Abstract
Colorectal cancer (CRC) is a common digestive malignancy and emerging studies have closely linked its initiation and development with gut microbiota changes. Fusobacterium nucleatum (Fn) has been recently identified as a pathogenic bacteria for CRC; however, its prognostic significance for patients is poorly investigated and is less for patients within late stage. Therefore, in this study, we made efforts to analyze its level and prognostic significance in a retrospective cohort of 280 stage III/IV CRC patients. We found that the Fn level was abnormally high in tumor tissues and correlated with tumor invasion, lymph node metastasis status, and distant metastasis. We also identified it as an independent adverse prognostic factor for cancer-specific survival (CSS) and disease-free survival (DFS). The following subgroup analysis indicated that Fn level could stratify CSS and DFS in stage IIIB/C and IV patients but failed in stage IIIA patients. In addition, stage III/IV patients with low Fn level were found to benefit more from adjuvant chemotherapy than those with high Fn level, in terms of DFS. Finally, we analyzed the expression and clinical significance of epithelial-to-mesenchymal transition (EMT) markers (E-cadherin and N-cadherin) and cancer stem cell (CSC) markers (Nanog, Oct-4, and Sox-2) in CRC tissues. The results indicated that N-cadherin, Nanog, Oct-4, and Sox-2 were adverse prognostic factors in these patients, while the opposite was true for E-cadherin. More importantly, expression of E-cadherin, N-cadherin, and Nanog was significantly correlated with Fn level in tumor tissues, suggesting the potential involvement of Fn in EMT-CSC cross talk during CRC progression. Taken together, these findings indicate that Fn is a novel predictive biomarker for clinical management in stage III/IV patients, and targeting Fn may be an effective adjuvant approach for preventing CRC metastasis and chemotherapy resistance.
Introduction
Colorectal cancer (CRC) is a fatal digestive malignancy that is commonly diagnosed in both males and females worldwide.Citation1 In USA, it is the third most common form of cancer and will account for an estimated 135,430 newly diagnosed cases and 50,260 CRC-specific deaths in 2017.Citation2 In China, its incidence has reached ~37.63 per 100,000 in 2015 according to the latest report.Citation3 The pathogenesis of CRC is a complicated multistep process involving various inherent and environmental factors such as genetic predisposition and unhealthy lifestyles.Citation4 Although dramatic reduction has been achieved in CRC mortality because of the introduction of screening programs and multidisciplinary treatments, ~60% of CRC patients are still diagnosed with advanced stage with their 5-year survival rate ranging from 14% to 71%.Citation5 In addition, there are few effective therapeutical approaches and prognostic biomarkers available for metastatic CRC currently, frequently leading to inappropriate decision making.Citation6 Targeted therapy (such as epidermal growth factor receptor antagonists) represents an emerging clinical strategy for these patients; however, primary and acquired therapy resistance limit its actual efficiency.Citation7 Molecular biomarker tests hold promise for personalized therapy, while a considerable proportion of them may be overestimated and fail to be recommended for prognosis prediction or therapy selection due to insufficient evidence.Citation8,Citation9 Therefore, it can be concluded that our existing achievements appear to be insufficient to improve the clinical outcome of CRC patients and therefore substantial efforts are still essential to identify other potential CRC-related driving factors.
Recently, increasing studies have suggested that gut microbiota dysbiosis is correlated with tumor initiation and development.Citation10 Microbiota dysbiosis may contribute to the malignant progression of cancer cells through various mechanisms such as metabolism signals, inflammation induction, and immunosuppression.Citation11 Furthermore, microbiota is also crucial for the therapeutical efficacy of some anticancer drugs such as cyclophosphamide, which may associate with its regulation of T-cell responses.Citation12 In gastrointestinal malignancies, a close correlation between microbiota and carcinogenesis has been well established in gastric cancer, where Helicobacter pylori is most extensively studied and has been identified as a risk factor for screening.Citation13 However, with regard to CRC, related studies are emerging although advanced metagenomic techniques are able to provide more potential pathogenic microbiota.Citation14 For example, Tsoi et al proved that Peptostreptococcus anaerobius is increased in CRC tissues and promotes the growth of CRC cells through inducing intracellular cholesterol synthesis.Citation15 Wang et al demonstrated that Enterococcus faecalis can drive the malignant transformation in normal colon epithelial cells via its bystander effect.Citation16 Despite increasing evidences supporting the oncogenic role of some specific bacteria in CRC, their clinical significance is still poorly investigated and whether these bacteria can be further developed as clinical biomarkers for patient management remains unknown.
Previously, using pyrosequencing, we found that Fusobacterium nucleatum (Fn) is abnormally abundant in 1,2-dimethylhydrazine-induced CRC animal models as compared with healthy controls.Citation17 Then, we used the same method to further confirm that it is also significantly more abundant in human CRC tissues than in adjacent normal tissues, suggesting its potential correlation with CRC development.Citation18 Further investigation revealed that Fn promotes the proliferation and invasiveness of CRC cells through activating toll-like receptors/MyD88/NF-Kb/miR-21 signaling.Citation19 Given these findings, we speculate that Fn may be a promising clinical biomarker for CRC patients. Therefore, in this study, we aimed to investigate the level and clinical significance of Fn in stage III/IV CRC patients, who are clinically characterized with positive regional/distant metastasis and have a dramatically worse outcome than those within stage I/II. Since epithelial-to-mesenchymal transition (EMT) and cancer stem cell (CSC) are both widely considered as major molecular factors driving cancer development, we also made efforts to detect the expression of representative EMT and CSC markers in these patients and identify their potential correlations with Fn.Citation20,Citation21 Taken together, our findings not only suggest Fn as a novel therapeutical target and prognostic biomarker for CRC patients within late stage, but also highlight the crucial link between dysregulated microbiota and oncogenic molecular events in CRC progression.
Materials and methods
Patient data and specimens
A total of 280 pairs of tumor and adjacent normal tissues were collected from stage III/IV CRC patients who underwent radical surgery at Department of General Surgery, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital and Shanghai Tenth People’s Hospital between October 1, 2007 and September 25, 2015. All the patients were pathologically confirmed as CRC with positive lymph node metastasis (LNM). Preoperative distant metastasis (including lung, liver, and ovary) was identified by enhanced computed tomography (CT) scanning. Tumor-node-metastasis (TNM) stage was determined according to the latest guidelines of the Union for International Cancer Control (8th edition). Neither preoperative chemotherapy nor radiotherapy was performed on patients. For postoperative chemotherapy, a standard FOLFOX scheme (5-fluorouracil [5-fu] [Shanghai Xudong Haipu Pharmaceutical Co., LTD, Shanghai, China] + oxaliplatin [Jiangsu HengRui Medicine Co., LTD, Lianyungang, Jiangsu, China] + leucovorin [Jiangsu HengRui Medicine Co., LTD, Lianyungang, Jiangsu, China]) was applied. Regular follow-up was conducted according to the Clinical Practice Guidelines in Oncology proposed by the National Comprehensive Cancer Network. In brief, patients were recommended to undergo physical examination, carcinoembryonic antigen (CEA) test, and enhanced CT scan every 3–6 months for the first 2 years, and then 6–12 months for the following 3 years. Patient prognosis was assessed by cancer-specific survival (CSS) and disease-free survival (DFS). CSS was calculated from the date of surgery to the date of death caused by CRC, while DFS was calculated from the date of surgery to the date of local recurrence or regional/distant metastasis. The basic clinical features of patients are summarized in . This study was approved by the ethics committees of both the hospitals mentioned above. Written informed consents were obtained from patients or their legal guardians for using their specimens in medical researches.
Table 1 Correlations between Fn level and clinicopathological parameters in stage III/IV CRC patients
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
The levels of Fn in human CRC and adjacent normal tissues were detected by qRT-PCR. Briefly, paraffin-embedded tissues were deparaffinized in xylene and lysed in buffer ATL (Qiagen NV, Venlo, the Netherlands) and Proteinase K (Qiagen NV). Then, the genomic DNAs were extracted using QIAamp DNA FFPE Tissue Kit according to the manufacturer’s instructions (Qiagen NV). The quality of obtained DNAs was verified by an ultraviolet spectrophotometer and eligible DNA samples were preserved at −20°C. The PCR reaction was performed on a 7500 Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA) using SYBR Premix Ex Taq (TaKaRa, Kusatsu, Shiga, Japan). The reaction conditions were applied as follows: initial denaturation at 95°C for 10 minutes, denaturation at 95°C for 1 minute, primer annealing at 60°C for 20 seconds, and primer extension at 56°C for 60 seconds. The sequences of primers were as follows: Fn, forward: 5′-CTTAGGAATGAGACAGAGATG-3′ and reverse: 5′-TGATGGTAACATACGAAAGG-3′; β-actin, forward: 5′-CCTCCATCGTCCACCGCAAATG-3′ and reverse: 5′-TGCTGTCACCTTCACCGTTCCA-3′. The 2−ΔΔCT method was utilized to calculate the relative level of Fn gene and β-actin served as an internal control gene. All the experiments were repeated in triplicate.
Immunohistochemistry (IHC) and staining evaluation
Experimental procedures of IHC were carried out according to our previous study.Citation22 In brief, paraffin-embedded tissues were continuously cut into 4-μm-thick sections, dewaxed in xylene, and rehydrated in gradient concentrations of ethanol. Antigen retrieval was achieved by microwave heating and endogenous peroxidase activity was blocked by incubation with 3% H2O2 solution. Then, sections were incubated with the primary antibody against E-cadherin (1:250; Abcam, Cambridge, UK), N-cadherin (1:250; Abcam), Nanog (1:200; Abcam), Sox-2 (1:200; Abcam), and Oct-4 (1:200; Abcam) at 4°C overnight. Sections incubated with only antibody dilution buffer were utilized as negative controls. Following several washes with phosphate-buffered saline solution, sections were treated with the secondary antibody (1:250; Abcam) at 37°C for 30 minutes. Finally, protein staining was visualized by incubating sections with a diaminobenzidine kit (Thermo Fisher Scientific) for 5 minutes. The sections were counterstained with hematoxylin (Thermo Fisher Scientific) for 10 minutes, dehydrated, sealed, and transferred for microscopic examination.
Staining evaluation was independently carried out by two investigators who were blind to the clinical features and outcome of patients. Any controversial cases were determined by a well-skilled pathologist. The evaluation criteria were based on staining intensity (SI) and percentage of positive cells (PP). SI is scored as follows: 0, negative; 1, weak; 2, moderate; 3, strong. PP is scored as follows: 0, 0%–10%; 1, 11%–25%; 2, 26%–50%; 3, 51%–75%; 4, 76%–100%. A final staining score was calculated by multiplying the PP score with SI score. The cutoff value of the final score was determined by receiver operating characteristic (ROC) curve analysis. The sections that scored more or less than the cutoff value were regarded as high or low expression cases, respectively.
Statistical analysis
Data are presented as mean ± standard deviation and statistical analyses were performed on SPSS 20.0 statistical software (IBM Corporation, Armonk, NY, USA). The Fn level between CRC and adjacent normal tissues was compared by Mann–Whitney test. The cutoff value of the ROC curve was estimated by Youden index. The correlations between biomarkers and clinicopathological parameters were analyzed by chi-square test. The CSS and DFS curves based on Kaplan–Meier model were depicted using GraphPad Prism 5 (GraphPad Software, Inc, La Jolla, CA, USA) and intergroup difference was compared by log-rank test. Independent factors affecting CSS/DFS were identified by univariate and multivariate analysis based on Cox proportional hazards regression model. The impact of Fn level on chemotherapy benefits was evaluated using treatment-by-biomarker interaction analysis in a 2×2 factorial design.Citation23 The correlations of Fn level with expression of EMT/CSC markers in CRC tissues were evaluated by Spearman’s rank correlation coefficient. A p-value <0.05 was considered statistically significant.
Results
Fn level in CRC and adjacent normal tissues of stage III/IV CRC patients
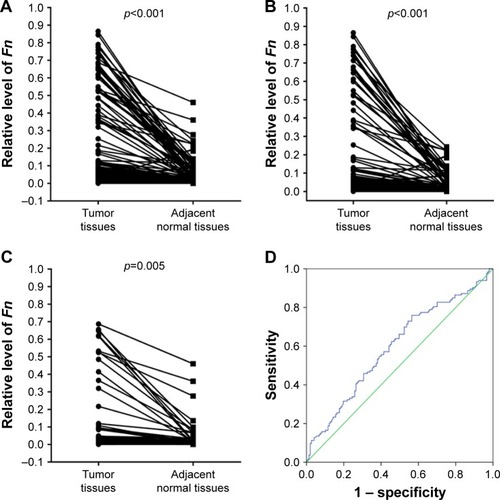
The relative level of Fn in CRC and adjacent normal tissues was detected by qRT-PCR. As shown in , for the whole study cohort, Fn level is significantly higher in CRC tissues than in adjacent normal tissues (CRC vs normal: 0.1092±0.2150 vs 0.0245±0.0553, n=280, p<0.001). In subgroups classified by tumor stage, this difference remains statistically significant in both stage III patients (CRC vs normal: 0.1043±0.2165 vs 0.0216±0.0450, n=218, p<0.001, ) and stage IV patients (CRC vs normal: 0.1266±0.2106 vs 0.0348±0.0817, n=62, p=0.005, ). Then, the ROC curve was used to calculate an optimal cutoff value for defining the Fn level (). The optimal cutoff value of Fn level in CRC tissues was 0.0282. Therefore, we classified the entire cohort into a high level group (n=187) and a low level group (n=93) according to this cutoff value.
Figure 1 Fn level in tumor and adjacent normal tissues of stage III/IV CRC patients.
Abbreviations: Fn, Fusobacterium nucleatum; CRC, colorectal cancer; ROC, receiver operating characteristic.
Correlations between Fn level and clinicopathological parameters in stage III/IV CRC patients
As shown in , Fn level was found to significantly associate with tumor invasion (p=0.015), LNM status (p=0.008), and distant metastasis (p=0.020). No significant association was observed between Fn level and other clinicopathological parameters including age (p=0.822), gender (p=0.705), tumor location (p=0.579), tumor size (p=0.357), tumor differentiation (p=0.650), body mass index (p=0.202), preoperative serum CEA level (p=0.274), and Ki-67 positive rate (p=0.381).
Prognostic significance of Fn in stage III/IV CRC patients
The impact of Fn on patient prognosis was illustrated using Kaplan–Meier survival curves. For the whole cohort, patients with low Fn level had a significantly better CSS and DFS than those with high Fn level (CSS, p<0.001; DFS, p<0.001, ). As shown in and , the univariate analysis suggested that Fn level, tumor invasion, LNM status, distant metastasis, and serum CEA level were significant factors for CSS (p<0.001, p=0.015, p=0.002, p<0.001, p=0.046), while Fn level, tumor differentiation, tumor invasion, LNM status, distant metastasis, and serum CEA level were for DFS (p<0.001, p=0.009, p=0.005, p=0.015, p<0.001, p=0.018). The multivariate analysis suggested that Fn level, LNM status, distant metastasis, and serum CEA level were independent factors affecting CSS (p<0.001, p=0.001, p<0.001, p=0.031), while Fn level, tumor differentiation, tumor invasion, LNM status, distant metastasis, and serum CEA level were affecting DFS (p<0.001, p=0.003, p=0.022, p=0.008, p<0.001, p=0.027). To further identify whether Fn has the capacity to stratify patient prognosis within the same stage, subgroup analysis was performed according to LNM status and distant metastasis. Surprisingly, we found that stage IIIA patients with low Fn level had no better CSS and DFS than those with high Fn level (CSS: p=0.247; DFS: p=0.371, ). But, high Fn level was still significantly associated with worse CSS and DFS in other stage III patients (stage IIIB: CSS: p=0.038, DFS: p=0.029, ; stage IIIC: CSS: p=0.035, DFS: p=0.048, ). With regard to its prognostic role in stage IV patients, a statistically significant association between high Fn level and worse clinical outcome is also obviously found (CSS: p=0.042; DFS: p=0.019, ).
Figure 2 Prognostic significance of Fn in stage III/IV CRC patients.
Abbreviations: Fn, Fusobacterium nucleatum; CRC, colorectal cancer; CSS, cancer-specific survival; DFS, disease-free survival.
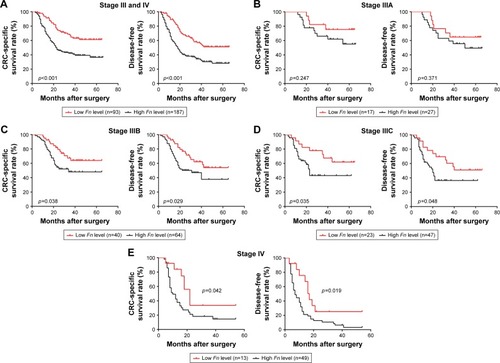
Table 2 Univariate and multivariate analysis for prognostic factors in cancer-specific survival of stage III/IV CRC patients
Table 3 Univariate and multivariate analysis for prognostic factors in disease-free survival of stage III/IV CRC patients
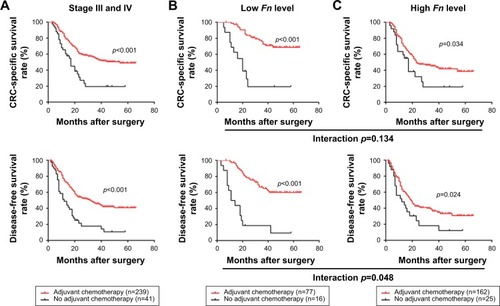
Adjuvant chemotherapy (AC) is the primary therapeutical modality for surgically treated CRC patients, especially for those within stage III/IV. Hence, we next made efforts to identify whether Fn level is associated with AC benefits in stage III/IV patients. In this study, majority of patients (n=239) received standard AC treatment postoperatively, while the rest (n=41) failed due to some factors such as poor physical condition and financial problems. As shown in , the survival analysis demonstrated that patients receiving AC had a dramatically better CSS and DFS than those receiving no AC (CSS: p<0.001; DFS, p<0.001). In the subgroups classified by Fn level, we found that AC treatment was associated with a significantly better clinical outcome in both patients with low Fn level (CSS: p<0.001, DFS: p<0.001, ) and high Fn level (CSS: p=0.034, DFS: p=0.024, ). However, the interaction analysis based on factorial design indicated that patients with low Fn level benefit more from AC than those with high Fn level, in terms of DFS (CSS: p=0.134; DFS: p=0.048).
Figure 3 Correlations between Fn level and chemotherapy benefits in stage III/IV patients.
Abbreviations: Fn, Fusobacterium nucleatum; CRC, colorectal cancer; CSS, cancer-specific survival; DFS, disease-free survival.
Expression and clinical significance of EMT and CSC markers in stage III/IV CRC patients
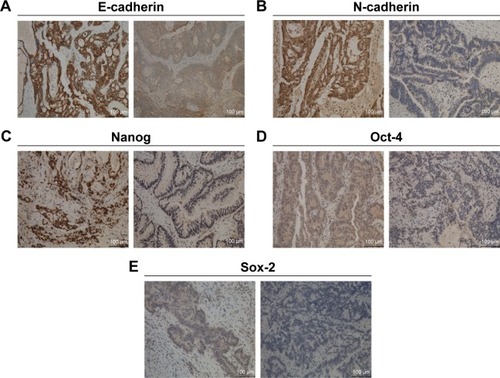
The representative images of IHC assay are shown in . ROC curves were employed to estimate the cutoff values of staining scores for these markers and the results are shown in Figure S1. The cutoff value is 2.5 for E-cadherin and Sox-2, 3.5 for Oct-4, and 5 for N-cadherin and Nanog. Therefore, we used these cutoff values for the following statistical analysis and the correlations between their expression and clinicopathological features are summarized in . We noted that expression of these markers was significantly correlated with prognosis-related clinical features. For instance, both E-cadherin and N-cadherin expression was correlated with LNM status and distant metastasis (all p<0.05). Naong and Sox-2 expression was correlated with LNM status, while Oct-4 expression was correlated with distant metastasis (all p<0.05).
Figure 4 Representative immunohistochemical staining images of EMT and CSC markers in CRC tissues.
Abbreviations: CRC, colorectal cancer; EMT, epithelial-to-mesenchymal transition; CSC, cancer stem cell.
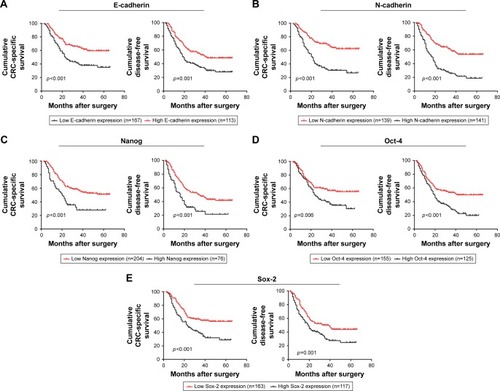
The prognostic significance of EMT and CSC markers was analyzed using Kaplan–Meier survival curves. Patients with high E-cadherin expression had a significantly better CSS and DFS than those with low E-cadherin expression (CSS: p<0.001, DFS: p=0.001, ), while the opposite was true for N-cadherin (CSS: p<0.001, DFS: p<0.001, ), Nanog (CSS: p<0.001, DFS: p<0.001, ), Oct-4 (CSS: p=0.006, DFS: p<0.001, ), and Sox-2 (CSS: p<0.001, DFS: p=0.001, ).
Figure 5 Prognostic significance of epithelial-to-mesenchymal transition and cancer stem cell markers in stage III/IV CRC patients.
Abbreviations: CRC, colorectal cancer; CSS, cancer-specific survival; DFS, disease-free survival.
Correlations of Fn with EMT and CSC markers in stage III/IV CRC patients
The correlations between Fn level and expression of EMT/CSC markers in CRC tissues are summarized in . Fn level was negatively correlated with E-cadherin expression (r=−0.301, p<0.001), but positively correlated with expression of N-cadherin (r=0.377, p<0.001) and Nanog (r=0.362, p<0.001). No significant association was observed between Fn level and Sox-2 expression (r=0.105, p=0.078) or Oct-4 expression (r=0.099, p=0.097).
Table 4 Correlations of Fn with EMT/CSC markers in stage III/IV CRC patients
Discussion
Fn is a gram-negative anaerobe that is enriched in the oral cavity but hardly detected in other body organs under physiological conditions.Citation24 However, under pathological conditions, it disseminates and colonizes into extraoral sites to function as pathogenic bacteria for various diseases such as inflammatory bowel disease, organ abscess, and adverse pregnancy outcome.Citation25–Citation27 In human malignancies, it is perhaps most relevant to CRC, although some emerging evidences have suggested its implication in esophageal and pancreatic cancer.Citation28,Citation29 Using RNA sequencing, Castellarin et al for the first time proposed that Fn infection might be prevalent in CRC patients.Citation30 Then, increasing studies made efforts to investigate its potential oncogenic mechanisms in CRC where its regulatory role in tumor immunity is the most extensively discussed.Citation31–Citation33 In addition, Fn is found to be abundant in premalignant lesions with positive CpG island methylator phenotype, implying its involvement in epigenetic changes of early tumorigenesis.Citation34 However, despite these novel findings about its oncogenic role, its prognostic significance in CRC patients remains unclear and whether it has the potential utility for improving the current TNM-based prognostic system still needs to be validated.
In this study, the level and clinical significance of Fn were analyzed in a cohort of 280 surgically treated stage III/IV patients. Firstly, we found that the Fn level is significantly higher in tumor tissues than that in adjacent normal tissues in both stage III and IV patients, supporting its promoting role in CRC initiation and development. A recent study proposed that this promoting role may be partly attributed to its participation in oncogenic biofilm formation.Citation35 The following correlation analysis demonstrated that Fn level is significantly correlated with tumor invasion, LNM status, and distant metastasis. This further confirmed our previous finding that Fn enhances the malignant characteristics of CRC cells in vitro and in vivo.Citation19 Li et al proved that Fn level is positively associated with the presence of LNM but not with tumor invasion in a relatively smaller cohort of CRC patients (n=101), partly consistent with our present result.Citation36 Furthermore, Castellarin et al found that 74.4% (29/39) of CRC patients with high Fn level had positive LNM as compared with 44.8% (26/58) of those with low Fn level, also indicating a close correlation between Fn and LNM.Citation30 Therefore, given these evidences, we concluded that Fn level might be a promising indicator for CRC metastasis in CRC patients, especially for those with positive LNM.
Although Fn level has been identified as an unfavorable prognostic factor in several studies, its specific prognostic significance for stage III/IV patients remains unknown.Citation37,Citation38 Using the Kaplan–Meier model, our survival analysis showed that stage III/IV patients with high Fn level had a significantly worse CSS and DFS than those with low Fn level. The following univariate and multivariate analysis not only further confirmed a significant correlation between Fn level and patient survival, but also revealed its independence in prognosis prediction. Given these results, we preliminarily proposed that Fn level might serve as a predictor for clinical outcome of stage III/IV patients. Several studies have recently suggested the limitation of traditional LNM status in prognosis stratification of stage III patients, strongly urging us to investigate whether the Fn level has the capacity to provide an accurate stratification for these patients.Citation39,Citation40 We therefore subsequently performed a subgroup analysis and found that Fn level could stratify the CSS and DFS of both stage IIIB and IIIC patients, but failed in stage IIIA patients. This result suggested that Fn level might be an effective prognostic indicator only for stage IIIB or IIIC patients. We also speculate that this result is partly attributed to the survival paradox that stage IIIA patients, clinically characterized as T1–2N1–2aM0, have a significantly better prognosis than other stage III and even most stage II patients, with a 5-year overall survival rate ranging from 81.6% to 85.6% as reported.Citation41,Citation42 This abnormally favorable prognosis may contribute to the failed prognostic stratification of Fn level in stage IIIA patients and we therefore suggest that detecting the Fn level in these patients may provide limited beneficial information for clinical management. Furthermore, we found that high Fn level is associated with worse outcome in stage IV patients despite the limited samples, implying its potential to be a prognostic predictor for surgically treated patients with distant metastasis. Finally, it should be noted that our study was unable to investigate the prognostic value of fecal Fn level in CRC patients, although its diagnostic potential has been highly advocated in several previous studies.Citation43,Citation44 Hence, whether its fecal level has any prognostic value or serves as a dynamic noninvasive marker like CEA in CRC surveillance still requires our extensive clinical validations in future.
Increasing evidences have supported that gut bacteria play a major role in modulating the anticancer efficacy of various CRC-related chemotherapeutic drugs such as 5-Fu, irinotecan, and oxaliplatin.Citation45 To identify the correlation between Fn level and chemotherapy benefits in stage III/IV patients, a subgroup analysis was carried out based on Fn level and we found that patients receiving chemotherapy had a significantly better prognosis than those receiving no chemotherapy in both the high and low Fn level group. However, the following interaction analysis on DFS indicated that patients with low Fn level benefited more from chemotherapy than those with high Fn level, suggesting that Fn might be a predictive biomarker for chemotherapy response in stage III/IV patients. These results also implied its potential involvement in chemotherapy resistance of metastatic CRC cells. Yu et al have recently found that Fn can induce chemotherapy resistance of CRC cells through modulating autophagy via toll-like receptor/microRNAs signaling cascade, strongly supporting our results.Citation46 Furthermore, it is reported that chemotherapy may in turn influence the gut bacteria of cancer patients.Citation47 Therefore, whether the Fn level is changed during chemotherapy treatment and this change has any impact on therapy efficacy or even drug toxicity is also worthy of further investigation.
Finally, we analyzed the expression and clinical significance of EMT and CSC markers in stage III/IV patients, based on the consideration that both the molecular events play a major part in disease progression and therapy resistance of cancer patients.Citation48 Our results showed that these markers are correlated with not only some clinicopathological features, but also CSS and DFS in stage III/IV patients. These findings are consistent with those of previous studies regarding their clinical significance in CRC patients.Citation49–Citation51 More importantly, through correlation analysis, we found that the Fn level was negatively correlated with E-cadherin expression, but positively correlated with N-cadherin expression in CRC tissues. Since loss of E-cadherin and gain of N-cadherin are defined as classical hallmarks of EMT, we speculated that Fn might contribute to CRC development partly by inducing this oncogenic molecular phenotype.Citation52 This speculation is partly supported by a recent study that proved that Fn promotes CRC growth and invasion through regulating E-cadherin/β-catenin signaling.Citation53 Our previous study also found that Fn upregulates miR-21 level to induce colitis-associated cancer by repressing E-cadherin, implying that Fn may induce EMT through upregulating miR-21.Citation19,Citation54 Then, we observed a positive correlation between Fn level and Nanog expression in CRC tissues, indicating that Fn might be involved in CSC phenotype. Nanog, as a well-established CSC marker, is also found to participate in the EMT program in cancer development, suggesting that Fn may partly induce EMT through regulating CSC phenotype.Citation55,Citation56 However, for further clarifying the correlation of Fn with EMT and CSC phenotype, extensive cellular assays are needed. In addition, it is reported that statins enhance the chemosensitivity of CRC cells through impairing CSC phenotype and whether Fn screening may be useful to discriminate between patients who most likely benefit from statins during chemotherapy still requires more clinical validations.Citation57
In summary, our study indicates that Fn level is positively correlated with malignant progression and may serve as an independent prognostic indicator in stage III/IV patients. In addition, our findings also suggest that the Fn level is helpful for predicting chemotherapy benefits in these patients. Finally, we found that Fn level is correlated with several EMT and CSC markers in their tumor tissues, suggesting its potential involvement in EMT-CSC cross talk during CRC development. These findings not only suggest the immense potential of Fn as a clinically actionable biomarker for precise treatment in stage III/IV patients, but also provide a promising adjuvant therapeutic strategy for them that targeting Fn may be helpful for preventing CRC metastasis and improving chemotherapy efficacy.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (no 81230057, 81372615, 81472262, and 81200264); Emerging Cutting-Edge Technology Joint Research projects of Shanghai (SHDC12012106), Tongji University Subject Pilot Program (no 162385), and China Postdoctoral Science Foundation (no 2017M610278). We thank Dr Ruting Xie (Department of Pathology, Shanghai Tenth People’s Hospital, Tongji University School of Medicine) for her crucial assistance in sample collection and IHC assay.
Supplementary materials
Figure S1 The ROC curve analysis is used to determine the cutoff values of staining scores of epithelial–mesenchymal transition and cancer stem cell markers.
Notes: (A) E-cadherin; (B) N-cadherin; (C) Nanog; (D) Oct-4; (E) Sox-2.
Abbreviation: ROC, receiver operating characteristic.
Table S1 Correlations between epithelial-to-mesenchymal transition/cancer stem cell markers and clinicopathological characteristics
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- SiegelRLMillerKDJemalACancer statistics, 2017CA Cancer J Clin201767173028055103
- ChenWZhengRBaadePDCancer statistics in China, 2015CA Cancer J Clin201666211513226808342
- AranVVictorinoAPThulerLCFerreiraCGColorectal cancer: epidemiology, disease mechanisms and interventions to reduce onset and mortalityClin Colorectal Cancer201615319520326964802
- SiegelRLMillerKDFedewaSAColorectal cancer statistics, 2017CA Cancer J Clin201767317719328248415
- De GreefKRolfoCRussoAMultidisciplinary management of patients with liver metastasis from colorectal cancerWorld J Gastroenterol201622327215722527621569
- TroianiTNapolitanoSDella CorteCMTherapeutic value of EGFR inhibition in CRC and NSCLC: 15 years of clinical evidenceESMO Open201615e00008827843640
- ParikhRBPrasadVBlood-based screening for colon cancer: a disruptive innovation or simply a disruption?JAMA2016315232519252027305625
- SepulvedaARHamiltonSRAllegraCJMolecular biomarkers for the evaluation of colorectal cancer: guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical OncologyJ Clin Oncol201735131453148628165299
- TsilimigrasMCFodorAJobinCCarcinogenesis and therapeutics: the microbiota perspectiveNat Microbiol201721700828225000
- GagnaireANadelBRaoultDNeefjesJGorvelJPCollateral damage: insights into bacterial mechanisms that predispose host cells to cancerNat Rev Microbiol201715210912828045107
- DartATumour microenvironment: that gut feelingNat Rev Cancer20161612756757
- O’ConnorAO’MorainCAFordACPopulation screening and treatment of Helicobacter pylori infectionNat Rev Gastroenterol Hepatol201714423024028053340
- WangJJiaHMetagenome-wide association studies: fine-mining the microbiomeNat Rev Microbiol201614850852227396567
- TsoiHChuESZhangXPeptostreptococcus anaerobius induces intracellular cholesterol biosynthesis in colon cells to induce proliferation and causes dysplasia in miceGastroenterology201715261419143328126350
- WangXYangYHuyckeMMCommensal bacteria drive endogenous transformation and tumour stem cell marker expression through a bystander effectGut201564345946824906974
- ZhuQJinZWuWAnalysis of the intestinal lumen microbiota in an animal model of colorectal cancerPLoS One201496e9084924603888
- GaoZGuoBGaoRZhuQQinHMicrobiota disbiosis is associated with colorectal cancerFront Microbiol201562025699023
- YangYWengWPengJFusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear factor-κB, and up-regulating expression of microRNA-21Gastroenterology2017152485186627876571
- PradellaDNaroCSetteCGhignaCEMT and stemness: flexible processes tuned by alternative splicing in development and cancer progressionMol Cancer2017161828137272
- ChenTYouYJiangHWangZZEpithelial–mesenchymal transition (EMT): a biological process in the development, stem cell differentiation and tumorigenesisJ Cell Physiol2017232123261327228079253
- YanXLiuLLiHDual specificity phosphatase 5 is a novel prognostic indicator for patients with advanced colorectal cancerAm J Cancer Res20166102323233327822421
- DalerbaPSahooDPaikSCDX2 as a prognostic biomarker in stage II and stage III colon cancerN Engl J Med2016374321122226789870
- HanYWFusobacterium nucleatum: a commensal-turned pathogenCurr Opin Microbiol20152314114725576662
- TaharaTShibataTKawamuraTFusobacterium detected in colonic biopsy and clinicopathological features of ulcerative colitis in JapanDig Dis Sci201560120521025102986
- AtanasovaKRYilmazOPrelude to oral microbes and chronic diseases: past, present and futureMicrobes Infect201517747348325813714
- StockhamSStamfordJERobertsCTAbnormal pregnancy outcomes in mice using an induced periodontitis model and the haematogenous migration of Fusobacterium nucleatum sub-species to the murine placentaPLoS One2015103e012005025806806
- YamamuraKBabaYNakagawaSHuman microbiome Fusobacterium nucleatum in esophageal cancer tissue is associated with prognosisClin Cancer Res201622225574558127769987
- MitsuhashiKNoshoKSukawaYAssociation of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosisOncotarget2015697209722025797243
- CastellarinMWarrenRLFreemanJDFusobacterium nucleatum infection is prevalent in human colorectal carcinomaGenome Res201222229930622009989
- SaitoTNishikawaHWadaHTwo FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancersNat Med201622667968427111280
- GurCIbrahimYIsaacsonBBinding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attackImmunity201542234435525680274
- MimaKSukawaYNishiharaRFusobacterium nucleatum and T cells in colorectal carcinomaJAMA Oncol20151565366126181352
- ItoMKannoSNoshoKAssociation of Fusobacterium nucleatum with clinical and molecular features in colorectal serrated pathwayInt J Cancer201513761258126825703934
- LiSKonstantinovSRSmitsRPeppelenboschMPBacterial biofilms in colorectal cancer initiation and progressionTrends Mol Med2017231183027986421
- LiYYGeQXCaoJAssociation of Fusobacterium nucleatum infection with colorectal cancer in Chinese patientsWorld J Gastroenterol201622113227323327004000
- MimaKNishiharaRQianZRFusobacterium nucleatum in colorectal carcinoma tissue and patient prognosisGut201665121973198026311717
- FlanaganLSchmidJEbertMFusobacterium nucleatum associates with stages of colorectal neoplasia development, colorectal cancer and disease outcomeEur J Clin Microbiol Infect Dis20143381381139024599709
- HuangBChenCNiMMoSCaiGCaiSLog odds of positive lymph nodes is a superior prognostic indicator in stage III rectal cancer patients: a retrospective analysis of 17,632 patients in the SEER databaseInt J Surg201632243027321382
- LiQLiangLJiaHNegative to positive lymph node ratio is a superior predictor than traditional lymph node status in stage III colorectal cancerOncotarget2016744722907229927474167
- ChuQDZhouMMedeirosKPeddiPPositive surgical margins contribute to the survival paradox between patients with stage IIB/C (T4N0) and stage IIIA (T1-2N1, T1N2a) colon cancerSurgery201616051333134327425043
- ChuQDZhouMMedeirosKLPeddiPKavanaughMWuXCPoor survival in stage IIB/C (T4N0) compared to stage IIIA (T1-2 N1, T1N2a) colon cancer persists even after adjusting for adequate lymph nodes retrieved and receipt of adjuvant chemotherapyBMC Cancer20161646027412163
- WongSHKwongTNChowTCQuantitation of faecal Fuso-bacterium improves faecal immunochemical test in detecting advanced colorectal neoplasiaGut20176681441144827797940
- LiangQChiuJChenYFecal bacteria act as novel biomarkers for noninvasive diagnosis of colorectal cancerClin Cancer Res20172382061207027697996
- AlexanderJLWilsonIDTeareJMarchesiJRNicholsonJKKinrossJMGut microbiota modulation of chemotherapy efficacy and toxicityNat Rev Gastroenterol Hepatol201714635636528270698
- YuTGuoFYuYFusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagyCell2017170354856328753429
- RajagopalaSVYoosephSHarkinsDMGastrointestinal microbial populations can distinguish pediatric and adolescent acute lymphoblastic leukemia (ALL) at the time of disease diagnosisBMC Genomics201617163527527070
- IshiwataTCancer stem cells and epithelial–mesenchymal transition: novel therapeutic targets for cancerPathol Int2016661160160827510923
- YanXYanLLiuSShanZTianYJinZN-cadherin, a novel prognostic biomarker, drives malignant progression of colorectal cancerMol Med Rep20151222999300625936636
- MengHMZhengPWangXYOver-expression of Nanog predicts tumor progression and poor prognosis in colorectal cancerCancer Biol Ther20109429530220026903
- ZhouHHuYUWangWExpression of Oct-4 is significantly associated with the development and prognosis of colorectal cancerOncol Lett201510269169626622555
- Serrano-GomezSJMaziveyiMAlahariSKRegulation of epithelial–mesenchymal transition through epigenetic and post-translational modificationsMol Cancer2016151826905733
- RubinsteinMRWangXLiuWHaoYCaiGHanYWFusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesinCell Host Microbe201314219520623954158
- ShiCYangYXiaYNovel evidence for an oncogenic role of microRNA-21 in colitis-associated colorectal cancerGut20166591470148125994220
- PanQMengLYeJTranscriptional repression of miR-200 family members by Nanog in colon cancer cells induces epithelial–mesenchymal transition (EMT)Cancer Lett2017392263828163188
- MigitaTUedaAOhishiTEpithelial–mesenchymal transition promotes SOX2 and NANOG expression in bladder cancerLab Invest Epub2017227
- KodachLLJacobsRJVoorneveldPWStatins augment the chemosensitivity of colorectal cancer cells inducing epigenetic reprogramming and reducing colorectal cancer cell ‘stemness’ via the bone morphogenetic protein pathwayGut201160111544155321551187
