Abstract
Intrahepatic cholangiocarcinoma (ICC) arises from the biliary epithelium and is a relatively rare and highly fatal neoplasm. The prognosis is poor, and survival is limited to a few months. Here, we report a case of advanced ICC that was successfully treated with apatinib, a new oral tyrosine kinase inhibitor that targets the intracellular domain of vascular endothelial growth factor receptor-2. To the best of our knowledge, this is the first case report of the successful use of apatinib for advanced ICC; this treatment has demonstrated fewer toxic effects than traditional cytotoxic chemotherapy. The progression-free survival time was 8 months. The only toxicity observed was mild hand–foot syndrome. Therefore, apatinib may be an additional option for the treatment of advanced ICC, but further prospective studies are needed to optimize the treatment.
Introduction
Intrahepatic cholangiocarcinoma (ICC) arises from the biliary epithelium and is a relatively rare and highly fatal neoplasm. Patients who experience ICC usually present at a late stage of the disease, and the outcomes are poor; as few as 5%–10% of ICC patients with unresectable disease are still alive after 5 years.Citation1 Surgery may be the only potentially curative treatment, but for advanced and metastatic ICC, systemic chemotherapy and radiotherapy remain poorly defined and have only a modest therapeutic effect.Citation2,Citation3 Therefore, patients with ICC require newer, safer, and more effective treatments.
Apatinib (Hengrui Pharmaceutical Co. Ltd, Shanghai, People’s Republic of China), which is a small-molecule tyrosine kinase inhibitor that is taken orally, selectively inhibits vascular endothelial growth factor receptor-2 (VEGFR-2). This is currently a treatment for patients with advanced gastric cancer. Apatinib plays antiangiogenic and antitumor roles through the inhibition of the VEGFR-2 signaling pathway, which prevents tumor growth. The effects of apatinib on advanced gastric cancerCitation4,Citation5 and hepatocellular carcinoma (HCC)Citation6 have been demonstrated by several clinical trials. This drug also shows a wide potential efficacy in a variety of solid tumors including metastatic lung, colon, and breast cancers.Citation7,Citation8 Moreover, apatinib is currently used to treat breast and lung cancer patients.Citation9 Here, we report a unique case of ICC that was treated with apatinib at our hospital. To the best of our knowledge, this is the first case report of a patient with ICC who responded to apatinib.
Case report
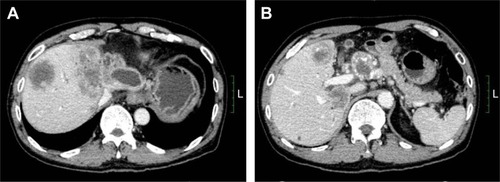
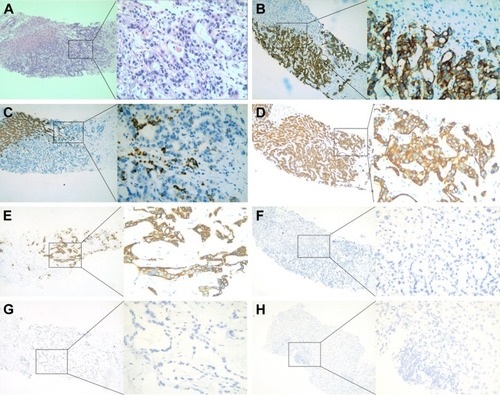
The patient was a 59-year-old male with a 2-month history of upper abdominal pain. He had a history of chronic hepatitis B for >30 years, but no family history of cancer. Physical examination showed no positive signs. The Eastern Cooperative Oncology Group (ECOG) performance status was 0. The concentration of CA19–9 (carbohydrate antigen 19–9) was 60.2 U/mL (0–37 U/mL). He did not appear jaundiced, and his initial liver function was scored as Child-Pugh A. The patient underwent abdominal computed tomography (CT) on January 27, 2015, which demonstrated several irregular and low-density masses in the liver as well as abdominal para-aortic lymph node metastasis, with slight delay enhancement. These masses were located in the left and right lobes of the liver and had a maximum volume of 8.1×7.8 cm, while the volume of the abdominal para-aortic lymph node was 5.4×4.1 cm (). He underwent a liver puncture, and the mass was histologically confirmed as ICC (). The immunohistochemical analysis showed that the neoplastic cells were positive for CK 18, CK 19, CK 7, and Cox-2 and negative for CDX 2, CK 20, Glypican 3, and hepatocyte markers ().
Figure 1 Abdominal CT images show that one of the lesions was located in the left lobe of the liver and the other was located in the para-aortic lymph node (A and B). In the venous phase, the mass was of low density and was irregular.
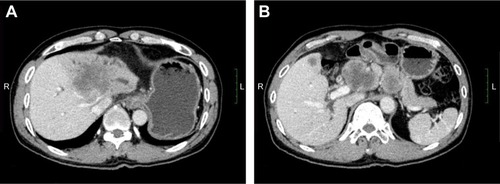
Figure 2 Pathologic evidence of intrahepatic cholangiocarcinoma (A ×100, ×400) showing adenocarcinoma infiltration of normal liver tissue. Immunohistochemical staining showed that CK 18, CK 19, CK 7, and Cox-2 were positive, while CDX 2, CK 20, Glypican 3, and Hep-1 were negative, which support adenocarcinoma from the intrahepatic bile duct (B, CK 19; C, Hep-1; D, CK 18; E, CK 7; F, Cox-2; G, CK 20; H, Glypican 3; ×100, ×400).
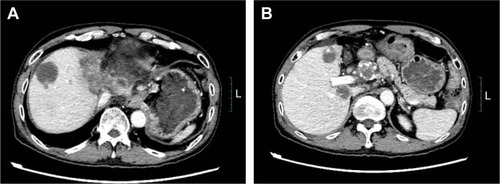
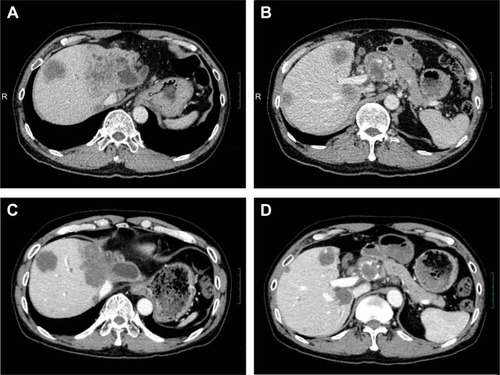
This patient did not undergo surgery or radiotherapy because of extensive lesions and metastasis to the para-aortic lymph node. There was no remote organ metastasis. The patient was initially treated with a traditional chemotherapeutic regimen that consisted of six cycles of gemcitabine/albumin–bound paclitaxel (gemcitabine, 1,000 mg/m2, days 1 and 8, albumin-bound paclitaxel 125 mg/m2, days 1, 8, and 15). A CT scan of the upper abdomen was done after every two cycles of treatment and showed that the regimen was effective. After six cycles of treatment, the mass measured 4.8×6.2 cm (left lobe of the liver, decreased 23.5%), while the abdominal para-aortic lymph node measured 2.7×2.8 cm (decreased 48.1%) on CT (August 5, 2015), which indicated a 33.33% decrease compared with the baseline measurement. This also met the RECIST (Response Evaluation Criteria in Solid Tumors) criteria for a partial response (PR) (). During and after the treatment, thrombocytopenia was observed, and no improvement was seen after repeated treatment of the symptoms. Cytotoxic chemotherapy was not given at that time due to the severe thrombocytopenia and was thereafter discontinued. However, the upper abdominal CT scan (January 9, 2016) indicated clear evidence of disease progression, with more intrahepatic metastases and larger metastasis in the abdominal para-aortic lymph node. Since progressive disease (PD) was apparent, the therapy regimen was changed to Keytruda® (Merck Sharp & Dohme Corp, Kenilworth, NJ, USA), a programmed death-1 (PD-1) inhibitor. During 4 months of the treatment, an upper abdominal CT scan (June 3, 2016) revealed that the disease continued to progress ().
Figure 3 Confirmation of tumor shrinkage. A CT scan on August 5, 2015, revealed the tumor to be smaller after chemotherapy. (A), lesion located in the left lobe of the liver, (B) lesion located in the para-aortic lymph node.
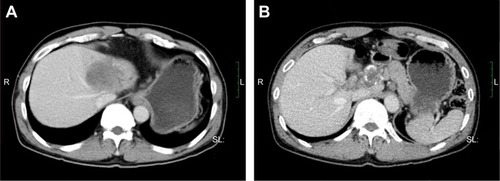
Figure 4 Tumor size before and after 1 month of treatment with apatinib.
Abbreviations: CT, computed tomography; PD-1, programmed death-1.
After written informed consent was provided by the patient to have the case details and any accompanying images published, and the study was approved by The Ethics Committee of the First Affiliated Hospital of Guangxi Medical University, the regimen was then changed to apatinib, at a dose of 500 mg/day (June 4, 2016). The liver function had been scored as Child-Pugh A before the treatment with apatinib. Following 1 month of therapy, a CT scan suggested stable disease (SD) on July 16, 2016 (). After that, the patient stopped using apatinib, and a CT scan after 2 months suggested PD (). The patient then began treatment with apatinib once again. At that time, an upper abdominal CT scan (May 11, 2017) obtained after 10 months of apatinib treatment suggested SD (), and the progression-free survival was 8 months. The ECOG performance status was 1 and the CA19–9 level was 181 U/mL (0–37 U/mL). The only toxicity observed was mild hand–foot syndrome. The entire timeline of treatment is presented in .
Discussion
ICC is a relatively rare disease, but its incidence continues to increase worldwide.Citation1,Citation10 For patients with local and early ICC, surgery is a potentially curative therapy. It is often asymptomatic until later stages of the disease, and patients are frequently diagnosed at an advanced and unresectable stage. However, in patients with advanced ICC, treatment options are complicated and limited due to recurrence, metastasis, and poor patient outcome, as the median reported survival is 3–6 months.Citation11 No standard treatment guidelines exist for advanced ICC, and the role of adjuvant chemotherapy is limited and controversial. Regimens are primarily based on 5-fluorouracil or gemcitabine as first-line treatments, which have slightly prolonged survival times.Citation12,Citation13 However, the use of 5-fluorouracil or gemcitabine has not improved the 1-year median survival times.
Considering that the prevalence of ICC is usually in late adulthood and that the toxicities of combined chemotherapeutic regimens often lead to treatment cessation, ICC requires novel, effective, and safer treatment options, especially for older patients.Citation1 An understanding of the molecular mechanisms of malignancies will help in the development of novel targeted therapies for this disease. Targeted VEGFR-2 therapies represent a potential treatment of choice. Apatinib promotes apoptosis in ICC via the inhibition of vascular endothelial growth factor (VEGF) signaling in vitro and delayed xenograft tumor growth in vivo.Citation14
VEGF mediates angiogenesis, which plays an essential role in the process of malignant tumor growth.Citation15 The proliferation of the vascular endothelium is activated by the downstream signals that are generated when VEGF binds to vascular epidermal growth factor receptor (VEGFR). Proteins of the VEGFR family are membrane receptor tyrosine kinases that include VEGFR-1, VEGFR-2, and VEGFR-3.Citation16 VEGFR-2 is the major mediator of the mitogenic, angiogenic, and permeability-enhancing effects of VEGF.Citation17 The inhibition of VEGFR-2 might be a promising strategy to inhibit tumor-induced angiogenesis.
Apatinib is a novel oral small-molecule tyrosine kinase inhibitor that targets VEGFR-2 that can suppress tumor angiogenesis. Several studies have confirmed the efficacy of the inhibition of VEGFR-2, which is a promising therapeutic option for a variety of tumor types since this treatment inhibits angiogenesis.Citation14,Citation18,Citation19 In several Phase II clinical trials, it has been demonstrated to be effective in diverse tumor types.Citation20–Citation22 In a Phase II clinical trial, apatinib, which showed a survival benefit in patients with gastric cancer, also demonstrated a manageable toxicity profile.Citation20 However, the successful use of apatinib in ICC has not yet been reported.
In the Phase I study of apatinib, the maximum-tolerated dose was 850 mg once daily, and the recommended dose was 750 mg. The most frequent adverse events of apatinib were hypertension (70%), proteinuria (50%), and hand–foot syndrome (46%).Citation19
In this case report, 6 months after the end of chemotherapy, CT indicated disease progression. Due to older age and thrombocytopenia, the patient could not tolerate toxic chemotherapy, and apatinib was then used to control the disease. Considering his older age and thrombocytopenia, a dose of 500 mg was given. The disease was controlled successfully with an SD and a progression-free survival time of 8 months. In addition, the administration of apatinib resulted in no obvious side effects.
Gene expression analysis revealed that VEGFR-2 is overexpressed in the tissues of patients with ICC.Citation23 Apatinib inhibits VEGF signaling and promotes apoptosis in ICC,Citation17 which suggests that apatinib leads to a clinical response via the inhibition of VEGFR-2 tyrosine kinase activity.
Conclusion
Apatinib may serve as an additional option for the treatment of ICC, especially for older patients and those with poor performance status. In this case report, apatinib exhibited good efficacy and safety in the treatment of the patient. Further large-scale prospective studies are required to optimize the use of apatinib in the treatment of ICC and to identify which patients will benefit from the agent.
Acknowledgments
We are grateful to the patient for participating in this study. We also thank Wen-guang Bao, Zhi-gang Peng, and all of the clinicians for their contributions to the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- ShaibYEl-SeragHBThe epidemiology of cholangiocarcinomaSemin Liver Dis200424211512515192785
- FargesOFuksDBoleslawskiEInfluence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study groupAnn Surg2011254582483022042474
- MechteldCde JongNathanHariIntrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessmentJ Clin Oncol201129233140314521730269
- LiJinQinShukuiXuJianmingApatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trialJ Clin Oncol201331263219322523918952
- LiJQinSXuJRandomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junctionJ Clin Oncol201634131448145426884585
- QinSApatinib in Chinese patients with advanced hepatocellular carcinoma: a phase II randomized, open-label trialJ Clin Oncol201432Suppl 5 abstract 4019
- HuXZhangJXuBMulticenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancerInt J Cancer201413581961196924604288
- ScottAJMessersmithWAJimenoAApatinib: a promising oral antiangiogenic agent in the treatment of multiple solid tumorsDrugs Today (Barc)201551422322926020064
- FontanellaCOngaroEBolzonelloSClinical advances in the development of novel VEGFR2 inhibitorsAnn Transl Med201421212325568876
- DohertyBNambudiriVEPalmerWCUpdate on the diagnosis and treatment of cholangiocarcinomaCurr Gastroenterol Rep2017191228110453
- CunninghamSCChotiMABellavanceECPawlikTMPalliation of hepatic tumorsSurg Oncol200716427729117935975
- LimKHHanSWOhDYImSAKimTYBangYJOutcome of infusional 5-fluorouracil, doxorubicin, and mitomycin-C (iFAM) chemotherapy and analysis of prognostic factors in patients with refractory advanced biliary tract cancerOncology2012832576622760079
- ValleJWasanHPalmerDHCisplatin plus gemcitabine versus gemcitabine for biliary tract cancerN Engl J Med2010362141273128120375404
- PengHZhangQLiJApatinib inhibits VEGF signaling and promotes apoptosis in intrahepatic cholangiocarcinomaOncotarget2016713172201722926967384
- FerraraNVascular endothelial growth factor as a target for anticancer therapyOncologist20049Suppl 121015178810
- FerraraNGerberHPLeCouterJThe biology of VEGF and its receptorsNat Med20039666967612778165
- Glade-BenderJKandelJJYamashiroDJVEGF blocking therapy in the treatment of cancerExpert Opin Biol Ther20033226327612662141
- ScottAJMessersmithWAJimenoAApatinib: a promising oral antiangiogenic agent in the treatment of multiple solid tumorsDrugs Today (Barc)201551422322926020064
- LiJZhaoXChenLSafety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignanciesBMC Cancer20101052920923544
- LiJQinSXuJApatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trialJ Clin Oncol201331263219322523918952
- HuXCaoJHuWMulticenter phase II study of apatinib in non-triple-negative metastatic breast cancerBMC Cancer20141482025376790
- LangerCJMokTPostmusPETargeted agents in the third/fourth-line treatment of patients with advanced (stage III/IV) non-small cell lung cancer (NSCLC)Cancer Treat Rev201339325226022703830
- SangHLiTLiHLiuJGab1 regulates proliferation and migration through the PI3K/Akt signaling pathway in intrahepatic cholangiocarcinomaTumor Biol2015361183678377