Abstract
Programmed death-ligand 1 (PD-L1) is an immune checkpoint that is often activated in cancer and plays a pivotal role in the initiation and progression of cancer. However, the clinicopathologic significance and prognostic value of PD-L1 in pancreatic cancer (PC) remains controversial. In this study, we conducted a meta-analysis to retrospectively evaluate the relationship between PD-L1 and PC. PubMed and other databases were searched for the clinical studies published up to March 21, 2017, to be included in the meta-analysis. Hazard ratios and their 95% CIs were calculated. Risk ratios (RRs) were extracted to assess the correlations between the clinicopathologic parameters and PD-L1 expression. Ten studies including 1,058 patients were included in the meta-analysis. The pooled results indicated that positive PD-L1 expression was correlated with a poor overall survival outcome in PC patients (hazard ratio =1.76, 95% CI: 1.43–2.17, P<0.00001). Interestingly, high PD-L1 expression was correlated with poor pathologic differentiation (RR =1.57, 95% CI: 1.25–1.98, P=0.0001) and neural invasion (RR =1.30, 95% CI: 1.03–1.64, P=0.03). However, there were no significant correlations between PD-L1 expression and other clinicopathologic characteristics. In summary, our meta-analysis implied that PD-L1 could serve as a negative predictor for the overall survival of PC patients, and high expression of PD-L1 was correlated with poor differentiation and neural invasion, indicating that anti-PD-L1 treatments should be evaluated in PC patients, especially in those who exhibit these two characteristics.
Introduction
Pancreatic cancer (PC) is a lethal malignancy with an overall 5-year relative survival rate of 5%.Citation1 In 2015, an estimated 90,100 new patients were diagnosed with PC in China, with an estimated 79,400 deaths occurring as a result of delayed diagnosis and treatment resistance.Citation2 At the time of clinical diagnosis, most cancers are either locally advanced or metastatic, and surgical resection is very difficult.Citation3 In addition, PC is resistant to radiotherapy and chemotherapy.Citation4 One of the reasons that the PC responds poorly to treatment is due to its ability to evade host immune surveillance.Citation5 Emerging evidence has shown that the coinhibitory receptors, such as programmed death 1 (PD-1), play a critical role in cancer immunoediting.Citation6
Programmed death-ligand 1 (PD-L1 or B7-H1), the major ligand for PD-1, plays a crucial role in PD-1-dependent immune suppression, which is mediated by an antigen-specific T-cell response.Citation7,Citation8 PD-L1 is expressed on various tumor cells, including PC cells, and immune cells, including activated CD4+ and CD8+ T cells, dendritic cells, macrophages, and regulatory T cells.Citation9–Citation12 PD-L1 is not highly expressed in normal tissues; however, PD-L1 is upregulated in many tumors to attenuate the antitumor immune response via downregulating antitumor T-cell activity or suppressing apoptosis.Citation13
Accumulating studies related to tumoral PD-L1 have been performed on malignancies, such as esophageal, liver, colorectal, breast, lung, glioblastoma, and blood cancers.Citation14–Citation18 However, the prognostic role of PD-L1 is still debated. Overexpression of PD-L1 indicates a poor outcome in gastric cancer and non-small-cell lung cancer;Citation19,Citation20 in contrast, a better prognosis was observed in glioblastoma patients.Citation18 The association between aberrant PD-L1 expression and PC survival has also been evaluated. Due to the relatively small sample sizes, it is necessary to evaluate the association between PD-L1 and the prognosis of PC patients using a meta-analysis of a large cohort of up-to-date reports. In this study, we aimed to conduct a meta-analysis to reveal the association between PC and the clinicopathologic significance and prognostic value of PD-L1.
Materials and methods
Literature search strategy
A literature search was performed up to March 21, 2017, for published articles using the electronic databases PubMed, Web of Science, Scopus, and the Cochrane Library. We also searched the Chinese databases of Wanfang Data, China National Knowledge Infrastructure (CNKI), and SinoMed. Searches were limited to human studies without language restriction. The following terms and their combinations were searched in the title/abstract field: “programmed cell death-ligand 1”, “PD-L1”, “CD274”, “B7-H1”, “pancreatic cancer”, and “pancreatic adenocarcinoma”. We also carried out manual searches of references cited in the retrieved articles and preceding reviews. In addition, we searched meeting abstracts and virtual presentations of the American Society of Clinical Oncology annual meetings and the European Society of Medical Oncology congresses from 2010 to 2017.
Inclusion and exclusion criteria
Qualified studies meeting the following eligibility criteria were included: 1) the histologic target was PC; 2) the association between PD-L1 expression, prognosis, and clinicopathologic features was investigated; 3) the expression of PD-L1 was categorized into high (positive) and low (negative) groups; and 4) relevant information could be acquired from the full-text study. Exclusion criteria included 1) duplicates, ongoing studies, letters, and reviews; 2) studies about PC cell lines, animal experiments, and other types of cancer; 3) studies not about PD-L1; and 4) incomplete data.
Data extraction and outcomes interest
Data from the included studies were acquired and summarized independently by two reviewers (Yongxun Zhuan-Sun and Fengting Huang). Disagreement was resolved by a discussion among the authors. The following information was extracted from the included studies: first author’s name, year of publication, sample size, and survival time.
The primary outcome measure was the relationship between PD-L1 expression and overall survival. The secondary outcomes were the associations between PD-L1 expression and clinicopathologic characteristics.
Quality assessment and statistical analysis
The quality of trials was evaluated by the Newcastle–Ottawa scale,Citation21 which consists of three factors: patient selection, comparability, and assessment of outcome. A score of 0–9 (allocated as stars) was allocated to each study; those that achieved six or more stars were considered to be high quality.
All the meta-analyses were performed by Review Manager 5.3 (Cochrane Collaboration, Oxford, UK). We addressed time-to-event outcomes by pooling hazard ratios (HRs) from Cox proportional hazards models. If the article supplied the HR with a 95% CI, we used the data. If the study did not supply the HR with a 95% CI, we calculated the HR and 95% CI according to Kaplan–Meier survival curves using Engauge Digitizer version 4.1 (Engauge Digitizer Software). The data were used following the method proposed by Tierney et al.Citation22 The generic inverse-variance method was performed to summarize the data. Pooled dichotomous data from other secondary outcomes were presented as risk ratios (RRs). Statistical heterogeneity between studies was assessed using chi-square tests with P<0.10 as significance, and heterogeneity was quantified using the I2 statistic. The random-effects model was used if there was heterogeneity between studies; otherwise, the fixed-effects model was used.
Results
Characteristics of included studies
A total of 10 studies including 1,058 PC patients were used in the final analysis. The initially included studies comprised seven from Asia and three from Europe. All of these studies were cohort studies. Eight articles were published in English and two were in Chinese. PD-L1 expression was examined by immunohistochemistry in eight studies, while the other two studies used quantitative reverse transcription polymerase chain reaction. Based on multivariate analysis, four studiesCitation23–Citation26 provided HRs with 95% CIs, while another five studiesCitation27–Citation31 provided survival curves with which to calculate HRs with 95% CIs. However, the remaining one studyCitation32 provided neither survival curves nor HRs with 95% CIs and only provided the correlation between clinicopathologic characteristics and PD-L1 expression. Therefore, it was excluded from the overall survival analysis. The characteristics of the included studies are shown in .
Table 1 Characteristics of included studies
Effect of PD-L1 expression on the overall survival of PC patients
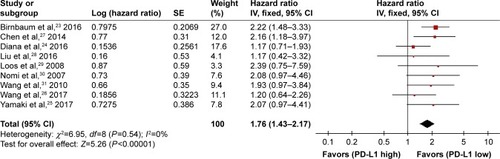
Nine studies were included. A fixed-effects model was applied in the meta-analysis for the HR, since the heterogeneity analysis showed that there was no significant heterogeneity among the studies (I2=0%, P=0.54). A significant difference was indicated by the pooled HR of 1.76 (95% CI: 1.43–2.17, P<0.00001) between high and low PD-L1 expression groups ().
Figure 1 Forest plot and meta-analysis of the overall survival of the PD-L1 high expression group compared with the low expression group.
Abbreviations: df, degrees of freedom; HR, hazard ratio; IV, generic inverse variance method; PC, pancreatic cancer; PD-L1, programmed death-ligand 1; SE, standard error.
Correlation between PD-L1 expression and clinicopathologic characteristics
The clinicopathologic characteristics analyzed included tumor status, pathologic stage (TNM stage), metastatic status, differentiation, lymph node metastasis, vascular invasion, and neural invasion. Interestingly, high-level PD-L1 expression was correlated with poor differentiation (RR: 1.57, 95% CI: 1.25–1.98, P=0.0001) and neural invasion (RR: 1.30, 95% CI: 1.03–1.64, P=0.03), whereas other clinicopathologic characteristics were not significantly correlated with PD-L1 expression. The combined RRs for tumor status, pathologic (TNM) stage, metastatic status, lymph node metastasis, and vascular invasion were 1.04 (RR for higher tumor status, 95% CI: 0.85–1.27, P=0.74), 1.22 (RR for higher pathologic stage, 95% CI: 0.20–7.64, P=0.83), 1.40 (RR for M1, 95% CI: 0.88–2.23, P=0.16), 1.09 (RR for lymph node metastasis, 95% CI: 0.97–1.23, P=0.14), and 0.96 (RR for vascular invasion, 95% CI: 0.73–1.26, P=0.76), respectively ().
Table 2 Meta-analysis: correlation between PD-L1 overexpression and clinicopathologic characteristics
Subgroup analysis
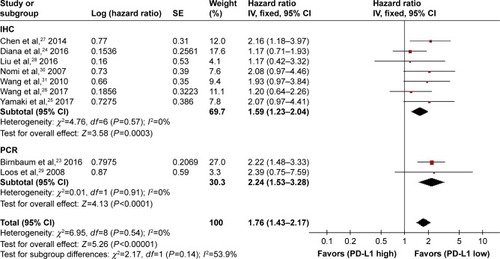
Subsequently, we carried out a subgroup analysis according to the methods detecting the expression of PD-L1. As shown in , a statistical significance of the pooled HR was observed in both the subgroup analyzed by polymerase chain reaction (HR: 2.24, 95% CI: 1.53–3.28, P<0.0001) and the subgroup analyzed by immunohistochemistry (HR: 1.59, 95% CI: 1.23–2.04, P=0.0003) between high and low PD-L1 expression groups. The heterogeneity analysis detected no significant heterogeneity (I2=0%, P=0.54).
Figure 2 Subgroup analysis based on the methods used to detect PD-L1 expression.
Abbreviations: df, degrees of freedom; HR, hazard ratio; IHC, immunohistochemistry; IV, generic inverse variance method; PCR, polymerase chain reaction; PD-L1, programmed death-ligand 1; SE, standard error.
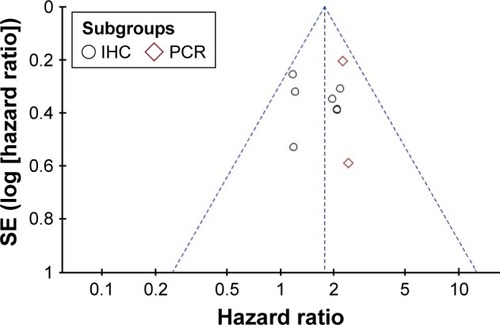
Publication bias
shows a funnel plot of the studies included in this meta-analysis, which illustrates the overall survival. There was no obvious publication bias.
Discussion
To date, the association between PD-L1 and PC patients remains inconclusive.Citation24,Citation30,Citation33 Using meta-analysis, we examined data from a total of 1,058 patients from 10 independent studies.Citation23–Citation32 We assessed the clinicopathologic significance and prognostic value of PD-L1 in PC patients. Intriguingly, the pooled results of our analysis showed that positive PD-L1 expression was highly correlated with a poorer overall survival in PC patients. Moreover, high-level PD-L1 expression was correlated with poor differentiation and neural invasion, which is in accordance with a study on lung adenocarcinoma.Citation34 However, the analysis found no significant correlations between PD-L1 expression and other clinicopathologic characteristics, including tumor status, pathologic (TNM) stage, metastatic status, lymph node metastasis, and vascular invasion.
Two previous meta-analyses evaluated the relationship of PD-L1 expression and survival in solid tumors and digestive system cancers, both of which included PC patients with a limited number of studies and participants.Citation35,Citation36 Our result was comparable to that of Dai et al who analyzed the survival in PC patients.Citation36 In addition, our data provided more reliable evidence with more participants and a lower publication bias. Moreover, we analyzed the correlations between PD-L1 expression and other clinicopathologic characteristics, which were not included in the two aforementioned meta-analyses.
Smoking contributes to tumor development and progression in various types of cancer, including lung cancer, oral squamous cell carcinoma, and PC.Citation37–Citation39 Smoking cessation is one of the most effective strategies to reduce the risk of tumorigenicity. Interestingly, PD-L1 positivity is associated with smoking history in lung cancer and oral squamous cell carcinoma.Citation34,Citation40 High PD-L1 expression was a poor prognostic marker in the patients who were smokers, whereas this was not the case in those who never had a smoking habit among lung adenocarcinoma and oral squamous cell carcinoma patients.Citation40,Citation41 In addition, it was demonstrated that B7-H3, another member of the B7 family that is also known as an immune modulator, was associated with higher mortality in moderate/heavy smoking patients, but not in nonsmoking/light smoking patients in a study on lung adenocarcinoma.Citation42 It seemed that the impact of PD-L1 positivity on the survival in PC patients should be evaluated by stratification of smoking status. However, there were no data about the impact of PD-L1 positivity on the survival in PC patients stratified by smoking status in the studies included in this meta-analysis. Further studies are recommended to better interpret the impact of smoking on PD-L1 expression.
The molecular mechanism of overexpressed PD-L1 in PC remains obscure. Upregulation of PD-L1 could be stimulated by cytokines produced by infiltrating immune cells, such as interferon-γ (IFN-γ), interleukin (IL)-4, IL-10, vascular endothelial growth factor, and growth cell stem factor, in some solid tumors.Citation43–Citation46 In addition, PD-L1 overexpression was observed in acute myeloid leukemia through IFN-γ or toll-like receptor stimulation in blast cells from patients via the mitogen-activated protein kinase kinase/extracellular signal-related kinase pathway and the myeloid primary differentiation response 88/tumor necrosis factor receptor-associated factor 6 pathway.Citation47 Moreover, constitutive oncogene pathway activation could also promote PD-L1 expression, for instance, in non-small-cell lung cancer, and specifically, the anaplastic lymphoma kinase/signal transducer and activator of transcription 3, phosphoinositide 3-kinase, and mitogen-activated protein kinase kinase/extracellular signal-related kinase/signal transducer and activator of transcription 1 pathways can activate PD-L1 expression.Citation48,Citation49 It was reported that the expression of PD-L1 in PC cells was induced via CD8+ T-cell IFN-γ-secretion.Citation50,Citation51 Therefore, PD-L1 expression might be modulated via different pathways among different tumor types.Citation43,Citation44,Citation49
Due to conventional treatment resistance in PC, new immunotherapeutic strategies are urgently needed.Citation52,Citation53 Immunotherapy with immune checkpoint inhibition has shown promise as a therapeutic approach.Citation54 PD-L1, a critical immune checkpoint, is the primary PD-1 ligand, and it can decrease cytokine secretion and attenuate the biologic function of PD-1+ cells and tumor-infiltrating CD4+ and CD8+ T-cells.Citation55 These properties of PD-L1 may allow it to serve as a potentially promising target for cancer immunotherapy.Citation54 PD-L1 monoclonal antibodies exhibited an in vivo antitumor effect on murine PC by increasing CD8+ T-cell infiltration and triggering local immune function.Citation56 Furthermore, an effective outcome on murine PC was observed with the combined treatment of an anti-PD-L1 monoclonal antibody and gemcitabine. In addition, several clinical trials using an anti-PD-L1 monoclonal antibody, such as durvalumab and BMS-936559, were carried out and exhibited an appealing effect.Citation57
High-level PD-L1 expression was correlated with a poorer overall survival in PC patients in our meta-analysis, which suggested that an anti-PD-L1 monoclonal antibody may be a new, promising treatment strategy for PC patients. Additional clinical trials are needed to evaluate the efficacy and safety of anti-PD-L1 monoclonal antibodies in patients with PC. The positive correlations between high-level PD-L1 expression and poor differentiation and PC neural invasion may provide an additional indication for the application of anti-PD-L1 treatment strategies for PC patients with poorly differentiated PC and neural invasion.
Despite the promising result from this meta-analysis, there are several limitations that need to be further addressed. First, the number of studies in this meta-analysis was moderate with limited statistical power. Second, some studies were not included due to a lack of data about the association between PD-L1 expression and clinicopathologic characteristics.Citation50,Citation58 Moreover, studies included in this meta-analysis were mostly single-center retrospective studies and did not include multicenter prospective cohort studies.Citation23,Citation29,Citation30 Finally, meta-analysis is a retrospective method that is subject to methodological limitations. Therefore, further studies are recommended to better interpret the available datasets.
Conclusion
Our meta-analysis implied that PD-L1 may act as a negative predictor for the overall survival of PC patients, and high expression of PD-L1 was correlated with poor differentiation and neural invasion. However, further studies are recommended to better interpret the available datasets.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No 81572348 and No 81602123), the Science and Technology Planning Project of Guangdong Province (No 2014A020212386), the Guangdong Province Natural Science Foundation (No 2015A030313115 and No 2016A030313363), and the Foundation of Guangzhou Science and Technology Bureau (No 201510010206).
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2015CA Cancer J Clin201565152925559415
- ChenWZhengRBaadePDCancer statistics in China, 2015CA Cancer J Clin201666211513226808342
- ZhouBZhanHTinLTUFT1 regulates metastasis of pancreatic cancer through HIF1-Snail pathway induced epithelial-mesenchymal transitionCancer Lett20163821112027566398
- KaderaBETostePAWuNLow expression of the E3 ubiquitin ligase CBL confers chemoresistance in human pancreatic cancer and is targeted by epidermal growth factor receptor inhibitionClin Cancer Res201521115716525348515
- DelittoDPerezCHanSDownstream mediators of the intratumoral interferon response suppress antitumor immunity, induce gemcitabine resistance and associate with poor survival in human pancreatic cancerCancer Immunol Immunother201564121553156326423423
- XiaoGDengALiuHGeGLiuXActivator protein 1 suppresses antitumor T-cell function via the induction of programmed death 1Proc Natl Acad Sci U S A201210938154191542422949674
- TassiEGraziaGVegettiCEarly effector T lymphocytes coexpress multiple inhibitory receptors in primary non-small cell lung cancerCancer Res201777485186127979840
- ChatterjeeJDaiWAzizNHAClinical use of programmed cell death-1 and its ligand expression as discriminatory and predictive markers in ovarian cancerClin Cancer Res201731334533460
- DaleyDZambirinisCPSeifertLGammadelta T cells support pancreatic oncogenesis by restraining alphabeta T cell activationCell2016166614851499.e141527569912
- IraolagoitiaXLSpallanzaniRGTorresNINK cells restrain spontaneous antitumor CD8+ T cell priming through PD-1/PD-L1 interactions with dendritic cellsJ Immunol (Baltimore, Md: 1950)20161973953961
- ShaabaniNDuhanVKhairnarVCD169+ macrophages regulate PD-L1 expression via type I interferon and thereby prevent severe immunopathology after LCMV infectionCell Death Dis2016711e244627809306
- LiZDongPRenMPD-L1 expression is associated with tumor FOXP3(+) regulatory T-cell infiltration of breast cancer and poor prognosis of patientJ Cancer20167778479327162536
- HendriksDHeYKoopmansIProgrammed death ligand 1 (PD-L1)-targeted TRAIL combines PD-L1-mediated checkpoint inhibition with TRAIL-mediated apoptosis inductionOncoimmunology201658e120239027622071
- OhigashiYShoMYamadaYClinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancerClin Cancer Res20051182947295315837746
- ChenJLiGMengHUpregulation of B7-H1 expression is associated with macrophage infiltration in hepatocellular carcinomasCancer Immunol Immunother201261110110821853301
- KimJHParkHEChoNYLeeHSKangGHCharacterisation of PD-L1-positive subsets of microsatellite-unstable colorectal cancersBr J Cancer2016115449049627404452
- MuenstSSchaerliARGaoFExpression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancerBreast Cancer Res Treat20141461152424842267
- LiuYCarlssonRAmbjornMPD-L1 expression by neurons nearby tumors indicates better prognosis in glioblastoma patientsJ Neurosci20133335142311424523986257
- CooperWATranTVilainREPD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinomaLung Cancer (Amsterdam, Netherlands)2015892181188
- BogerCBehrensHMMathiakMKrugerSKalthoffHRockenCPD-L1 is an independent prognostic predictor in gastric cancer of Western patientsOncotarget2016717242692428327009855
- WellsGSheaBO’ConnellDThe Newcastle-Ottawa Scale (nos) for assessing the quality of nonrandomised studies in meta-analysesOttawa Hospital Research Institute Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.aspAccessed March 28, 2017
- TierneyJFStewartLAGhersiDBurdettSSydesMRPractical methods for incorporating summary time-to-event data into meta-analysisTrials2007811617555582
- BirnbaumDJFinettiPLoprestiAPrognostic value of PDL1 expression in pancreatic cancerOncotarget2016744711987121027589570
- DianaAWangLMD’CostaZPrognostic value, localization and correlation of PD-1/PD-L1, CD8 and FOXP3 with the desmoplastic stroma in pancreatic ductal adenocarcinomaOncotarget2016727409924100427329602
- YamakiSYanagimotoHTsutaKRyotaHKonMPD-L1 expression in pancreatic ductal adenocarcinoma is a poor prognostic factor in patients with high CD8+ tumor-infiltrating lymphocytes: highly sensitive detection using phosphor-integrated dot stainingInt J Clin Oncol201724726733
- WangYLinJCuiJPrognostic value and clinicopathological features of PD-1/PD-L1 expression with mismatch repair status and desmoplastic stroma in Chinese patients with pancreatic cancerOncotarget2017869354936528030840
- ChenYSunJZhaoHThe coexpression and clinical significance of costimulatory molecules B7-H1, B7-H3, and B7-H4 in human pancreatic cancerOnco Targets Ther201471465147225170273
- LiuJWLuYShenGJYaoWFHuangDSThe relationship of B7-H1 with clinicopathologic characteristics and prognosis of pancreatic carcinomaChin J Gen Pract2016144571574
- LoosMGieseNAKleeffJClinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancerCancer Lett200826819810918486325
- NomiTShoMAkahoriTClinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancerClin Cancer Res20071372151215717404099
- WangLMaQChenXGuoKLiJZhangMClinical significance of B7-H1 and B7-1 expressions in pancreatic carcinomaWorld J Surg20103451059106520145927
- ChuDDChuZHZhangJLClinical significance of PTEN and B7-H1 expressions in pancreatic carcinomaChin J Exp Surg2011285723726
- SongXLiuJLuYJinHHuangDOverexpression of B7-H1 correlates with malignant cell proliferation in pancreatic cancerOncol Rep20143131191119824378899
- InamuraKYokouchiYSakakibaraRRelationship of tumor PD-L1 expression with EGFR wild-type status and poor prognosis in lung adenocarcinomaJpn J Clin Oncol2016461093594127511990
- WuPWuDLiLChaiYHuangJPD-L1 and survival in solid tumors: a meta-analysisPLoS One2015106e013140326114883
- DaiCWangMLuJPrognostic and predictive values of PD-L1 expression in patients with digestive system cancer: a meta-analysisOnco Targets Ther2017103625363428769571
- AlexandrovLBJuYSHaaseKMutational signatures associated with tobacco smoking in human cancerScience (New York, NY)20163546312618622
- MaisonneuvePLowenfelsABRisk factors for pancreatic cancer: a summary review of meta-analytical studiesInt J Epidemiol201544118619825502106
- YadavDLowenfelsABThe epidemiology of pancreatitis and pancreatic cancerGastroenterology201314461252126123622135
- LinYMSungWWHsiehMJHigh PD-L1 expression correlates with metastasis and poor prognosis in oral squamous cell carcinomaPLoS One20151011e014265626562534
- MoriSMotoiNNinomiyaHHigh expression of programmed cell death 1 ligand 1 in lung adenocarcinoma is a poor prognostic factor particularly in smokers and wild-type epidermal growth-factor receptor casesPathol Int2017671374427976463
- InamuraKYokouchiYKobayashiMTumor B7-H3 (CD276) expression and smoking history in relation to lung adenocarcinoma prognosisLung Cancer (Amsterdam, Netherlands)20171034451
- AbikoKMatsumuraNHamanishiJIFN-gamma from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancerBr J Cancer201511291501150925867264
- QuandtDJasinski-BergnerSMullerUSchulzeBSeligerBSynergistic effects of IL-4 and TNFalpha on the induction of B7-H1 in renal cell carcinoma cells inhibiting allogeneic T cell proliferationJ Transl Med201412115124885059
- Rodriguez-GarciaMPorichisFde JongOGExpression of PD-L1 and PD-L2 on human macrophages is up-regulated by HIV-1 and differentially modulated by IL-10J Leukoc Biol201189450751521097698
- FukudaTKamaiTMasudaAHigher preoperative serum levels of PD-L1 and B7-H4 are associated with invasive and metastatic potential and predictable for poor response to VEGF-targeted therapy and unfavorable prognosis of renal cell carcinomaCancer Med2016581810182027292320
- KronigHKremmlerLHallerBInterferon-induced programmed death-ligand 1 (PD-L1/B7-H1) expression increases on human acute myeloid leukemia blast cells during treatmentEur J Haematol201492319520324175978
- ChenNFangWZhanJUpregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutationJ Thorac Oncol201510691092325658629
- OtaKAzumaKKawaharaAInduction of PD-L1 expression by the EML4-ALK oncoprotein and downstream signaling pathways in non-small cell lung cancerClin Cancer Res201521174014402126019170
- SoaresKCRuckiAAWuAAPD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumorsJ Immunother (Hagerstown, Md: 1997)2015381111
- WilkeCMWeiSWangLKryczekIKaoJZouWDual biological effects of the cytokines interleukin-10 and interferon-gammaCancer Immunol Immunother201160111529154121918895
- IrelandLSantosAAhmedMSChemoresistance in pancreatic cancer is driven by stroma-derived insulin-like growth factorsCancer Res201676236851686327742686
- YangSHGuoJCYehKHTienYWChengALKuoSHAssociation of radiotherapy with favorable prognosis in daily clinical practice for treatment of locally advanced and metastatic pancreatic cancerJ Gastroenterol Hepatol201631122004201227059987
- AzadAYin LimSD’CostaZPD-L1 blockade enhances response of pancreatic ductal adenocarcinoma to radiotherapyEMBO Mol Med20179216718027932443
- GibsonAOgeseMSullivanANegative regulation by PD-L1 during drug-specific priming of IL-22-secreting T cells and the influence of PD-1 on effector T cell functionJ Immunol (Baltimore, Md: 1950)2014192626112621
- MaceTAShakyaRPitarresiJRIL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancerGut Epub20161021
- AntoniaSGoldbergSBBalmanoukianASafety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b studyLancet Oncol201617329930826858122
- BassoDFogarPFalconiMPancreatic tumors and immature immunosuppressive myeloid cells in blood and spleen: role of inhibitory co-stimulatory molecules PDL1 and CTLA4. An in vivo and in vitro studyPLoS One201381e5482423359812