous disease with multiple subtypes, each with distinct biological properties.Citation7–Citation13 The molecular classifications of The Cancer Genome Atlas (TCGA) cohort, ie, Epstein–Barr virus+, MSI, genome stable, and chromosomal instability (CIN),Citation11 and the ACRG cohort,Citation10 ie, MSI, MSS/EMT, MSS/TP53+, and MSS/TP53−, add to what is known of GC etiology and pathogenesis. The two molecular classifications probably reflect different underlying properties. When tumors from the original ACRG cohort were classified using the TCGA genomic criteria, the association between molecular subtype and prognosis had decreased.Citation10 In addition, CIN detection is complicated and requires in-depth knowledge of the underlying mechanism. Gonzalez et alCitation24 found that it was difficult to identify CIN subtype GC tumors with wild-type TP53 using IHC for p53 detection. In addition, the ACGR samples were from an Asian population, but the TCGA samples were from Europe and the USA. Because the ACRG molecular classification may be easier to use in clinical practice, especially in Chinese populations, we classified this series of 69 EGJ carcinoma patients using the four ACTRG molecular subtypes using IHC.
MSI is a genetic alteration consisting of the expansion or contraction of regions of microsatellites, which are repetitive nucleotide sequences caused by the inactivation of DNA mismatch repair genes (eg, MLH1 or MSH2). MSI has been reported in various tumors and can be detected by IHC assay of mismatch repair proteins or by profiling the Bethesda markers.Citation25,Citation26 It has the best overall prognosis and the lowest frequency of recurrence of the four subtypes. The prevalence of MSI in GC is estimated at 15%–30% of GC, and MSI-type tumors are more frequent in the antrum, in women and in older patients.Citation10,Citation11,Citation27,Citation28 In this cohort, 17.40% of the EGJ carcinomas were MSI, were the rarest subtype, and had the best prognosis. This was consistent with the percentage of MSI in the original ACRG cohort (12.5%), but in that cohort, MSS/EMT subtype was the least frequent. It is possible that using only MLH1 expression for typing may underestimate the prevalence of MSI because PMS2, MSH2, and MSH6 may also be deficient and independent of MLH1 expression.Citation29
A small minority of GC cases are associated with a germline mutation in CDH1,Citation30 which is downregulated in epithelial tumorigenesis and is categorized as a tumor suppressor gene.Citation31 The loss or downregulation of E-cadherin is a characteristic of EMT.Citation32 In this study, the MSS/EMT subtype occurred in 18.84% of the EGJ carcinomas and had the worst prognosis. Li et alCitation33 reported that in diffuse-type GC, CDH1 mutation was associated with shortened survival, independent of disease stage. However, molecular analysis by next-generation sequencing and IHC showed that E-cadherin expression was not significantly associated with intestinal-type GC.Citation34 The prognostic value of CDH1 and its potential as a candidate therapeutic target in GC deserve further study.
Next-generation sequencing and molecular profiling in GC found that TP53 was the most frequently mutated gene and that it contributed to the occurrence and development of GC.Citation10,Citation11,Citation29,Citation34 Consequently, the ACRG stratified the remaining (non-MSI and non-EMT) tumors by the presence of TP53 activation. In both this cohort and the ACRG cohort, the non-MSI and non-MSS/EMT subtypes were the most common, comprising 63.76% and 62% of the total cases, respectively. The difference was that in the ACRG cohort, MSS/TP53+ was the most common subtype (14 of 32, 43.75%) followed by MSS/TP53− (9 of 32, 28.13%), whereas in this cohort, MSS/TP53− was the most common (36.23%), followed by MSS/TP53+ (27.53%). In this cohort, the prognosis of MSS/TP53−, with a median OS of 39 months, was little better than that of MSS/TP53+, which had a median OS of 36 months. In the ACRG cohort, MSS/TP53+ had a better prognosis than MSS/TP53−.
In the ACRG cohort study, GC molecular analysis was significantly associated with clinical phenotypes, such as age, grade, World Health Organization classification, AJCC stage, tumor invasion, and lymph node metastasis.Citation10 In this study, the EGJ carcinoma molecular analysis did not correlate with those clinical variables. On the one hand, the difference may be a consequence of the small sample sizes or the different tumor types. On the other hand, it is the recurrence but not the depth of invasion and nodal status that correlate with molecular classification, but due to limited information about the local and distant recurrences, we failed to further do the work. To sum up, from the above results, we concluded that EGJ carcinoma and GC may have different molecular characteristics and that the ACRG molecular classification can be determined by IHC analysis, but the method needs to further be evaluated.
Disclosure
The authors report no conflicts of interest in this work.
References
- Van CutsemESagaertXTopalBHaustermansKPrenenHGastric cancerLancet2016388100602654266427156933
- SoerjomataramILortet-TieulentJParkinDMGlobal burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regionsLancet201238098561840185023079588
- MalekiSSRockenCChromosomal instability in gastric cancer biologyNeoplasia201719541242028431273
- ColquhounAArnoldMFerlayJGoodmanKJFormanDSoerjomataramIGlobal patterns of cardia and non-cardia gastric cancer incidence in 2012Gut201564121881188825748648
- PohlHSirovichBWelchHGEsophageal Adenocarcinoma incidence: are we reaching the peak?Cancer Epidemiol Biomarkers prev20101961468147020501776
- BuasMFVaughanTLEpidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this diseaseSemin Radiat Oncol20132313923207041
- MaedaHOkabayashiTNishimoriIClinicopathologic features of adenocarcinoma at the gastric cardia: is it different from distal cancer of the stomach?J Am Coll Surg2008206230631018222384
- LiuKZhangWChenXComparison on clinicopathological features and prognosis between esophagogastric junctional adenocarcinoma (Siewert II/III types) and distal gastric adenocarcinoma: retrospective cohort study, a single institution, high volume experience in ChinaMedicine (Baltimore)20159434e138626313779
- ShahMAKhaninRTangLMolecular classification of gastric cancer: a new paradigmClin Cancer Res20111792693270121430069
- CristescuRLeeJNebozhynMMolecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomesNat Med201521544945625894828
- Cancer Genome Atlas Research NetworkComprehensive molecular characterization of gastric adenocarcinomaNature2014513751720220925079317
- TaySTLeongSHYuKA combined comparative genomic hybridization and expression microarray analysis of gastric cancer reveals novel molecular subtypesCancer Res200363123309331612810664
- TanIBIvanovaTLimKHIntrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapyGastroenterology20111412476485485.e471e41121684283
- DengNGohLKWangHA comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targetsGut201261567368422315472
- WangKYuenSTXuJWhole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancerNat Genet201446657358224816253
- LobodaANebozhynMVWattersJWEMT is the dominant program in human colon cancerBMC Med Genomics20114921251323
- Cancer Genome Atlas NetworkComprehensive molecular characterization of human colon and rectal cancerNature2012487740733033722810696
- Cancer Genome Atlas NetworkComprehensive molecular portraits of human breast tumoursNature20124907418617023000897
- RockenCMolecular classification of gastric cancerExpert Rev Mol Diag2017173293301
- RiceTWGressDMPatilDTHofstetterWLKelsenDPBlackstoneEHCancer of the esophagus and esophagogastric junction: major changes in the American Joint Committee on Cancer eighth edition cancer staging manualCA Cancer J Clin201767430431728556024
- SinicropeFARuanSBClearyKRStephensLCLeeJJLevinBbcl-2 and p53 oncoprotein expression during colorectal tumorigenesisCancer Res19955522372417812951
- ThibodeauSNFrenchAJRochePCAltered expression of hMSH2 and hMLH1 in tumors with microsatellite instability and genetic alterations in mismatch repair genesCancer Res19965621483648408895729
- ThibodeauSNFrenchAJCunninghamJMMicrosatellite instability in colorectal cancer: different mutator phenotypes and the principal involvement of hMLH1Cancer Res1998588171317189563488
- GonzalezRSMessingSTuXMcMahonLAWhitney-MillerCLImmunohistochemistry as a surrogate for molecular subtyping of gastric adenocarcinomaHum Pathol201656162127342907
- DudleyJCLinMTLeDTEshlemanJRMicrosatellite instability as a biomarker for PD-1 blockadeClin Cancer Res201622481382026880610
- VilarEGruberSBMicrosatellite instability in colorectal cancer-the stable evidenceNat Rev Clin Oncol20107315316220142816
- PedrazzaniCCorsoGVelhoSEvidence of tumor microsatellite instability in gastric cancer with familial aggregationFam Cancer20098321522019152022
- VelhoSFernandesMSLeiteMFigueiredoCSerucaRCauses and consequences of microsatellite instability in gastric carcinogenesisWorld J Gastroenterol20142044164331644225469011
- KimHSShinSJBeomSHComprehensive expression profiles of gastric cancer molecular subtypes by immunohistochemistry: implications for individualized therapyOncotarget2016728446084462027331626
- RichardsFMMcKeeSARajparMHGermline E-cadherin gene (CDH1) mutations predispose to familial gastric cancer and colorectal cancerHum Mol Genet19998460761010072428
- CavallaroUChristoforiGCell adhesion and signalling by cadherins and Ig-CAMs in cancerNat Rev Cancer20044211813214964308
- BrunerHCDerksenPWBLoss of E-cadherin-dependent cell-cell adhesion and the development and progression of cancerCold Spring Harb Perspect Biol Epub2017515
- LiXWuWKXingRDistinct subtypes of gastric cancer defined by molecular characterization include novel mutational signatures with prognostic capabilityCancer Res20167671724173226857262
- BriaEPilottoSSimboloMComprehensive molecular portrait using next generation sequencing of resected intestinal-type gastric cancer patients dichotomized according to prognosisSci Rep201662298226961069
